Biology of the T Lymphocyte
Introduction
Previous chapters focused on the characteristics of B lymphocytes and their receptor for antigen, immunoglobulin (Ig). In Chapter 9 we introduced the role of immune responses involving T lymphocytes (T cells) and discussed the key roles of MHC molecules in the recognition of antigen by T cells. Why do we need two sets of antigen-specific lymphocytes if we have B cells? B cells and their products—antibodies—are extremely effective at dealing with pathogens such as viruses, bacteria, and parasites in the body fluids but are not effective once the pathogens infect or are taken into cells. We believe that T cells evolved to deal with the crucial phase of the response to pathogens that takes place inside host cells. Because proteins are either major components of pathogens or are synthesized by pathogens, T cells play a critical role in the response to nearly all the harmful agents—and “harmless” antigens—to which an individual is exposed.
In this chapter we focus on the major characteristics of T cells. First we describe the T cell’s receptor for antigen—the TCR—and compare its characteristics with those of the B-cell receptor (BCR), Ig, and then we will describe other important molecules on the T-cell surface. Next we explain the key steps in T-cell development in the thymus, the organ in which the developing T cell acquires its TCR, and describe the critical role played by MHC molecules in this process.
The Antigen-Specific T-Cell Receptor
Molecules That Interact with Antigen
Like B cells, T cells express an antigen-specific receptor that is clonally distributed, i.e., every clone of T cells expresses a TCR with a unique sequence. The huge repertoire of TCR molecules, calculated to be 109 or more different possible structures, is generated by the same V(D)J gene rearrangement strategies described for Ig molecules in Chapter 7. The unique aspects of TCR gene rearrangement are discussed in more detail later in the chapter.
The αβ TCR.
Figure 10.1 shows the form of the TCR expressed on the majority of mature T cells in humans and many other species. It comprises two disulfide-linked polypeptide chains, α and β, that span the cell membrane. A minor population of human T cells expresses an alternative TCR made of γ and δ chains, which we discuss later in the chapter.
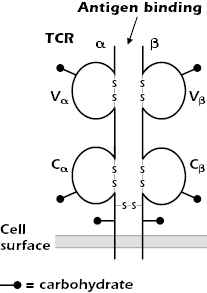
The TCR α and β chains are both glycoproteins with short cytoplasmic tails; because of differences in the carbohydrate composition, the molecular weights of the chains vary between 40 and 60 kDa. The TCR α and β chains consist of variable (V) and constant (C) regions, analogous to the V and C regions of Ig molecules (see Figures 5.3 and 5.14 in Chapter 5). Each TCR V and C region folds into an Ig-like domain.
As shown in Figure 10.2, the TCR Vα and Vβ regions, like the Ig VH and VL regions, contain three complementarity-determining regions (CDR1, CDR2, and CDR3) that come together in the three-dimensional structure to form the antigen-binding site. (The β chain has an additional hypervariable region [HV4] that does not bind MHC + peptide, but does bind to superantigens, discussed in Chapter 11.)
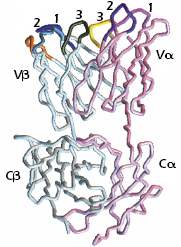
The similarities between the structures of the TCR and Ig and the organization of the genes that code for these molecules suggest that the TCR and Ig evolved from a common ancestral gene. As we described in Chapter 5 and illustrated in Figure 5.14, these genes belong to the Ig gene superfamily, and the molecules are referred to as members of the Ig superfamily.
Comparison of the αβ TCR and Ig.
Although the structures of the TCR and Ig are similar, they differ in several important ways (see Table 10.1).
Table 10.1. Comparison of the Properties of the TCR and the B Cell Receptor for Antigen, Immunoglobulin
| T-Cell Receptor | B-Cell Receptor |
|---|---|
| Two chains | Four chains |
| One antigen binding site (monovalent) | Two antigen binding sites (bivalent) |
| Predominantly protein antigens (more specifically, peptides) recognized | Can recognize carbohydrate, DNA, lipid, or protein |
| Peptide recognized must be bound to an MHC molecule | Can recognize free antigen |
| Restricted to interacting with other cells | Can deal with any antigen in body fluids |
| Not secreted on activation | Secreted on activation |
| No change in TCR during response to antigen | Can undergo somatic hypermutation and class switch recombination |
Antigen Recognition.
Ig binds to many different types of antigen (carbohydrates, DNA, lipids, and proteins), which it encounters in fluids such as serum (see Chapters 5 and 6). Ig can also respond to linear and conformational epitopes in an antigen; thus, both the three-dimensional shape and the sequence of an antigen are important in eliciting antibody responses. By contrast, the αβ TCR interacts predominantly with small linear fragments of proteins (peptides) bound to an MHC molecule expressed on the surface of a host cell. Consequently, in contrast to the variety of structures and shapes recognized by Ig molecules, the antigen recognized by the αβ TCR is generally a combination of major histocompatibility complex (MHC) molecule (either MHC class I or II) and a small peptide (8–9 amino acids long for peptides bound to MHC class I or 12–17 amino acids long for peptides bound to MHC class II).
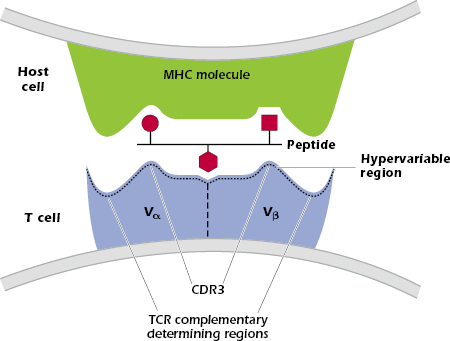
The TCR makes critical interactions with both the MHC molecule and the peptide bound in the MHC groove—the complex of MHC and peptide interacts with the CDRs of the TCR Vα and Vβ chains (Figure 10.3). Some sequences in the TCR contact the MHC molecule, while other sequences contact the peptide. In the TCR-peptide–MHC interaction shown in Figure 10.3, the TCR CDR3 contacts amino acids in the center of the peptide, with CDRs 1 and 2 interacting with the MHC molecule (not shown). This pattern is found in many TCRs but some show the reverse pattern, in which CDRs 1 and 2 interact with the peptide.
Valence and Conformation.
The four-chain Ig molecule has a hinge and two antigen-binding sites. When expressed at the surface of the B cell or in solution, these properties give the Ig molecule a flexibility that allows it to bind bivalently to antigens of different shapes and sizes. The TCR is a two-chain structure that forms a single binding site for antigen; in other words, the TCR is monovalent and resembles the monovalent Fab fragment of an antibody. The TCR also has a rigid conformation, which is appropriate for binding to the surface of other host cells.
Secretion of Receptor.
Unlike Ig, the TCR does not exist in a specifically secreted form and is not secreted as a consequence of T-cell activation. Thus, unlike Ig, the TCR does not have an “effector” function. Rather, T-cell activation results in cytokine secretion and/or the killing of infected host cells. By contrast, after antigen binds to membrane Ig and activates the B cell, the B cell differentiates into a plasma cell that secretes Ig with the same antigenic specificity expressed by the B cell that initially bound the antigen (see Chapters 7 and 8). Thus the effector molecule of the B cell is antigen specific, but the effector molecules secreted by T cells are not.
No Change in TCR During Response to Antigen.
Over the course of a response to antigen, Ig molecules undergo somatic hypermutation (with associated affinity maturation) and class switching, the linking of one set of genes coding for a particular V region to different C region genes. These mechanisms are unique to B cells: Once a T cell has acquired its TCR in the thymus, it does not undergo any further genetic alteration. Thus, the TCR does not change during the response to antigen.
The T-Cell Receptor Complex
The TCR is expressed on the surface of T cells in tight, noncovalent association with the molecule CD3 and with two identical ζ (zeta) chains (CD247, molecular weight 16 kDa; Figure 10.4).
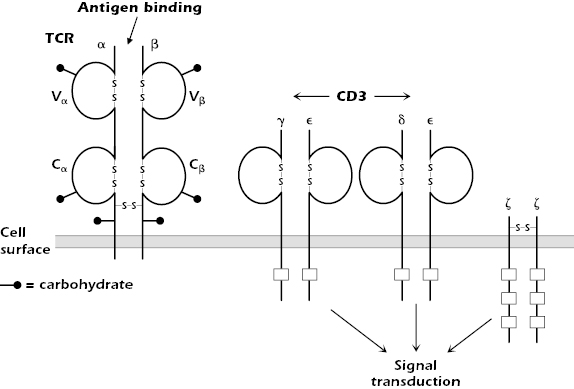
The combination of TCR and CD3 and ζ is referred to as the TCR complex. CD3 consists of three polypeptides γ, δ, and ε (molecular weights 25, 20, and 20 kDa, respectively), which are expressed in the membrane as two sets of molecules: γε and δε. Note that the CD3 γ and δ molecules differ from the γ and δ chains of the TCR expressed by γδ T cells.
CD3 and ζ do not bind antigen. They are signal transduction molecules activated after antigen binding to the TCR; thus, they are analogous to the Igα/Igβ molecules associated with Ig in the B-cell antigen-specific receptor complex (Chapter 8).
Each chain of the CD3 complex contains one tyrosine-containing sequence referred to as an immunoreceptor tyrosine-based activation motif (ITAM) (also found in Igα and Igβ), and the ζ chain contains three. After antigen binds to the antigen-binding chains of the TCR, the ITAMs of the CD3 and ζ chains play important roles in the early phases of T-cell activation (see Chapter 11).
CD3 has a second important function: It acts as a “chaperone” to transport a newly synthesized TCR molecule through the cell to the cell surface. Thus, it is always found in association with the TCR at the T-cell surface. Although the CD3ε chain is detectable in the cytoplasm of natural killer (NK) cells, the entire CD3 molecule is expressed exclusively only on the surface of T cells. Thus, surface expression of all CD3 polypeptides can be used as a marker to distinguish T cells from all other cells.
Co-Receptors
The TCR is expressed on the T-cell surface in close association with either CD4 or CD8, which are known as co-receptors (Figure 10.5). CD4 is a single-chain glycoprotein, and CD8 is a two-chain glycoprotein. CD4 and CD8 do not bind antigen but, as shown in Figures 10.5A and 10.5B, they bind to MHC class II and MHC class I molecules, respectively. Expression of either CD4 or CD8 splits the αβ TCR-expressing T-cell population into two major subsets, known as CD4+ T cells and CD8+ T cells; only immature T cells differentiating in the thymus express both CD4 and CD8. In addition to their expression on these major subsets of T cells, CD4 and CD8 are also expressed on other cells: CD4, by monocytes, macrophages and dendritic cells, and CD8 by dendritic cells and NK cells.
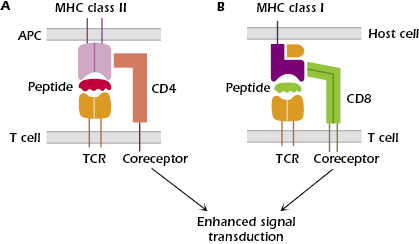
The function of CD4+ T cells is predominantly in producing cytokines and thus interacting with many other cell types. The function of CD8+ T cells is predominantly to kill infected cells. In healthy people, the ratio of CD4+ T cells to CD8+ T cells in the circulation is approximately 2 : 1, but this ratio is decreased during viral infections (due to an increased number of CD8+ T cells) and in conditions such as acquired immune deficiency syndrome (AIDS), in which the level of CD4+ T cells is depleted (see Chapter 18).
CD4 and CD8 have several important functions, as follows:
- They act as specific adhesion molecules in T-cell interactions with host cells, and thus tighten the binding of T cells to host cells that express peptide in combination with an MHC molecule.
CD4 binds selectively to all cells expressing MHC class II molecules (Figure 10.5A); CD4 binds outside the peptide-binding groove of the MHC class II molecules, to the invariant portion of the molecule. MHC class II molecules are expressed constitutively on antigen-presenting cells (APCs): dendritic cells, macrophages, B cells, and thymic epithelial cells. Thus, CD4+ T cells interact with APCs expressing antigen bound to MHC class II.
CD8 binds selectively to cells expressing MHC class I molecules (Figure 10.5B), which are expressed on the surface of all nucleated cells in the body. Thus, CD8+ T cells interact with all host cells expressing antigen associated with MHC class I.
- They are signal transduction molecules that enhance the signal through the TCR after antigen binding. In this way, the co-receptor enhances the ability of antigen to activate T cells; that is, expression of the co-receptor lowers the threshold for antigen responses; therefore, less antigen is needed to stimulate the T cell than in the absence of the co-receptor. Thus, the T-cell co-receptor is analogous to the B-cell co-receptor, the complex of CD19, CD21, CD81, and CD225 (Chapter 8). The signaling function of CD4 and CD8 is carried out by the intracellular portion of each molecule, which is linked to protein tyrosine kinases that are important early components of the T-cell activation pathways. This concept will be discussed more fully in Chapter 11.
- A unique characteristic of the human CD4 molecule is that HIV-1 binds to it. This binding allows the virus to infect cells expressing CD4, eventually leading to AIDS (see Chapter 18). Because CD4 is expressed by monocytes, macrophages and dendritic cells as well as T cells, all these cell types may be infected by HIV-1.
Other Important Molecules Expressed on the T-Cell Surface
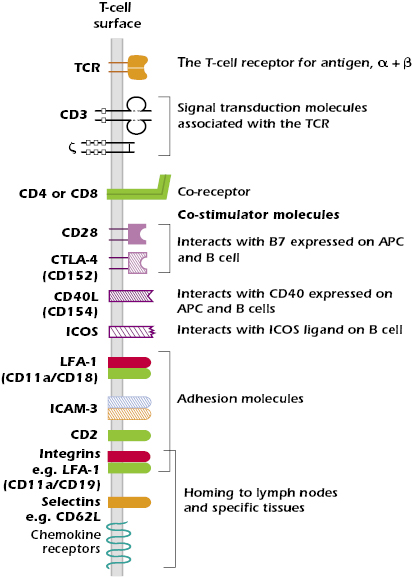
In the following paragraphs and in Figure 10.6 we describe molecules in addition to those associated with the TCR complex and T-cell co-receptors that play important roles in T-cell function.
Co-Stimulator Ligands.
In order to be activated, naïve T cells (T cells that have not previously encountered antigen) need two signals. The first signal is the interaction between peptide and MHC expressed on the APCs and the TCR expressed by the T cell. Second signals, also known as co-stimulator interactions, are needed for full T-cell activation. These co-stimulator interactions enhance the signal delivered by the TCR complex.
Multiple pairs of such co-stimulator molecules have been defined on the T cell and APC surfaces. The best understood interaction is between CD28 expressed on the T cell and the B7 family of molecules expressed on APCs. This interaction is critical for antigen-activated T cells to synthesize the cytokine IL-2, a T-cell growth factor, which is required for the proliferation of T cells (also see Chapter 11). We now know that there are several molecules in the CD28 family on T cells. These interact with either B7 or other molecules on the surface of an APC and have functions other than simply activation. One important member of the CD28 family is CTLA-4 (CD152) (Chapters 11 and 13). It is expressed on activated T cells and interacts with B7 molecules to impart a negative signal to the activated T cell, which helps to terminate the response.
T cells also express CD40 ligand (CD40L or CD154), which interacts with CD40 expressed on macrophages, dendritic cells, and B cells. This interaction enhances the B7-CD28 co-stimulator interaction. The CD40–CD40L interaction also plays a critical role in T-cell-dependent class switching and somatic hypermutation in B cells.
Molecules Involved in Adhesion and Homing
Adhesion.
T cells make several adhesive interactions with molecules expressed on the surface of APCs. None of these molecules is specific to the T cell or APC. These interactions are important when the T cell enters a lymph node; the adhesive interactions slow down the movement of the T cell near the APCs, particularly dendritic cells, allowing the T cell time to “scan” the dendritic cells for the “correct” peptide + MHC combination. Key adhesion molecules expressed on the T cell include the following:
- CD2, which binds to LFA-3 (CD58) expressed on APCs and many other cells; CD2 is expressed almost exclusively on T cells and is expressed by all mature and immature T cells, as well as NK cells.
- LFA-1 (CD11aCD18), a member of the two-chain integrin family, which binds to several ligands, including ICAM-1 (CD54), expressed on endothelial cells and APCs such as macrophages and dendritic cells.
- ICAM-3 (CD50), which binds to LFA-1 expressed on APC.
Homing.
Like B cells, T cells express surface molecules associated with homing, the preferential entry of cells into specific tissues. Homing is tightly regulated by the expression pattern of adhesion molecules and chemokine receptors; their pattern of expression changes with the activation state of the cell. Naïve T cells, like naïve B cells, circulate through peripheral and mucosal lymph nodes. This enhances their ability to interact with antigen because naïve lymphocytes are not normally resident in tissues, where an individual is likely to be exposed to pathogens.
Entry of naïve T lymphocytes to lymph nodes is mediated by the expression of the same set of adhesion molecules and chemokine receptors expressed by naïve B cells. The critical adhesion molecules are L-selectin (CD62L) for entry to peripheral lymph nodes (those outside the mucosal associated lymphoid tissue) and the integrin α4β7 for lymphoid tissue in the mucosa. These adhesion molecules bind to glycoproteins known as addressins, which are expressed on cells of the high endothelial venules, the specialized region of the vascular endothelium at the boundary of the nodes. Naïve T cells also express the chemokine receptor CCR7, which binds chemokines expressed by high endothelial venules of peripheral and mucosal nodes. Binding of the appropriate combination of adhesion molecule and chemokine receptor to their ligands expressed by high endothelial venules allows naïve T cells to leave the circulation and enter a particular lymph node. The mechanisms by which lymphocytes enter tissues from the bloodstream are similar to those described for other leukocytes in Chapter 12.
Once T cells are activated in the nodes, the resulting effector cells and memory T cells change their pattern of expressing adhesion molecules and chemokine receptors, migrate out of the nodes, and home to specific tissues. The expression pattern of adhesion molecules and chemokine receptors provides an “address code” that identifies the tissue to which the T cell migrates. Generally, this homing takes the effector or memory T cell back to the region in which the T cell was originally activated; for example, cells that were initially activated in nodes draining the gut will home back to the gut.
γδ T Cells
Most human T cells use αβ chains as their TCR but a minor population expresses a distinct two-chain TCR known as γδ; the cells expressing this receptor are referred to as γδ T cells
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree