Cytokines
Introduction
As we have already discussed, the immune system is regulated by soluble mediators collectively called cytokines. These low-molecular-weight proteins are produced by virtually all cells of the innate and adaptive immune systems and, in particular, by T cells, which orchestrate many effector mechanisms. Some cytokines possess direct effector functions of their own. A simple way to understand how cytokines work is to compare them with hormones, the chemical messengers of the endocrine system. Cytokines serve as chemical messengers within the immune system, although they also communicate with cells in other systems, including those of the nervous system. Thus, they can function in an integrated fashion to facilitate homeostasis. By contrast, they also play a significant role in driving hypersensitivity and inflammatory responses, and in some cases they can promote acute or chronic distress in tissues and organ systems.
As we will discuss later in this chapter, cells regulated by a particular cytokine must express a receptor for that factor. Cells are positively and/or negatively regulated by the quantity and type of cytokines to which they are exposed and by the expression or downregulation of cytokine receptors. Normal regulation of innate and adaptive immune responses is largely controlled by a combination of these methods.
The History of Cytokines
In the late 1960s, when the activities of cytokines were first discovered, it was believed that they served as amplification factors that acted in an antigen-dependent fashion to elevate proliferative responses of T cells. Gery and colleagues were the first to demonstrate that macrophages released a thymocyte mitogenic factor termed lymphocyte activating factor (LAF). This view changed radically when it was found that supernatants of mitogen-stimulated peripheral blood mononuclear cells promoted the long-term proliferation of T cells in the absence of antigens and mitogens. Soon afterward, it was found that this factor was produced by T cells and could be used to isolate and clonally expand functional T cell lines. This T-cell-derived factor was given several names by different investigators, most notably, T-cell growth factor (TCGF). Cytokines produced by lymphocytes were collectively called lymphokines, whereas those produced by monocytes and macrophages were called monokines. To complicate matters, studies of the cellular sources of lymphokines and monokines ultimately revealed that these factors were not the exclusive products of lymphocytes and monocytes/macrophages. Thus, the more appropriate term cytokine was adopted as a generic name for these glycoprotein mediators. In 1979, an international workshop was convened to address the need to develop a consensus regarding the definition of these macrophage- and T-cell-derived factors. Since they mediated signals between leukocytes, the term interleukin was coined. The macrophage-derived LAF and T-cell-derived growth factors were given the names interleukin-1 (IL-1) and interleukin-2 (IL-2), respectively. Currently, numbers have been assigned to more than 40 interleukins, and this will undoubtedly continue to grow as research efforts continue to identify new members of this cytokine family. To further illustrate the degree to which the cytokine field has outgrown the terminology established in 1979, knowledge of the functional properties of various cytokines has engendered a more liberal meaning of terms originally designed to define what a given factor does.
Pleiotropic and Redundant Properties of Cytokines
It is well known that many cytokines have important biologic effects on cell types other than those of the immune system. Thus, cytokines commonly have pleiotropic properties, since they can affect the activity of many different cell types. For example, the development and differentiation of bone-forming cells known as osteoblasts is regulated by a host of different cytokines. IL-2 produced by activated T lymphocytes also drives differentiation of human bone marrow stromal cells toward an osteoblastic phenotype. In contrast, tumor necrosis factor (TNF)-α inhibits the differentiation of osteoblasts and has been shown to be proapoptotic for these cells. IL-4 and IL-13 suppress osteoblast prostaglandin synthesis in bone and are chemoattractants for these cells.
Cytokines are also linked to human diseases that involve bone. Perhaps the most extensive studies have been on the role of cytokines in the development of osteolytic lesions sometimes observed in severe rheumatoid arthritis (RA) and other inflammatory bone diseases, including periodontal disease. A hallmark of RA is the rapid erosion of periarticular bone, which is often followed by general secondary osteoporosis (osteopenia).
Knowledge regarding such intersystem crosstalk will no doubt contribute to our understanding of how the immune system regulates cells in other systems in a physiologic context, both at the molecular level and at the level of organ systems. In the case of bone formation, this will lead to better treatments for human diseases involving both systems, including various inflammatory and metabolic bone diseases, as well as tumor-induced bone lysis.
In addition to their pleiotropic properties, there is a great deal of functional redundancy among cytokines. This redundancy is explained, in part, by the common use of cytokine receptor signaling subunits (e.g., common γ chain used by receptors for IL-2, IL-4, IL-7, IL-9, and IL-15) by certain groups of cytokines discussed later in this chapter. Finally, cytokines rarely, if ever, act alone in vivo. Under physiologic and, in some cases, pathophysiologic conditions, cells responding to cytokines do so within a milieu containing multiple cytokines, which often exhibit additive, synergistic, or antagonistic properties. In the case of synergism, the combined effect of two or more cytokines is sometimes greater than the additive effects of the individual cytokines. Conversely, antagonism occurs when one or more cytokines inhibit the biologic activity of one or more other cytokines.
The convenient nomenclature originally developed to define the origin or the functional activities of certain cytokines has, in general, not held up. Nevertheless, occasionally, the field recognizes that common functional features of several glycoproteins merit the creation of yet another collective term to help define a family of cytokines. In particular, the term chemokines was adopted in 1992 to describe a family of chemotactic cytokines with conserved sequences, known to be potent attractors for various leukocyte subsets, such as lymphocytes, neutrophils, and monocytes. As students of immunology, learning about the rapidly expanding list of cytokines with diverse functional characteristics may appear to be a formidable task. However, by focusing on some that deserve special mention, we hope that this will be interesting and manageable exercise.
General Properties of Cytokines
Common Functional Properties
Cytokines have several functional features in common. Some, like interferon-γ (IFN-γ) and IL-2, are synthesized by cells and rapidly secreted. Others such as TNF-α and TNF-β may be secreted or expressed as membrane-associated proteins. Most cytokines have very short half-lives; consequently, cytokine synthesis and function typically occur in a burst.
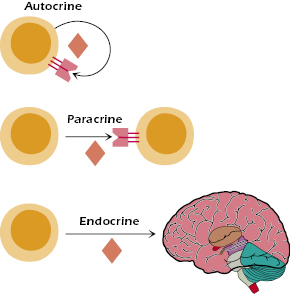
Similar to the actions of polypeptide hormones, cytokines facilitate communication between cells and do so at very low concentrations (typically 10−10 to 10−15 M). Cytokines may act locally either on the same cell that secreted it (autocrine), on other nearby cells (paracrine), or, like hormones, they may act systemically (endocrine) (Figure 12.1). In common with other polypeptide hormones, cytokines exert their functional effects by binding to specific receptors on target cells. Thus, cells regulated by specific cytokines must have the capacity to express a receptor for that factor. In turn, the activity of a responder cell may be regulated by the quantity and type of cytokines to which they are exposed or by the upregulation or downregulation of cytokine receptors, which, themselves, may be regulated by other cytokines. A good example of the latter is the ability of IL-1 to upregulate IL-2 receptors on T cells. As noted earlier, this illustrates one common feature of cytokines, namely, their ability to act in concert with one another to create synergistic effects that reinforce the other’s action on a single cell.
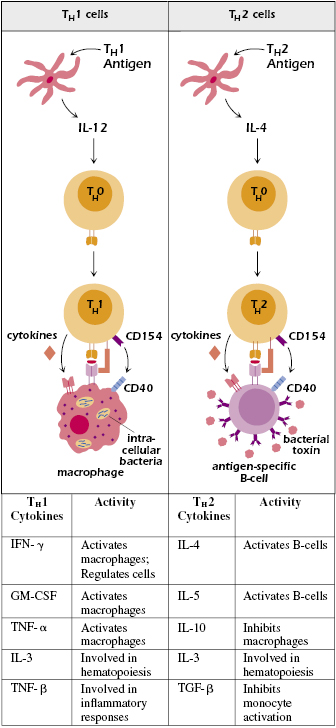
Alternatively, some cytokines behave antagonistically toward one or more other cytokines and thus inhibit each other’s action on a given cell. The TH1 and TH2 lineage choices have become pivotal examples for the regulation (positive or negative) of cell differentiation. TH1 and TH2 cells are characterized by distinct cytokine profiles and transcription factor expression (discussed later in this chapter), and also by the types of pathogens they control. Perhaps the classic example of differential cytokine expression by TH1 and TH2 cells is the ability of TH1cells to produce high levels of the effector cytokine IFN-γ as compared with TH2 cells, which produce IL-4, and, in some cases, IL-10. IFN-γ activates macrophages but inhibits B cells and is directly toxic for certain cells. In contrast, IL-4 activates B cells, and IL-10 inhibits macrophage activation and suppresses allergic inflammation (Figure 12.2). TH2 cells that express IL-10 have been termed inflammatory TH2 cells to distinguish them from canonical noninflammatory TH2 cells. Additional information regarding TH1 and TH2 transcription factor profiles and how they have evolved to protect the host against a spectrum of pathogens is provided in Chapters 10 and 21, respectively.
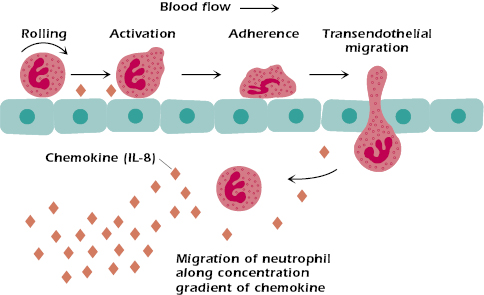
When cells produce cytokines in response to various stimuli (e.g., infectious agents), they establish a concentration gradient that serves to control or direct cell migration patterns, also known as chemotaxis (Figure 12.3). Cell migration (e.g., neutrophil chemotaxis) is essential to the development of inflammatory responses resulting from localized injury or other trauma. Chemokines play a key role in providing signals that upregulate expression of adhesion molecules expressed on endothelial cells to facilitate neutrophil chemotaxis and transendothelial migration.
Common Systemic Activities
Cytokines can act over both short range and long range, with consequent systemic effects. Cytokines thus play a crucial role in the amplification of the immune response because the release of cytokines from just a few antigen-activated cells results in the activation of multiple different cell types, which are not necessarily antigen specific or located in the immediate area. This is apparent in a response such as delayed-type hypersensitivity, discussed in detail in Chapter 17, in which the activation of rare antigen-specific T cells is accompanied by the release of cytokines. As a consequence of cytokine effects, monocytes are recruited into the area in great numbers, dwarfing the original antigen-activated T-cell population. It is also worth noting that the production of high levels of cytokines by a powerful stimulus can trigger deleterious systemic effects, such as toxic-shock syndrome, as discussed later in this chapter. Similarly, therapeutic manipulation of the immune system using recombinant cytokines or cytokine antagonists can affect multiple physiologic systems depending on the range of biologic activity associated with a particular cytokine.
Common Cell Sources and Cascading Events
A given cell may make many different cytokines. Moreover, one cell may be the target of many cytokines, each binding to its own specific cell-surface receptor. Consequently, one cytokine may affect the action of another, which may lead to an additive, synergistic, or antagonistic effect on the target cell.
Interactions of the multiple cytokines produced during a typical immune response are often referred to as the cytokine cascade. This cascade largely determines whether a response to an antigen will be primarily antibody-mediated (and, if so, which classes of antibodies will be made) or cell-mediated (and, if so, whether cells engaged in delayed hypersensitivity or cytotoxicity will be activated). Later in this chapter, we will discuss the cytokine-mediated control mechanisms that help determine the pattern of cytokines that develop following CD4+ T-cell activation. The antigenic stimulus appears to play a key role in the initiation of cytokine responses by these cells. Thus, depending on the nature of the antigenic signal, and the cytokine milieu associated with T-cell activation, naïve effector CD4+ T cells will generate a particular cytokine profile, one that ultimately controls the type of immune response generated (humoral versus cellular). The cytokine cascade associated with immune responses also determines what other systems are activated or suppressed, as well as the level and duration of the response.
Functional Categories of Cytokines
We will not attempt to list all of the currently well-characterized and molecularly cloned cytokines in this chapter. The major cytokines that play a role in the immune response and a brief description of their functions are listed in Table 12.1. A convenient way to begin is to classify cytokines into the functional categories discussed below based on shared functional properties. It is important to state that this subdivision, although convenient, is somewhat arbitrary, given the pleiotropic effects of many cytokines.
Table 12.1. Selected Cytokines and Their Functions
| Cytokine | Produced By | Major Functions |
|---|---|---|
| IL-1 | Monocytes; many other cell types | Produces fever; stimulates acute-phase protein synthesis; promotes proliferation of TH2 cells |
| IL-2 | TH0 and TH1 cells | T-cell growth factor |
| IL-3 | TH cells, NK cells, mast cells | Growth factor for hematopoietic cells |
| IL-4 | TH2, CD4+ T cells, mast cells | Growth factor for B cells and TH2 CD4+ T cells; promotes IgE and IgG synthesis; inhibits TH1 CD4+ T cells |
| IL-5 | TH2 cells, mast cells | Stimulates B-cell growth and Ig secretion; growth and differentiation factor eosinophils |
| IL-6 | T cells, many other cell types | Induces acute-phase protein synthesis, T-cell activation, and IL-2 production; stimulates B-cell Ig production and hematopoietic progenitor cell growth |
| IL-7 | Bone marrow, thymic stromal cells, some T cells | Growth factor for pre-T and pre-B cells |
| IL-9 | T cells | Mast cell activation |
| IL-10 | TH2 cells, macrophages | Inhibits production of TH1 cells and macrophage function |
| IL-11 | Fibroblasts | Stimulates megakaryocyte (platelet precursor) growth |
| IL-12 | B cells and macrophages | Activates NK cells and promotes generation of TH1 CD4+ T cells |
| IL-13 | T cells | Shares characteristics with IL-4 (e.g., Ig switch to IgE synthesis) but does not affect T cells; growth factor for human B cells |
| IL-14 | T cells | Involved in development of memory B cells |
| IL-15 | T cells and epithelial cells | T-cell growth factor; similar to IL-2 |
| IL-16 | T cells, eosinophils, mast cells | Chemotactic for T cells; proinflammatory |
| IL-17 family (IL-17A-F) | T cells (TH17 lineage) | Proinflammatory cytokine, promotes neutrophil migration and differentiation, plays decisive role in autoimmunity and immune-mediated tissue damage |
| IL-18 | Macrophages, monocytes, dendritic cells, many other cell types | Induces IFN-γ production; enhances NK-cell lytic activity |
| IL-23 | Activated dendritic cells | Acts on memory T cells to stimulate IL-17 secretion |
| IFN-γ | TH1 cells | Activates NK cells and macrophages; inhibits TH2 CD4+ T cells; induces expression of MHC class II on many cell types |
| TGF-β | Lymphocytes, macrophages, platelets, mast cells | Enhances production of IgA; inhibits activation of monocyte and T-cell subsets; active in fibroblast growth and wound healing |
| TNF-α | Macrophages, mast cells | Involved in inflammatory responses; activates endothelial cells and other cells of immune and nonimmune systems; induces fever and septic shock |
| TNF-β (lymphotoxin) | T cells | Involved in inflammatory responses; also plays role in killing target cells by cytotoxic CD8+ T cells |
| GM-CSF | T cells, monocytes | Promotes growth of granulocytes and macrophages; growth of dendritic cells in vitro |
| M-CSF | T cells, monocytes | Promotes macrophage growth |
| G-CSF | T cells, monocytes | Promotes granulocyte growth |
Cytokines That Facilitate Innate Immune Responses
Several cytokines facilitate innate immune responses stimulated by viruses and microbial pathogens. Included in this group are IL-1, IL-6, TNF-α, interferon-α (IFN- α), and IFN-β. IL-1, IL-6, and TNF-α initiate a wide spectrum of biologic activities that help coordinate the host’s responses to infection. They are produced largely by phagocytes (e.g., macrophages and neutrophils) and are termed endogenous pyrogens because they cause fever. Elevated body temperature is beneficial to host defenses because adaptive immune responses are more intense and most pathogens grow less efficiently at raised temperatures. Another important effect of IL-1, IL-6, and TNF-α is their initiation of a response known as the acute-phase response characterized by production of acute-phase proteins by hepatocytes. As discussed below, acute inflammation (e.g., in response to infections) is generally accompanied by a systemic acute-phase response. Typically, changes in acute-phase protein plasma levels occur within 2 days following infection. One of these proteins, C-reactive protein (CRP), binds to phosphorylcholine on bacterial surfaces, acts like an opsonin and also activates the classical complement pathway (Chapter 14). Another acute-phase protein with opsonin and complement-activating activity is mannan-binding lectin (MBL), which binds mannose residues accessible on many bacteria. Given these functional properties, they mimic the actions of antibodies, which opsonize bacteria and activate the complement cascade. In concert with the other members of the acute-phase protein family, CRP and MBL lead to bacterial clearance.
Another effect of the endogenous pyrogens is to induce an increase in circulating neutrophils that are summoned from the bone marrow and from the blood vessels where leukocytes attach loosely to endothelial cells. Finally, dendritic cells from peripheral tissues migrate to the lymph nodes in response to these cytokines. There, they serve as potent antigen-presenting cells (APCs) to facilitate adaptive immune responses needed to control infections.
The term interferon was coined because they interfere with viral replication thus blocking the spread of viruses to uninfected cells. Interferon-α (IFN-α) and interferon-β (IFN-β) (so-called type I interferons) are synthesized by many cell types following viral infection. Plasmacytoid dendritic cells (DCs) have been identified as being the most potent producers of type I IFNs in response to antigen and have thus been coined natural IFN-producing cells. They are distinguished from another glycoprotein called interferon-γ (the sole member of type II IFNs), which is produced by activated natural killer (NK) cells and effector T cells, and thus appear after the induction of adaptive immune responses. In addition to their antiviral activities, IFN-α and IFN-β induce increased MHC class I expression on most uninfected cells, thus enhancing their resistance to NK cells as well as making newly infected cells more susceptible to killing by CD8+ cytotoxic T cells. Finally, they activate NK cells, which contribute to early host responses to viral infections.
Cytokines That Regulate Adaptive Immune Responses
As discussed in Chapter 11, B- and T-cell activation in response to antigen stimulation is regulated by cytokines. Depending on the cytokines involved, regulation can be positive or negative and can impact cell proliferation, activation, and differentiation. Ultimately, cytokines regulate the intensity and duration of immune responses. A major feature of all adaptive immune responses is their antigen specificity. Given the potent immunoregulatory activities of cytokines, how does the immune system ensure that antigen-nonspecific B and T cells are not activated during an immune response? One mechanism to ensure the specificity of the immune response is through the selective expression of functional cytokine receptors only on lymphocytes that have been stimulated by antigen. As a consequence, cytokines tend to act only on antigen-activated lymphocytes. A second mechanism that protects antigen-nonspecific lymphocytes from being activated by cytokines involves the need for cells to interact with each other through cell-to-cell contact, also known as cognate interaction. Such interactions that might occur, say, between CD4+ helper T cells and APCs (e.g., DCs, macrophages, B cells), generate high concentrations of cytokines at the juncture between the interacting cells. In this way, only the target cell(s) participating in the interaction are affected by the cytokines produced. Finally, since the half-life of cytokines is very short, particularly in the bloodstream and extracellular spaces, they have a very limited period to act on other target cells.
Cytokines That Induce Differentiation of Distinct T-Cell Lineages
TH1 and TH2 Cell Lineages.
It is now clear that certain cytokines play a key role in determining the fate of naïve T cells by staging the signaling events involved in lineage-specific differentiation. As discussed in earlier chapters, activated naïve CD4+ T cells (TH0 cells) differentiate into TH1 cells under the influence of IL-12 produced by DCs and macrophages in the skin and mucosa (Figure 12.2). Numerous studies illustrate the importance of this pathway by establishing that IL-12 is required for the development of protective innate and adaptive responses to many intracellular pathogens. Given the central role of this factor in the development of cell-mediated immunity, it is not surprising that IL-12 has also been implicated in the development of various autoimmune inflammatory conditions. The advent of bioinformatics tools and protein sequence databases led to the discovery of two IL-12-related cytokines that had already been named as IL-23 and IL-27. Together, these cytokines constitute the IL-12 family of cytokines. As we shall see in the sections that follow, these discoveries have given us a much deeper understanding of how our immune system responds to pathogenic challenges.
In contrast with TH1-cell differentiation, polarization of the differentiation of naïve CD4+ T cells toward the TH2 pathway is promoted by IL-4 produced by DCs and other innate cell populations (e.g., mast cells) (see Figure 12.2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree