Omar K. Siddiqi, Igor J. Koralnik Keywords acute inflammatory demyelinating polyneuropathy (AIDP); cognitive disorders; distal sensory polyneuropathy (DSPN); human immunodeficiency virus–associated neurocognitive disorder (HAND); myopathy; neurodegenerative disorders; primary central nervous system lymphoma (PCNSL); progressive multifocal leukoencephalopathy (PML); vacuolar myelopathy
Neurologic Diseases Caused by Human Immunodeficiency Virus Type 1 and Opportunistic Infections
Neurologic manifestations are frequent in human immunodeficiency virus type 1 (HIV-1) infection. In the era before combined antiretroviral therapy (ART) or in settings where antiretroviral drugs are not available, neurologic disease constituted the initial presentation in 10% of patients, and neurologic complications developed in 30% to 50% during the course of the disease.1,2 Autopsies have shown involvement of the nervous system in up to 80% of cases.3,4 With the advent of ART, the overall incidence of the most frequent HIV-associated neurologic diseases has decreased. The most consistent impact has been a decreased incidence of acquired immunodeficiency syndrome (AIDS)-associated dementia, but decreases in HIV-associated polyneuropathy and central nervous system (CNS) opportunistic infections have also been observed.5–8
Diagnosis of neurologic complications in patients with HIV-1 infection poses a particular challenge for clinicians. Indeed, HIV-infected individuals are often severely debilitated and present with multiple constitutional symptoms related to systemic infections or tumors that might overshadow or mimic a primary neurologic condition. In addition, HIV-infected individuals are usually treated with a combination of prophylactic drugs and a rapidly growing number of antiretroviral medications. Drug interactions and neurologic side effects of these medications are common, which adds another level of complexity for care providers. However, certain rules can be applied to facilitate the understanding of these challenging cases:
4. Immune recovery associated with ART may be associated with an inflammatory reaction within the CNS.
Principal Neurologic Manifestations of HIV Type 1 Infection
Meningeal Syndrome
Patient Otherwise Asymptomatic, CD4+ T-Lymphocyte Counts Greater Than 200 cells/µL: Aseptic Meningitis
Clinical Presentation.
Headache, stiff neck, and fever, associated with nausea and vomiting, can be the first manifestations of HIV-1 infection. It is a self-limited illness that subsides spontaneously after several weeks. In some cases, transient cranial neuropathies may develop, affecting mostly the fifth, seventh, and eighth cranial nerves.9
In addition, patients may present with symptoms of encephalopathy, and postmortem examination of individuals who died of unrelated causes in this early stage showed mild meningeal inflammation, focal cerebral white matter myelin damage, and perivascular inflammatory infiltrates and gliosis.10,11
Laboratory Investigations.
Cerebrospinal fluid (CSF) analysis shows a moderate lymphocytic pleocytosis (10 to 100 cells/µL), which is typical of viral meningitis.12 This aseptic meningitis can occur as soon as 1 week after the primary infection, when HIV-1 conventional serology is still negative. HIV-1 RNA, however, should be detectable in blood and CSF, and the HIV-1 p24 antigen might be detected in the blood. Repeat serology testing after 6 weeks usually helps to clarify this situation.
Treatment.
Interestingly, early onset of aseptic meningitis has not been associated with late neurologic manifestations in HIV-1 infection, and patients may remain asymptomatic for many years before developing other symptoms of HIV-1 infection. If not properly diagnosed at the time of their acute illness, these patients may therefore unwittingly infect a number of sexual partners. It is therefore crucial to include HIV-1 in the differential diagnosis of aseptic meningitis. This condition might recur at any time throughout the course of the disease, although CSF pleocytosis becomes less common with advanced immunosuppression. Treatment is symptomatic. The decision to start on antiretroviral medications should be based on current guidelines that generally recommend treatment of initial HIV-1 infection (see Chapter 130).
Patient at Any Stage of HIV Type 1 Infection and CD4+ T-Lymphocyte Counts at Any Level: Syphilitic Meningitis
Clinical Presentation.
In the United States, up to 6% of HIV-infected individuals have a history of syphilis. Treponema pallidum infection can also occur at any time during HIV-1 infection and mimics neurologic complications of AIDS. Indeed, both conditions may cause acute or chronic meningitis, myelopathy, cranial or peripheral neuropathies, cerebrovascular disease, and dementia. Therefore, it is paramount to distinguish neurosyphilis from latent disease with positive serology and normal physical examination in HIV-infected patients because it has a direct impact on treatment. Neurosyphilis can occur as early as 1 year or as late as 30 years after initial infection. In this chapter, we discuss only syphilitic meningitis; other neurologic complications of this disease are covered in Chapter 239.
Laboratory Investigations.
Elevation of protein concentration and leukocyte counts can be found in the CSF of approximately 15% of patients with primary syphilis and up to 40% of patients with secondary syphilis. Some of these patients eventually experience spontaneous cure of this earlier CNS infection, but the persistence of asymptomatic CSF abnormalities for more than 5 years in the untreated patient is highly predictive of the development of clinical neurosyphilis.
Persistent mononuclear pleocytosis and elevated protein concentration can be found in the CSF of HIV-1-infected patients with neurosyphilis,13 as well as an elevated immunoglobulin G synthesis rate and oligoclonal bands. This is of no help in establishing the diagnosis of neurosyphilis in the context of HIV-1 infection because these findings occur as well in asymptomatic HIV-1 seropositive patients, especially when they have detectable HIV-1 RNA in their CSF. Using either the criterion of elevated CSF protein of greater than 50 mg/dL or white blood cells greater than 10 cells/µL in HIV-positive patients with reactive rapid plasma reagin may lead to overdiagnosis of neurosyphilis in the absence of clinical symptoms. Follow-up lumbar puncture after 12 months showed persistence of CSF abnormalities in 62% of cases.14 A positive CSF Venereal Disease Research Laboratories (VDRL) test result establishes the diagnosis of neurosyphilis if the tap is not bloody. However, this test may be negative in HIV-1 infection.15,16 A reactive CSF fluorescent treponemal antibody absorbed test increases the likelihood of T. pallidum infection but is less specific because it can result from treated neurosyphilis or from contamination of the CSF with small amounts of blood containing antibody at the time of the lumbar puncture.
Acute symptomatic syphilitic meningitis is usually the earliest clinical manifestation of neurosyphilis, which occurs within the first year of infection and can be associated with cranial nerve palsies, including isolated eighth nerve palsy, and signs and symptoms of hydrocephalus. CSF abnormalities are similar to those of asymptomatic neurosyphilis, except that the CSF VDRL test result is nearly always positive (also see Chapter 239).
Treatment.
HIV-1–infected individuals with a positive serum rapid plasma reagin (RPR) test result, unexplained CSF pleocytosis, and elevated protein concentration, as well as symptoms consistent with neurosyphilis, should be treated with intravenous penicillin even in the absence of a positive VDRL test result in the CSF. Treatment consists of intravenous penicillin G (3 to 4 million units IV every 4 hours for 10 to 14 days). In case of allergy to penicillin, ceftriaxone (2 g IV once daily for at least 14 days) is another option. A careful follow-up of these patients is necessary, and a repeat spinal tap 1 month after onset of treatment should show a normalization of the CSF cellularity and protein concentration. Normalization of serum RPR predicted normalization of CSF and clinical abnormalities 13 months after treatment in more than 90% of a cohort composed mostly of HIV-infected men. This was more frequent in those receiving ART than in untreated patients.17 Some patients have a transient increase in CSF HIV-1 viral load to more than 100,000 copies/mL associated with neurosyphilis, which subsides after antibiotic treatment. Neurosyphilis may amplify intrathecal HIV replication, possibly through immune activation that persists even after syphilis treatment.18 As is the case with other opportunistic infections of the brain, this viral burden is likely carried by activated circulating lymphocytes and monocytes coming to combat the CNS infection and should not be interpreted as a manifestation of HIV-1 encephalitis.
Patient with AIDS, CD4+ T-Lymphocyte Counts of Less Than 200 Cells/µL: Cryptococcal Meningitis
Clinical Presentation.
This is the most common opportunistic meningitis in AIDS, which affected 10% of patients before the ART era19 but has decreased in incidence since then (also see Chapter 264).20 Cryptococcal meningitis differs from aseptic meningitis in that meningismus is present in less than 40% of cases, and patients may present with only fever and headache, which become progressively more debilitating. Confusion, blindness, or altered state of consciousness occurs in severe cases.
Laboratory Investigations.
The detection of Cryptococcus neoformans antigen titers by enzyme immunoassay in the CSF provides a rapid diagnosis, which is confirmed subsequently by CSF culture. Because false-positive results occur with all the antigen tests, confirmation by culture is essential to establish the diagnosis of cryptococcal meningitis. Other CSF findings include markedly elevated opening pressure, mononuclear pleocytosis, elevated protein and decreased glucose concentration in 50% of the cases, and direct detection of the organism by India ink staining in 80% of the cases. Serum cryptococcal antigen is usually detected in cryptococcal meningitis in AIDS patients, and blood and urine cultures may also be positive.
Brain imaging is usually negative unless an associated cryptococcoma or hydrocephalus is present. Poor prognosis factors include altered mental status, absence of CSF pleocytosis, CSF antigen titer greater than 1 : 1024, a positive blood culture, and hyponatremia.
Treatment.
Treatment should begin with amphotericin B (0.7 mg/kg) with flucytosine (100 mg/kg PO in four divided doses per day) until the patient is clearly responding and for not less than 2 weeks, which is associated with significantly increased rates of yeast clearance from CSF and decreased mortality compared with amphotericin B alone. Combination of fluconazole with amphotericin B showed no survival benefit.21 Use of fluconazole alone as initial therapy is associated with much slower culture conversion and is not recommended.22 Consolidation therapy then consists of fluconazole (400 mg PO once or twice daily) administered for 8 weeks or until CSF cultures are sterile. Lipid formulations of amphotericin B may be used for patients with renal insufficiency, and itraconazole may be substituted for fluconazole if patients can tolerate fluconazole but is clearly inferior to the latter. Lifelong maintenance therapy using fluconazole 200 mg/day has been previously recommended to prevent relapses.23 Updated recommendations state that maintenance therapy can be discontinued if a patient has received a minimum of 12 months of antifungal treatment, CD4+ counts reach greater than 100 cells/µL, and there is a sustained undetectable or very low HIV RNA level for ≥3 months.23a
The outcome is generally favorable within 2 weeks, but early mortality still reaches 6%.26 In a study done in Botswana, the in-hospital mortality was significantly lower among those patients who were on ART at the time of diagnosis of cryptococcal meningitis compared with untreated patients (8% vs. 21%).27 Complications such as increased intracranial pressure greater than 200 mm H2O occur in nearly all patients. Such a complication should be recognized and treated aggressively with mechanical drainage, including repeat lumbar punctures, temporary external lumbar drainage, or intraventricular shunts.28,29 Corticosteroids, acetazolamide, and mannitol have not been shown to be effective. Neurologic deterioration after commencing ART occurs in 26% of patients and is caused by cryptococcosis-associated immune reconstitution inflammatory syndrome (C-IRIS).30 It is more frequent if ART is given 7 days after compared with 28 days after onset of antifungal therapy.31 Persistent CSF cryptococcal growth at ART initiation and poor CD4+ T-cell increases on ART are strong predictors of C-IRIS.30 Management of C-IRIS includes continuation of ART, antifungal therapy, and a course of corticosteroids.32
Differential Diagnosis of Meningitis
Other CNS infections in AIDS include tuberculosis, histoplasmosis, aspergillosis, coccidioidomycosis, amebiasis, Candida albicans infection, Trypanosoma cruzii infection, herpes simplex and herpes zoster infections, and Nocardia asteroides. Geographic differences account for variations in local prevalence. Estimates indicate that more than 1 million people worldwide have tuberculosis (TB) and HIV coinfection with the highest number of individuals in sub-Saharan Africa. CNS tuberculosis can present in numerous ways, including as a tuberculoma, abscess, spinal cord lesion, and most commonly as tuberculous meningitis (TBM). TBM is a highly treatable condition in resources-limited settings (RLS) because its presentation is commonly subacute and antituberculosis treatment is widely available. However, TBM diagnosis remains a challenge throughout the world. Acid-fast bacilli staining on CSF has a sensitivity of 10% to 20% that varies greatly on the basis of technical expertise.33 CSF culture has a sensitivity of approximately 50% and can take as long as 6 weeks to become positive.34 Neither of these techniques is practical in an RLS. As a result, treatment is often initiated empirically with minimal objective testing for guidance except for hypoglycorrachia and high CSF total protein, where biochemical tests are available.
Bacterial meningitis is more commonly a problem for HIV-infected patients in resource- limited settings compared with resource-rich settings.35,36 HIV-infected patients are at risk for pneumococcal infection,37 and pneumococcal meningitis usually arises from spread of an untreated primary infection. These cases are more likely in RLS where patients present at later stages of disease and have limited access to health services.
Cognitive and Motor Syndromes
HIV Type 1 Associated Neurocognitive Disorder
Clinical Presentation.
This common CNS complication of HIV-1 infection occurs in 15% of patients with AIDS and can be the first manifestation of the disease in 3% to 10%.38 In the past, this condition has also been named AIDS dementia complex, HIV-1 encephalopathy, or HIV-associated major cognitive disorder. A less severe entity named HIV-1-associated minor cognitive/motor disorder (MND) occurs in an additional 20% to 25%, and asymptomatic neurocognitive impairment is used to categorize individuals with subclinical impairment.39,40 Risk factors include an AIDS-defining illness, increased age and survival duration, lower nadir of CD4+ T-cell counts, and higher baseline HIV viral loads.41
The clinical characteristics of this disorder can be subdivided into three main categories: cognitive, behavioral, and motor (Table 127-1).
Initial symptoms are usually subtle. Patients often report difficulty with memory and note a slowness of thinking. They have trouble concentrating. Complex mental activities become more time consuming and difficult to perform. A loss of interest in social and professional activities soon follows, and such apathy and social withdrawal may be mistaken for depression. Although cognitive and behavioral symptoms are prominent in most patients, some mainly present with motor dysfunction, which includes decreased coordination, altered handwriting, loss of balance, and gait instability.
The mental status examination reveals minor psychomotor slowing, inattention, decreased short-term memory, inability to perform simple calculations, and frontal release signs. Ocular movement testing shows saccadic pursuit. Other frequent findings are brisk reflexes, mild postural tremor, slowing of rapid alternating movements, and gait instability when performing half-turns. If untreated, dementia becomes more global, profoundly impairing orientation, memory, and cognition. Confusional or psychotic episodes have been reported, but seizures are a rare occurrence. Despite the extent of the cerebral involvement, there is usually no aphasia, apraxia, or other signs of discrete cortical dysfunction, except in terminal stages. Therefore, this syndrome has been classified as a frontal-subcortical dementia. A rapid bedside evaluation can be performed with the HIV Dementia Scale.42 However, this screening test is not as sensitive as a more detailed neuropsychological evaluation, which should include tests of attention, memory, and psychomotor speed such as trail making, digit span, verbal fluency, grooved pegboard, symbol digit modalities, and Rey auditory verbal learning tests.43 An international dementia scale can be used to identify individuals at risk of HIV-associated dementia despite language barriers.44,45
Unfortunately, cognitive dysfunction persists despite ART. A cross-sectional study of 1555 HIV-infected adults in the United States showed neuropsychological impairment in 52%. Prevalence estimates for specific HIV-associated neurocognitive disorder (HAND) diagnoses were 33% for asymptomatic neurocognitive impairment, 12% for MND, and 2% for HIV-associated dementia.46
Laboratory Investigations.
Numerous groups have detected early neurologic dysfunction in HIV-1-infected asymptomatic individuals.47–49 Subtle electrophysiologic abnormalities can be found in early HIV-1 infection (on electroencephalography, evoked potentials, nerve conduction studies), but they do not seem to have a predictive value for the later onset of AIDS dementia, which occurs generally when the CD4+ T-lymphocyte counts are less than 200 cells/µL. CSF analysis shows mild lymphocytic pleocytosis in 25% and elevated protein in 55%,50 which can also be found in nondemented patients. Increased CSF levels of nonspecific markers of immune activation or neuronal injury have been reported, but their use in patients’ management is limited because these markers are also elevated during opportunistic infections of the CNS and because these assays are not readily available in the clinical setting.51
Measurement of the HIV-1 CSF viral load has been evaluated as a surrogate marker in HIV-1 infected individuals with cognitive dysfunction. A wide overlap was found between CSF HIV-1 viral load values of demented and nondemented individuals, and concomitant opportunistic infections of the CNS must be ruled out because they also contribute to transient elevation of HIV-1 viral burden in the CSF.52 These findings suggest that there may be two phases of HIV-1 infection in the CSF. (1) A transitory infection of the CNS may occur early in the disease via trafficking lymphocytes. These viral strains have a lymphotropic phenotype (using CXCR4 co-receptor) and may respond to therapy in parallel to plasma virus load. (2) A more autonomous infection of CNS monocytes/macrophages may take place at a later stage; with macrophage tropic strains (using CCR5 co-receptor).53 Such patients may need higher drug penetration in the CNS. Whether measurement of the HIV-1 CSF viral load provides an accurate representation of the viral replication occurring in the CNS is unclear. However, subsets of patients with higher CSF than plasma viral loads have been recognized in whom intrathecal viral replication correlates with neurologic deficits. Because HIV-1–associated dementia complex is a diagnosis of exclusion, CSF examination is indicated for bacterial, fungal, and acid-fast bacteria cultures; cryptococcal antigen; VDRL test; and cytology. In addition, CSF polymerase chain reaction (PCR) for JC virus (JCV), the agent of progressive multifocal leukoencephalopathy (PML), is indicated because this condition is often difficult to distinguish from HIV-1 encephalopathy on brain imaging studies (see later and also see Chapter 147).
Imaging Studies.
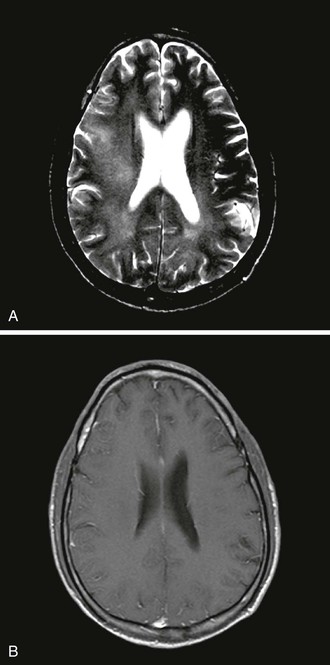
Computed tomography (CT) and magnetic resonance imaging (MRI) may show greater subcortical atrophy than cortical atrophy, which is not proportional to the degree of dementia. MRI may also demonstrate multiple hyperintense signals in T2-weighted images, which are nonenhancing, poorly demarcated, and localized bilaterally in the subcortical white matter (Fig. 127-1). MRI is superior to CT for distinguishing these abnormalities from confounding illnesses such as PML. Unlike lesions of PML, there is no associated hypointensity in T1-weighted images. The severity of global brain atrophy and signal changes in basal ganglia correlates with cognitive impairment.54
Brain Biopsy and Histologic Analysis.
There is no indication to perform a brain biopsy in patients with HAND, unless imaging studies suggest the presence of another process. Postmortem examination reveals encephalitis with multinucleated giant cells and microglial nodules, as well as astrocytosis and perivascular mononuclear cell infiltrates. HIV-1 has been found in microglial nodules, perivascular macrophages, and multinucleated giant cells. The latter are the result of the fusion of infected macrophages. The virus can also infect astrocytes.55 HIV-1 has also been found in endothelial cells,56 and abnormalities of the cerebral microcirculation are characterized by increased cellularity and pleomorphism of endothelial cells, as well as prominent perivascular aggregates of HIV-infected macrophages. These findings are sometimes related to microinfarcts and are postulated to give rise to increased vascular permeability.57 Indeed, early enhancement has been detected in the basal ganglia of patients with HAND, suggesting a disruption of the blood-brain barrier (BBB) in these regions.58 HIV-1 does not infect glial cells in adults, whereas a limited expression of viral regulatory gene products has been demonstrated in glial cells from children with AIDS.59 White matter pallor involving primarily the centrum semiovale is a hallmark of HIV-1 encephalopathy in adults, which may be caused by seepage of macromolecules and edema fluid in the brain parenchyma through the permeable BBB and may cause injury to the myelinated fibers. Finally, HIV-1 does not infect neurons. Therefore, quantitative neuronal loss, decrease in cell size, or dendritic injury found in the cortex of demented patients60 may only be secondary to infection of other cells and associated immune activation.61
Treatment.
Although the pathophysiology of HAND is not entirely elucidated, HIV-1 remains the main triggering factor, and it is therefore reasonable to prevent viral replication in the CNS. However, antiretroviral drugs have variable penetration through the BBB, and evaluation of CNS concentration and pharmacokinetics of these medications is incomplete. On the basis of available pharmacologic data, antiretrovirals can be classified according to their penetration through the BBB. A CSF penetration effectiveness (CPE) score62 is given to each medication going from 1 (low) up to 4 (high) (Table 127-2). The CPE score of an ART regimen is calculated by summing the score of each individual antiretroviral. Patients with higher penetration-effectiveness scores tended to have lower HIV RNA levels in the CSF. Drugs with the highest CPE scores include zidovudine, nevirapine, and ritonavir-boosted indinavir.
TABLE 127-2
Cerebrospinal Fluid Penetration Effectiveness Score*
| 4 | 3 | 2 | 1 | |
| Nucleoside analogue reverse-transcriptase inhibitors | Zidovudine | Abacavir | Didanosine | Tenofovir |
| Emtricitabine | Lamivudine | Zalcitabine | ||
| Stavudine | ||||
| Non-nucleoside analogue reverse-transcriptase inhibitors | Nevirapine | Delavirdine | Etravirine | |
| Efavirenz | ||||
| Protease inhibitors | Indinavir-r | Darunavir-r | Atazanavir-r | Nelfinavir |
| Fosamprenavir-r | Atazanavir | Ritonavir | ||
| Indinavir | Fosamprenavir | Saquinavir-r | ||
| Lopinavir-r | Saquinavir | |||
| Tipranavir | ||||
| Fusion/Entry inhibitors | Maraviroc | Enfuvirtide | ||
| Integrase inhibitors | Raltegravir |
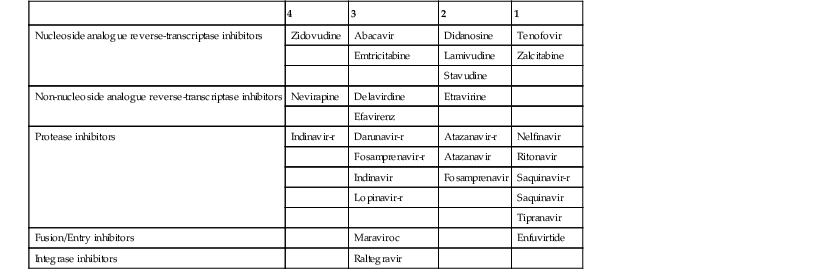
-r, ritonavir-boosted.
Since 1996, the availability of protease inhibitors (PIs) and the use of three or more drugs in ART regimens have made a major impact on the incidence of neurologic disease in HIV-1-infected patients.63–65 This is somewhat surprising; PIs in general have a very low CSF-to-plasma ratio because they are highly protein bound. Nevertheless, the incidence of HAND, which was estimated at 6.49 per 1000 person-years pre-1997, has decreased to 0.66 by 2003 to 2006, an approximately 10-fold decrease.63,66 Although ART is now the treatment of choice for advanced HIV-1 disease, the optimal drug combination for HAND has not been established. Whether regimens containing several drugs with good penetration through the BBB were advantageous has been investigated. In ART-experienced patients with CD4+ T-cell counts of less than 200 cells/µL, those who received multiple CSF penetrating agents had a greater reduction of CSF HIV-1 RNA.67,68 These results have been confirmed in recent studies, showing that higher CPE scores of long-term ART are associated with better viral suppression in CSF61–63and decreased risk of cognitive impairment.69,70 However, other studies have shown that discontinuation of efavirenz was associated with cognitive improvement in scheduled treatment interruption regimens71 and in otherwise asymptomatic HIV-infected individuals,72 suggesting that some antiretrovirals might have neurotoxic properties, which was also demonstrated in vitro.73
The hypothesis that discordant mutations are present in blood and CSF has been confirmed by the analysis of HIV-1 genotypes in paired samples. Mutations of the reverse transcriptase and protease genes of CSF strains, but not in the corresponding blood isolates, were detected in 10 of 23 subjects (43%). Interestingly, mutations conferring resistance to low or high CSF-penetrating agents occurred at similar frequencies.74 Reverse-transcriptase mutation patterns differed in 14 of 21 (67%) paired samples from plasma and CSF in one study, and HIV RNA responses and reverse-transcriptase genotypes were discordant between CSF and plasma in some subjects.75,76 In a study of HIV-infected children starting ART, 8 of 11 had identical resistance patterns in both CSF and plasma at baseline, whereas at week 48 of treatment, only 1 of 9 children had similar genotypes, suggesting discordant viral evolution in these two compartments.77 CSF viral escape was reported recently in 10 patients with progressive neurologic dysfunction despite well controlled plasma viral load and good immunologic response on ART. In general, these patients had a higher CSF viral load compared with plasma. The patients also had CSF pleocytosis and elevated protein concentration, MRI abnormalities, and unique CSF resistance mutations compared with plasma and improved clinically after optimization of ART.78 Long-term follow-up of a patient who developed HAND and elevated CSF viral load despite low plasma viral load and high CD4 counts confirmed the importance of concomitant genotype testing in CSF and plasma. Subsequent change of ART targeting CSF strains was associated with suppression of CSF HIV replication, as well as a marked clinical and radiologic improvement.78 Failure to recognize the occurrence of different genotypic resistance patterns in plasma and CSF may lead to uncontrolled viral replication in the CNS and worsening neurologic symptoms despite evidence of viral clearance in the periphery. These resistant isolates may, in turn, spill back into the circulation and contribute to the progression of HIV-1 disease. Therefore, genotypic analysis of CSF HIV-1 strains is indicated in patients with cognitive dysfunction who have good virologic response to ART in the plasma but persistence of an elevated HIV-1 CSF viral load.
In addition to antiretroviral treatment, other experimental compounds have been tested for the treatment of HAND, but without evidence of clear benefit so far. These include a calcium channel blocker (nimodipine); antioxidants (vitamin E, thioctic acid, CPI-1189); a tumor necrosis factor-α antagonist (pentoxifylline); a noncompetitive N-methyl-d-aspartate inhibitor (memantine); peptide T; selegiline; and, more recently, minocycline.79
Central Nervous System Mass Lesions
In patients with AIDS presenting with change in mental status or abnormal neurologic examination results, brain lesions are frequently revealed on CT or MRI. These can be quite extensive and may represent life-threatening emergencies.
Toxoplasma Encephalitis
At the beginning of the AIDS epidemic, Toxoplasma encephalitis (TE) was the most common cerebral mass lesion in patients with AIDS. TE is caused by a reactivation of latent infection by the protozoan Toxoplasma gondii as a result of progressive loss of cellular immunity. In the United States, where the incidence of seropositivity for T. gondii is approximately 30% in the adult population, TE formerly developed in 3% to 10% of patients with AIDS.80 In Europe and Africa, where the overall seroprevalence is higher, up to 25% to 50% of patients with AIDS presented with this condition. The incidence of TE has decreased initially thanks to widespread prophylaxis for Pneumocystis jirovecii pneumonia using trimethoprim-sulfamethoxazole, which also prevents CNS toxoplasmosis.81 Since the era of ART, there has been a further trend for a decreased incidence of this condition,20 and it accounted for only 28% of focal brain lesions occurring in patients with AIDS in 199882 and for 26% of patients with neurologic disorders in 2004.83 Nevertheless, TE continues to be a severe health problem. Patients who develop TE nowadays either did not receive or had not taken ART adequately.84 Therefore, toxoplasmosis still represents the most frequent complication affecting the CNS of patients with AIDS.
Clinical Presentation.
Almost 90% of patients with TE have CD4+ T-lymphocyte counts less than 200 cells/µL, and 75% have CD4+ counts less than 100 cells/µL at the time of clinical presentation. The most common symptoms include headache, confusion, fever, and lethargy. Seizures develop in up to 30% of patients.85 Seventy percent of patients have focal signs on the neurologic examination, such as hemiparesis, cranial nerve palsies, ataxia, and sensory deficits. The clinical presentation is usually subacute, ranging from a few days to a month (see Chapter 280).
Laboratory Investigations.
As is the case in the general population, serum anti-Toxoplasma immunoglobulin G antibodies can be detected in patients with TE, whereas immunoglobulin M antibodies are less commonly found, supporting the notion that most cases represent a reactivation of latent infection. Measurement of antibody titers is not helpful to establish the diagnosis. On enzyme-linked immunosorbent assay, only 7% of patients known to be seropositive for T. gondii had lost their antibodies at the time of presentation.80 Therefore, a negative serology orients toward another entity, whereas a positive serology is not diagnostic (see Chapter 280).
A slight increase in CSF protein concentration and a moderate mononucleated pleocytosis (<60 cells/µL) are common, but nonspecific, and may be due to the underlying HIV-1 infection. A slight decrease in the CSF glucose has been reported but is not a constant finding. The PCR technique has been less useful for detection of T. gondii DNA in the CSF compared with other pathogens, with a sensitivity of only 44% to 65% and a specificity of 100%.86 However, this low sensitivity may be improved by the use of stage-specific PCR primers.87 CSF analysis is therefore more useful to rule out other infectious processes than to confirm the diagnosis of TE.
Imaging Studies.
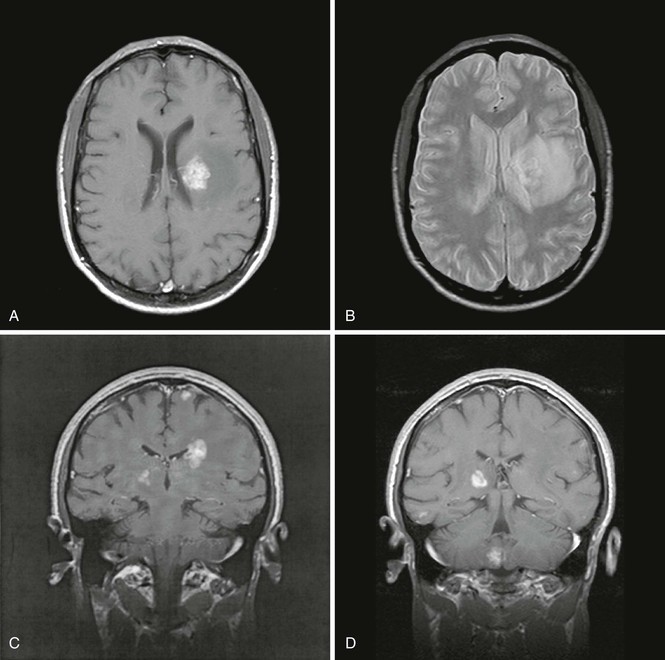
Neuroimaging with head CT or MRI demonstrates CNS lesions in almost all cases, with the exception of the rare diffuse encephalitic form of toxoplasmosis. Lesions are multiple in two thirds of the cases, and 90% of them display ring enhancement after administration of contrast material. MRI is more sensitive than CT in detecting multiple lesions. These are generally localized at the corticomedullary junction, in the white matter, or in the basal ganglia; are surrounded by edema; and induce mass effect on surrounding structures (Fig. 127-2). Unfortunately, the neuroradiologic characteristics of TE are not pathognomonic and may be observed in other conditions such as primary CNS lymphoma (PCNSL) (see later).
Brain Biopsy.
Because of the good response to therapy, histologic examination is not required for the diagnosis of TE, and an empirical therapeutic trial is recommended when the clinical and radiologic findings are consistent with this diagnosis. Histologic examination shows mainly necrotic abscesses with blood vessel thrombosis and necrosis. Cysts containing bradyzoites, the dormant form of T. gondii, coexist with numerous active tachyzoites.
Treatment.
Treatment consists of a combination of pyrimethamine and sulfadiazine, which cause a synergistic and sequential block on the folic acid metabolism necessary for the development of the parasite. Standard acute therapy consists of pyrimethamine 200 mg PO the first day of treatment, followed by 75 mg/day PO, sulfadiazine 4-6 g/day PO in four divided doses, and folinic acid 10 to 25 mg/day PO for 6 weeks.
Clindamycin 600 mg IV or 450 mg PO four times daily is an adequate alternative to sulfadiazine in the previous regimen for patients allergic to sulfonamides. Side effects, which consist of cytopenia, rashes, diarrhea, and increased liver enzymes, have been reported in 40% to 70% of patients receiving pyrimethamine and sulfadiazine and in 36% of those receiving pyrimethamine and clindamycin. These can cause early discontinuation of therapy. Azithromycin 900 to 1200 mg PO once daily or atovaquone 1500 mg PO two times daily can also be combined with pyrimethamine and folinic acid. Although corticosteroids are frequently prescribed to diminish cerebral edema, their use has been shown to be neither beneficial nor harmful in TE.88 Because high doses of steroids also decrease the size of CNS lymphoma lesions, they should be administered only in cases with impending cerebral herniation during the initial medical treatment of presumed TE so as not to cloud the diagnosis. Neurologic improvement is clinically apparent in more than half the cases by day 3 of therapy and in most cases by day 7. Nevertheless, the death rate at 1 year varies from 10% to 60%.83,89 Persistent neurologic sequelae remained in 37% of survivors.90 A failure to improve or a worsening of the symptoms should prompt repeat imaging studies by days 10 to 14 to determine the need for a brain biopsy. TE-IRIS presenting simultaneously with initiation of ART has been reported in six patients, including five who were supposed to take effective prophylaxis. Brain biopsy showed intense angiocentric infiltrate consisting predominantly of CD8+ T lymphocytes in two of them.91
Secondary Prophylaxis.
T. gondii is sensitive to treatment only when in tachyzoite form. Because dormant cystic forms may rupture and reinitiate the infectious process at any time, maintenance therapy is necessary to prevent a relapse, which is likely to occur after a delay of 6 to 8 weeks of interruption of treatment. Standard maintenance therapy consists of pyrimethamine 25 mg/day with folinic acid 10 to 25 mg/day and either sulfadiazine 2 g/day in four divided doses or clindamycin 450 mg PO four times daily. Patients with sustained CD4+ T-cell counts higher than 200 cells/µL for more than 3 months can discontinue their secondary prophylaxis.92 Relapse occurred in only one of 22 (5%) patients.93
If the diagnosis is made in a timely fashion and the patient does not become intolerant to the treatment, TE is currently an opportunistic infection with a relatively high therapeutic success rate, and death is usually caused by other complications of AIDS.
Primary Central Nervous System Lymphoma
This condition, which affected 2% of patients with AIDS at the beginning of the epidemic, has seen its incidence decrease considerably in the ART era.20 In 1998, it accounted for only 12% of AIDS-related focal brain lesions.82 Its radiographic appearance makes it the principal differential diagnosis of TE.
Clinical Presentation.
The onset of symptoms is generally subacute, lasting weeks to months. Confusion, lethargy, and memory loss are the most frequent symptoms. As the disease progresses, hemiparesis, aphasia, seizures, and cranial nerve palsies occur.94 Fever, headaches, and constitutional symptoms are generally absent, which helps distinguish PCNSL from TE. At the time of diagnosis, the average CD4+ T-lymphocyte counts are usually very low (≈50 cells/µL).
Laboratory Investigations.
A mild mononuclear pleocytosis (<30 cells/µL) and an increase in the protein concentration in the CSF are common findings in patients with PCNSL but are nonspecific and may be due to the underlying HIV-1 infection. High protein levels (≤590 mg/dL) have been reported in patients with extensive lymphomatous infiltration of both cerebral hemispheres. Hypoglycorrhachia is a rare finding.
It is important to perform cytologic analysis of the CSF because the presence of atypical or malignant lymphomatous cells can establish the diagnosis. Flow cytometry immunophenotyping has at least 25% higher sensitivity than conventional cytomorphologic methods for the detection of malignant cells.95 Systemic extracerebral lymphomas, which have an increased incidence in AIDS patients, can also cause lymphomatous meningitis but do not generally spread to the brain itself. Similarly, PCNSL does not metastasize systemically. The Epstein-Barr virus (EBV) genome can be detected in tumor cells of nearly all PCNSLs, but only in some systemic lymphomas of AIDS patients, and rarely in primary brain lymphoma tissue from patients without immunodeficiency.96 When testing is performed in a research setting, detection of EBV DNA by PCR in the CSF has a reported sensitivity of 80% to 90% and a specificity of 87% to 98% for the diagnosis of PCNSL. Measurement of the EBV CSF viral load by quantified PCR in the CSF may improve the sensitivity of this test and be clinically useful in monitoring responses to treatment.97 However, other groups have found EBV DNA in the CSF of patients who did not have PCNSL, which raises doubts regarding the true predictive value of this test when performed in the clinical setting.98,99
Imaging Studies.
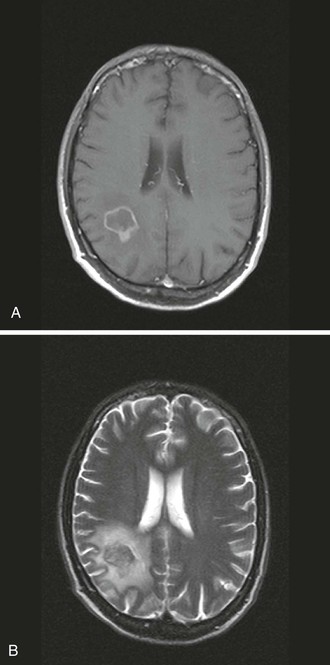
Head CT or MRI usually shows findings consistent with a CNS tumor. Solitary mass lesions are as frequent as multiple lesions.94 Most lesions display some degree of enhancement, which is usually nodular or patchy. Ring enhancement, identical to that commonly seen in TE, can occur and correlate with central tumor necrosis. Subependymal enhancement seems more specific of CNS lymphoma, but this is a rare feature. Lesions are frequently located in the corpus callosum, the periventricular white matter, or the cortex. Involvement of the posterior fossa occurs only in 10% of cases.100 Lesions can be surrounded by edema, which may induce variable mass effect on neighboring structures (Fig. 127-3).101 MRI is more sensitive than CT in revealing multiple lesions, which can be useful if a biopsy is being considered. Thallium 201 single-photon emission CT shows an accumulation of isotope in the tumor due to increased metabolic activity.102 In one series comparing enhancing brain lesions caused by toxoplasmosis and lymphoma, the thallium index of each lesion, measured as the ratio of the mean uptake in the lesion to that of the corresponding contralateral side, was increased and a significant predictor of lymphoma only in lesions 2 cm or larger, yielding 100% sensitivity and 89% specificity.103 However, the usefulness of this method in differentiating CNS lymphoma from opportunistic infections may depend heavily on the resolution of the images, and appropriate technology may not be available in every center. Fludeoxyglucose (FDG) positron emission tomography (PET) scan has also shown utility in differentiating TE from PCNSL in a small series.104
Brain Biopsy.
If the CSF cytology fails to reveal lymphomatous cells and if EBV PCR is negative in the CSF, an image-guided stereotactic brain biopsy using CT or MRI is the only way to ascertain the diagnosis. The macroscopic appearance of these tumors is generally that of a multifocal, diffusely infiltrating and expanding nonhemorrhagic mass, without well-demarcated borders, simulating the appearance of an infiltrating glioma. However, well-circumscribed, largely necrotic masses can also resemble abscesses. Microscopic analysis reveals a variety of patterns, including large cells, small noncleaved cells, and immunoblastic or mixed cellular components. These are generally of B-lymphocyte lineage. EBV is present in tumor cells, suggesting that this virus may have a role in tumorigenesis,105 as is the case in Burkitt’s lymphoma and in nasopharyngeal carcinoma.
Treatment.
The response to steroids seen in lymphomas of non-AIDS patients is not always seen in AIDS patients. In patients with altered mental status, debilitating focal symptoms, or impending herniation, dexamethasone 10 mg IV or PO followed by 4 mg IV or PO every 6 hours can provide temporary improvement. The treatment of CNS lymphoma in AIDS consists of whole-brain irradiation with a total of 3000 cGy over a 3-week period. Steroids are added to decrease peritumoral edema and mass effect.100 The palliative response rate before the ART era was 53%.106 In a series of 25 patients in the United States seen between 1995 and 2001, the median survival was 87 days. However, patients who received ART after diagnosis had a longer survival; 6 of 7 patients receiving ART were alive compared with 0 of 18 untreated patients at a median follow-up time of 1.8 years. Furthermore, the median survival was only 29 days for 11 patients who received neither radiation nor ART.107 In a recent study, the estimated 3-year overall survival (OS) rate of HIV-associated PCNSL after whole brain radiation was 64%. Results were influenced by the performance status at presentation, with patients with good performance status having a 100% 3-year OS vs. 38% in those with poor performance status.108 Combination treatment including radiation and chemotherapy is difficult to tolerate because of high toxicity. A pilot trial using a combination of zidovudine, ganciclovir, and interleukin-2 showed promising results.109 In any event, immune recovery induced by ART leads to dramatic improvement in survival of patients with AIDS-associated PCNSL.110
Progressive Multifocal Leukoencephalopathy
Before the AIDS era, PML was a rare disease affecting mainly patients with chronic lymphocytic leukemia, those with non-Hodgkin’s lymphoma, or organ transplant recipients.111 At the beginning of the AIDS epidemic, up to 5% of patients developed PML.112 Despite the availability of ART, up to 28% of focal brain lesions in AIDS patients were attributed to PML in 1998,82 which equals the number of TE cases. The incidence of PML in ART-treated patients is 0.6 to 1.3/1000 person-years.113,114 PML is caused by the polyomavirus JCV. This double-stranded DNA virus infects 90% of the normal adult population worldwide and remains quiescent in the kidneys without causing any disease. In the setting of immunosuppression, JCV is reactivated and induces a lytic infection of oligodendrocytes, causing multifocal demyelination of the CNS (see Chapter 147).115
Clinical Presentation.
Classic PML usually develops when CD4+ T-lymphocyte counts decrease to fewer than 200 cells/µL.112 The most common presenting symptoms are limb weakness (hemiparesis or monoparesis), altered mental status, gait ataxia, and visual symptoms, including hemianopsia, diplopia, and third nerve palsy. Approximately 80% of patients have focal neurologic findings.116 However, PML lesions can occur anywhere in the CNS white matter, particularly at the subcortical level, although the optic nerves and the spinal cord are rarely involved.117 Because subcortical lesions prevent the transmission of information to and from the cortical areas, presentation implying cerebral cortical dysfunction such as aphasia, apraxia, memory loss, and visual agnosia does not rule out this diagnosis. In fact, seizures, which are usually considered to be of cortical origin, occur in 18% of PML patients.118 Some symptoms can also be attributed to HAND, which is often superimposed on other CNS pathologies in the end stages of AIDS.
Laboratory Investigations.
Laboratory analyses are necessary to establish the diagnosis of PML.119 Conventional CSF analysis is normal or shows a moderate increase in protein concentration and a mild mononucleated pleocytosis (<25 cells/µL), which is nonspecific in the context of HIV-1 infection. Before the ART era, detection of JCV DNA in the CSF by PCR has a sensitivity of 74% to 92% and a specificity of 92% to 96% for the diagnosis of PML.120,121 In patients receiving ART, the sensitivity of this test has decreased to 58%, probably because of decreased JCV replication in the context of a recovering immune system.122 Nevertheless, measurement of JC viral load in the CSF appears to be of value as a correlate of PML disease activity and survival.121,123,124
Imaging Studies.
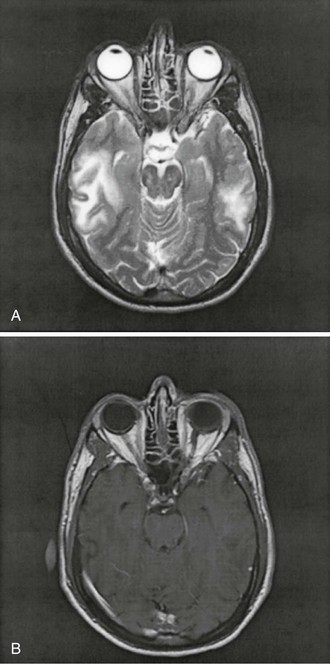
The hallmark of PML is patchy or confluent areas of low attenuation on CT or hyperintensity on T2-weighted MRI (Fig. 127-4). MRI is twice as sensitive as CT in distinguishing multiple lesions. These generally do not enhance after administration of contrast material, and they are not surrounded by edema, and, hence, substantial mass effect on surrounding structures is absent. However, 8% of lesions can show faint, peripheral, and irregular enhancement. Lesions are usually bilateral, asymmetrical, well demarcated, and localized preferentially in the periventricular areas and the subcortical white matter.125 Involvement of the deep gray structures, including the basal ganglia and thalamus, can nevertheless be found in up to 17% of cases. Normal findings on CT or MRI do not rule out PML because microscopic lesions might be smaller than the resolution power of these tests. One such case showed multiple small foci of demyelination disseminated among the cortical U fibers at autopsy.126 HIV-1 encephalopathy can be easily mistaken for PML on brain imaging studies. As in PML, white matter lesions without mass effect or contrast enhancement are the radiologic hallmarks of this disease. However, HIV-1 encephalopathy lesions tend to be symmetrical and less clearly demarcated than the lesions of PML and are not associated with focal neurologic deficits. Proton magnetic resonance spectroscopy and magnetization transfer studies have shown promising potential in differentiating PML lesions from HIV-1 encephalopathy.127 The typical proton magnetic resonance spectroscopy pattern in PML lesions includes a decreased N-acetylaspartate-to-creatine ratio, consistent with axonal compromise; increased choline/creatine ratio, indicating cell membrane breakdown and turnover; and an occasional increase in lipid/lactate and myo-inositol.128–130 In one study, patients who had the longest survival had the highest myo-inositol level, consistent with glial activity and inflammation in PML lesions.128 Metabolic alterations consistent with inflammation detected on proton magnetic resonance spectroscopy in PML lesions are associated with a cellular immune response against JCV. This inflammatory reaction, occurring early after disease onset, appears to be instrumental in the containment of PML.131