NEUROBORRELIOSIS: NERVOUS SYSTEM INVOLVEMENT WITH BORRELIA SPECIES
JOHN J. HALPERIN, SVEN BERGSTRÖM, AND GARY P. WORMSER
Relapsing fever and Lyme disease, infections most commonly acquired through bites of hematophagous arthropods, are caused by spirochetes in the genus Borrelia. Both can involve the nervous system; meningitis and cranial neuritis occur commonly. Relapsing fevers may cause more severe manifestations, leading in extreme cases to brain microhemorrhages and focal central nervous system (CNS) damage. Focal CNS involvement is quite rare in Lyme borreliosis; the details of its pathophysiologic mechanisms, although clearly inflammatory, remain to be elucidated.
RELAPSING FEVER
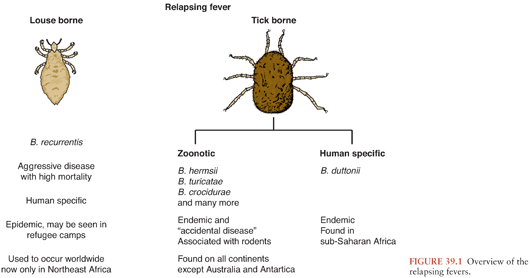
There are approximately 20 different borrelial species capable of causing relapsing fever (Fig. 39.1), an illness characterized by recurrent, several-day bouts of fever separated by periods of relative well-being. The episodic nature of this infection is due to sequential evasion of the host’s adaptive immunologic responses. In untreated patients, up to 10 to 15 recurrences may develop, although usually, the number is 1 to 3.
Epidemiology, Vectors, Agents, and Reservoirs
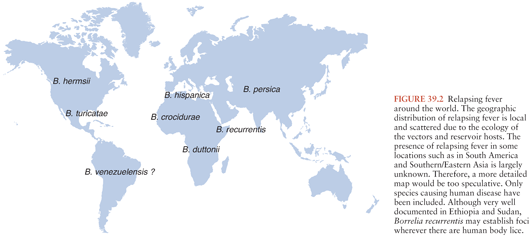
Borrelia recurrentis, for which the vector is the human body louse, Pediculosis humanus, is the only known species of relapsing fever Borrelia not vectored by ticks. This form of relapsing fever, referred to as louse-borne relapsing fever (LBRF), occurs in Africa (Fig. 39.2). Rather than being transmitted by an arthropod bite like other Borrelia, B. recurrentis infection occurs when scratching abraded skin inoculates organisms either from louse excrement or from lice crushed in the process of scratching (1,2). LBRF is the most severe form of relapsing fever, and in untreated patients, mortality rates may exceed 40%. There is no animal reservoir for B. recurrentis; epidemic transmission typically occurs when body lice spread extensively in badly overcrowded conditions such as in refugee camps resulting from war or famine. As the only relapsing fever that causes epidemics, LBRF is sometimes referred to as epidemic relapsing fever.
All other species of relapsing fever Borrelia are transmitted by tick bites. The resultant illness is referred to as endemic relapsing fever or tick-borne relapsing fever (TBRF). Soft-bodied Argasidae ticks transmit the vast majority of cases of TBRF. Transmission occurs within minutes of tick attachment as relapsing fever Borrelia persist in the tick salivary glands from which they are rapidly inoculated into a host. Most patients have no recollection of a tick bite because it is painless, typically occurs at night, and the blood meal is completed within 15 to 90 minutes (3).
Borrelia miyamotoi differs from other relapsing fever Borrelia in that it is transmitted by hard ticks in the Ixodes persulcatus family (Ixodidae). B. miyamotoi, first isolated in Japan from the hard tick I. persulcatus (4), has been found (5,6) in all Ixodes tick species that are epidemiologically important for transmission of Lyme disease, including I. ricinus and I. scapularis; the latter tick species frequently feed on humans.
B. miyamotoi infections are just being recognized as a cause of infection in the United States, where I. scapularis is the likely vector (7,8). Most cases of B. miyamotoi infection reported to date have not caused periodic fevers nor has B. miyamotoi been detected by examination of peripheral blood smears in the six infected patients for whom this diagnostic test was performed (9). B. miyamotoi infection has caused chronic meningoencephalitis in immunocompromised patients (8).
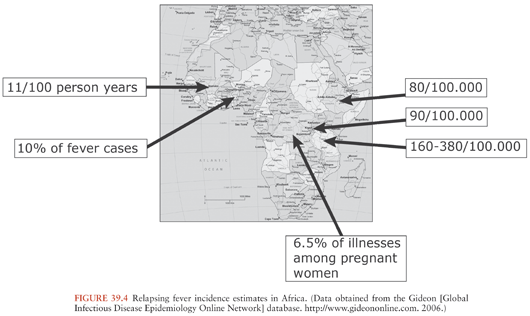
TBRF occurs nearly worldwide (Fig. 39.2). In North America, these infections occur primarily in the West and Southwest (Fig. 39.3) (3), where Borrelia hermsii, transmitted by the Ornithodoros hermsi tick, is the most common cause, followed by B. turicatae and B. parkeri. O. hermsi ticks are found in coniferous forests at altitudes of 1,500 to 8,000 feet (10). Consequently, in the United States, TBRF occurs primarily in the Rocky Mountain states, with infections acquired most commonly in rustic rodent-infested cabins. B. parkeri and B. turicatae infect ticks with corresponding names O. parkeri and O. turicata, which are found across a somewhat broader geography at lower altitudes, primarily in caves or burrows (3). In Eurasia, TBRF is most commonly caused by Borrelia persica but also by Borrelia caucasica and Borrelia latyschewii (11,12). In sub-Saharan Africa (Fig. 39.4), Borrelia duttonii (transmitted by Ornithodoros moubata) is the most common cause of TBRF (3). In rural sections of West Africa, Borrelia crocidurae is a prominent cause of infection, responsible for 5% to 25% of all febrile illnesses, depending on the year and location (13).
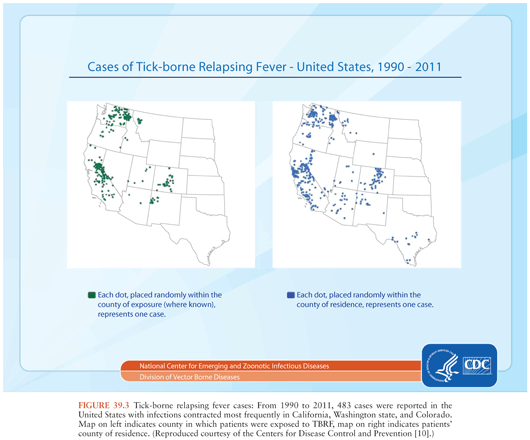
TBRF ecology varies among species. Most TBRF Borrelia infections are zoonoses, but no animal reservoir has been identified for B. duttonii, which is closely related genetically to B. recurrentis (1,3,14,15). For other species, the principal reservoirs are rodents, and other small animals. Infected Ornithodoros ticks may survive without a blood meal for more than a decade. That, along with the possibility of transovarial transmission of relapsing fever Borrelia in the tick vector (3), suggests that ticks themselves may potentially serve as reservoirs.
Although vector-borne transmission is by far the most common means of transmission of relapsing fever Borrelia, infection has also been transmitted through blood transfusion, sharing of needles among intravenous drug users, laboratory accidents, and transplacentally (3,12).
General Clinical Features and Pathogenesis
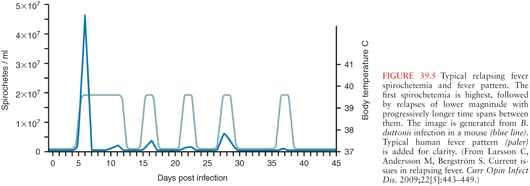
Symptom severity varies widely in patients infected with relapsing fever Borrelia (16); some remain completely asymptomatic, whereas others become critically ill. The hallmark of relapsing fever is periodic fevers. Approximately 7 days after inoculation, patients develop the sudden onset of chills and fever, typically between 38.7° and 41°C (Fig. 39.5), frequently with rigors, headache, fatigue, altered sensorium, arthralgias, and myalgias (16). Some patients develop a cough, nausea, vomiting, diarrhea, icterus, a maculopapular or petechial truncal skin rash, neurologic and ocular involvement, pneumonia, myocarditis, acute respiratory distress syndrome, abdominal pain, hepatosplenomegaly, icterus, hepatic failure, and splenic rupture (3). Ocular manifestations of TBRF include iritis, iridocyclitis, and choroiditis (17). In contrast to TBRF, eye involvement occurs rarely if ever in LBRF(17).
The most common causes of death are myocarditis, cerebral hemorrhage, and liver failure. Relapsing fever in pregnancy can result in miscarriage in one third of cases (12,18,19). B. duttonii infection is often associated with low birth weight, preterm delivery, spontaneous abortion, and perinatal death (20–25).
The number of inoculated bacteria required to cause infection can be quite low. In mice, a single bacterium may be sufficient to establish infection (1). Once relapsing fever Borrelia enter the blood, they multiply to large numbers, potentially reaching concentrations of 106 to 108 organisms per milliliter, at which point symptoms appear. Symptoms resolve spontaneously within about 3 days (range 1 to 9 days), as bactericidal T-cell independent immunoglobulin (Ig) M antibodies (26) damage the borrelial outer membrane, resulting in clearance of spirochetes from the blood. Both B-cell receptor and toll-like receptor signaling are involved in the development of this rapid T-cell independent antibody response (27,28). Neither phagocytic cells nor complement activation play an essential role in spirochete clearance; relapsing fever Borrelia are able to inactivate the complement cascade (26,29). Fever recurs in 7 or more days (range typically 3 to 10 days) with the emergence of an antigenically different strain of Borrelia, resistant to the bactericidal activity of the previously produced antibodies.
These antigenically different strains arise from alteration in the expression of the variable major surface lipoprotein, Vmp, through genetic rearrangement whereby silent genes move into an expression locus (3,30,31). This antigenic variation is not random; certain Vmps are more likely than others to be expressed in early infection (32), perhaps explaining why some neurologic and ophthalmologic manifestations usually do not occur during the first bout of fever (see the following discussion). Aside from the change in Vmp, the new strain of Borrelia is otherwise identical to the original infecting strain (30,31,33). In theory, at least 25 antigenically distinct serotypes can be generated from a single bacterium (27). Emergence of each successive serotype is associated with a fever relapse. Recurrences may be shorter in duration and associated with less intense symptoms and fewer spirochetes in the blood, especially for LBRF. In experimental animals, different serotypes not only confer antibody resistance but also appear to vary in their capacity to cause brain infection (34,35). This may, at least in part, be explained by different serotypes’ varying ability to bind and cross the brain microvascular endothelium (33).
Laboratory Findings
In patients with relapsing fever, the white blood cell count, erythrocyte sedimentation rate, and liver function tests are usually elevated. Anemia and thrombocytopenia are common (1,3), and although usually mild, the thrombocytopenia can be severe, potentially resulting in bleeding complications. One possible mechanism proposed for the development of thrombocytopenia, suggested by animal models (36,37), is through binding of Borrelia to the outer surface of platelets, a phenomenon that may also play a role in eradicating relapsing fever Borrelia from the blood. Certain species of relapsing fever Borrelia—such as B. crocidurae, B. duttonii, and B. hispanica—bind to erythrocytes, causing erythrocyte rosetting, which may contribute to the development of anemia (1,12,38). The resulting aggregates of bacteria and red blood cells may also disrupt the microcirculation, resulting in hemorrhage, reduced blood flow, hypoxia, and cell death (1,12,38,39).
Neurologic Manifestations
The most commonly reported neurologic manifestations are meningeal symptoms, meningitis, and facial nerve palsy.Delirium (encephalopathy) is also common. In severe cases, CNS microhemorrhages, focal abnormalities, seizures, and coma can occur. The frequency of neurologic involvement depends on the infecting species of relapsing Borrelia. B. duttonii, B. crocidurae, and B. turicatae are the TBRF species most likely to cause neurologic complications (17), particularly meningitis and facial nerve palsy.
Facial nerve palsy, although frequent, does not typically appear during the first febrile episode. It usually resolves within 2 months with or without antibiotic therapy (17). Ocular involvement is unilateral in two thirds of cases and, like facial nerve palsy, almost never occurs during the first febrile episode.
Although there are reports of other cranial nerve palsies, optic neuritis, radicular symptoms, neuropsychiatric abnormalities, and other findings, no large population-based studies are available to provide clear data regarding the frequency of most of these disorders or even compelling evidence of a causal relationship.
Microscopically, meninges and brain may show perivascular infiltrates composed of monocytes, lymphocytes, and plasma cells (17). Cerebral edema and subarachnoid and parenchymal brain hemorrhage may occur in fatal cases of LBRF. In experimental animals, cerebral microgliosis is a prominent feature (33,40,41). Both meningitis and meningismus occur frequently in TBRF. In LBRF, although as many as 50% of patients develop meningismus, far fewer have actual meningitis (as confirmed by cerebrospinal fluid [CSF] examination) (17). In experimentally infected rodents, some strains of relapsing fever Borrelia can cause persistent brain infection (15) that can be cleared successfully with ceftriaxone treatment (42). Persistent brain infection in untreated humans, however, has not been documented.
The CSF white cell count is typically elevated in relapsing fever patients with CNS involvement. CSF examination demonstrates a mononuclear-predominant pleocytosis with cell counts in the hundreds/mm3 and protein concentration of up to several hundred mg%. CSF glucose is typically normal. Organisms have been detected in CSF in a minority of cases by microscopic examination, culture, or animal inoculation. In fatal cases of TBRF, spirochetes have been detected histologically in perivascular areas of brain cortex (17). Testing for the production of intrathecal antibodies to Borrelia has not been established to be a useful diagnostic test in relapsing fever (32).
Diagnosis
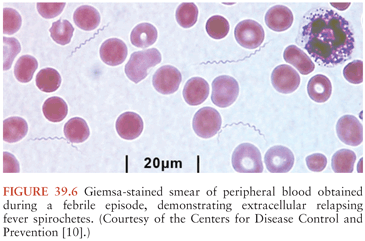
During febrile episodes, there may be up to 108 spirochetes per milliliter of blood; none are detectable during afebrile periods. As a result, extracellular spirochetes may be directly visualized in blood obtained during a febrile episode, either on Giemsa (Fig. 39.6) or Wright-stained blood smears or by dark-field or phase-contrast microscopy of plasma wet mounts. The sensitivity of these techniques is 70% or less, but this can be increased using fluorescent microscopy of acridine orange–stained smears or by examination of buffy coat smears.
Detection of relapsing fever borrelial DNA by PCR appears to be more sensitive than detection by blood smear but is only offered by a few laboratories. Serologic assays including enzyme-linked immunosorbent assays (ELISAs) or immunofluorescence assays (IFAs), followed by supplemental immunoblots using antigens from particular species of relapsing fever Borrelia, are available from a few laboratories but only for some borrelial species. Relapsing fever and Lyme disease patients often produce cross-reactive antibodies given the similarity of flagellin and other antigens. A specific serologic assay for relapsing fever Borrelia infections uses the surface protein glycerophosphodiester phosphodiesterase (GlpQ) as the antigen (3). Because this protein is not present in Lyme Borrelia, Lyme disease patients will be seronegative using this assay. Other testing modalities with even more limited availability include culture and animal inoculation.
Treatment
TBRF is treated with a 5- to 10-day course of oral tetracycline, doxycycline, erythromycin, penicillin, amoxicillin, or chloramphenicol (3). The preferred therapy for LBRF is a single oral dose of tetracycline or erythromycin, although some authorities favor extending treatment to 7 days (1). Meningitis or encephalitis is treated with intravenous penicillin G, ceftriaxone, or cefotaxime for at least 14 days.
A Jarisch-Herxheimer reaction, thought to result from the rapid killing of relapsing fever Borrelia by antibiotics with the sudden release of lipoproteins, occurs frequently in the first few hours following initial treatment. The resultant induction of cytokines such as tumor necrosis factor-α (TNF-α), interleukin-6 and -8 (IL-6, IL-8), and other chemical mediators causes rigors, leukopenia, fever, and hypotension, signs similar to those of a classic endotoxin reaction (1,3). Because of the potential severity of such reactions, patients should be kept under observation for approximately 2 hours after initiating antibiotic therapy. This is an “all or nothing” reaction, occurs in more than 50% of patients, and usually resolves spontaneously within 12 to 24 hours. However, cardiovascular collapse may occur, with a fatality rate of about 5% (43,44). Several studies have suggested that the choice of antibiotic affects the severity, but such data are inconclusive. Treatment with anti–TNF-α agents prior to antibiotics may reduce the severity of the reaction but is not used clinically at this time (45).
When tick bites and/or tick exposure has occurred with ticks at high risk for transmitting relapsing fever Borrelia, a 5-day course of doxycycline chemoprophylaxis during the infection’s incubation period is highly effective in prevention of TBRF (46).
LYME DISEASE
Lyme disease is caused by a group of related spirochetes—Borrelia burgdorferi sensu lato or Lyme Borrelia. Lyme disease is the most commonly reported vector-borne infectious disease in the United States and is also of public health importance in numerous countries with temperate climates in Eurasia (47). The term “Lyme disease” was coined in the mid-1970s, with the recognition of a cluster of cases of what appeared to be juvenile rheumatoid arthritis (48) in Lyme, Connecticut. Europeans, however, have been familiar with this disorder since the early twentieth century (49) and have been treating it successfully with antibiotics since the 1950s, three decades before the isolation of the causative organism (50). The history of European versus United States Lyme disease has resulted in the perception that these infections behave quite differently. However, they actually share more similarities than differences, making the century of clinical experience with European neurologic Lyme disease informative for our understanding of this aspect of Lyme disease in the United States.
Epidemiology, Vectors, Agents, and Reservoirs of Lyme Borrelia
In North America, the only species of Lyme Borrelia known to cause human disease is B. burgdorferi sensu stricto (which will be referred to as B. burgdorferi). In Europe, at least five species of Lyme Borrelia can cause disease, of which B. afzelii, B. garinii, and B. burgdorferi are the most common. Because of this species diversity, the range of clinical manifestations appears to be greater in Europe than in North America (47). In Europe, B. garinii is the species of Lyme Borrelia most closely associated with neurologic manifestations (47).
Lyme Borrelia is transmitted during feeding by Ixodes species ticks (Fig. 39.7). Only after ingested blood triggers spirochete proliferation and migration to the tick’s salivary glands can infection be transmitted. A feeding period of more than 36 hours is usually needed for transmission of B. burgdorferi by I. scapularis or I. pacificus ticks, whereas in experimental animals, transmission of B. afzelii by I. ricinus can occur in under 24 hours (47,51–54). The main vertebrate reservoirs for Lyme Borrelia are mice, other small mammals, and some species of birds.
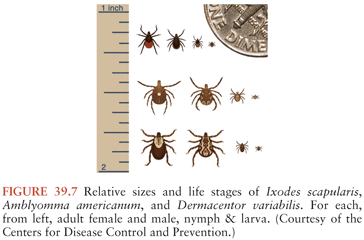
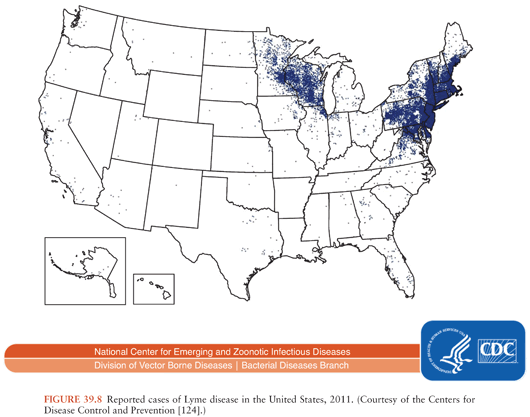
In the United States, about 30,000 cases of Lyme disease are identified by the Centers for Disease Control and Prevention (CDC) annually; cases occur predominantly in the northeast, middle/south Atlantic, and north central states (Fig. 39.8). In Europe and Asia, with an estimated 85,000 cases per year (accurate numbers are difficult to obtain as this is not a reportable disease in many European countries), infection occurs broadly across temperate central regions (55).
General Clinical Features and Pathogenesis
Lyme Borrelia resides in the midgut of unfed Ixodes ticks. When an infected tick takes a blood meal, the spirochetes present there increase in number and undergo phenotypic changes, including the expression of a particular outer surface protein, OspC, which allows them to disseminate within the tick and enter its salivary glands (47). This process takes several days and explains why transmission occurs only after a delay. Expression of OspC plays an essential part in the establishment of infection in a mammalian host, although the mechanism by which this occurs is unknown (56,57).
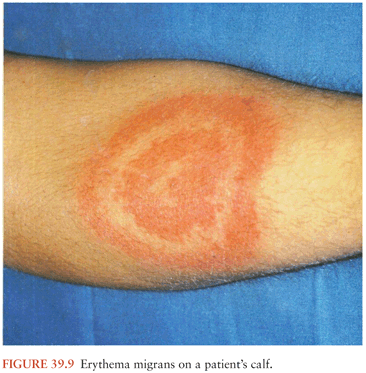
During feeding, the infected tick deposits spirochetes into the skin at the bite site. This may lead to the development of a type of bacterial cellulitis, referred to as erythema migrans (EM; Fig. 39.9), at that location. Lyme Borrelia may disseminate from that site to other locations in the body: through the blood or perhaps through tissue planes. The risk of hematogenous dissemination by B. burgdorferi is dependent on the strain of Borrelia (58).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree