Jeanne M. Marrazzo, Michael A. Apicella Keywords antigenic variation; antimicrobial resistance; arthritis; asialoglycoprotein receptor; carcinoembryonic antigen–related cell adhesion molecules (CEACAMs); cervicitis; complement; complement receptor 3 (CR3); conjunctivitis; disseminated gonococcal infection; epididymitis; gonorrhea; I-domain; lipo-oligosaccharide; Neisseria; Neisseria gonorrhoeae; Opa proteins; pelvic inflammatory disease; perihepatitis; pharyngitis; phase variation; pilus; porin; sexually transmitted diseases; urethritis
Neisseria gonorrhoeae (Gonorrhea)
Gonorrhea is a common bacterial infection that is transmitted almost exclusively by sexual contact or perinatally and primarily affects the mucous membranes of the urethra and cervix and, less frequently, those of the rectum, oropharynx, and conjunctivae. Ascending genital infection in women leads to endometritis and salpingitis—collectively called pelvic inflammatory disease (PID), the predominant complication and one of the most common causes of female infertility. Other complications include acute epididymitis; ophthalmitis; disseminated infection with arthritis, dermatitis, and sometimes endocarditis; and transmission to the neonate with attendant conjunctivitis (ophthalmia neonatorum).
Gonorrhea is one of the oldest known human illnesses, and references to sexually acquired urethritis can be found in ancient Chinese writings, the biblical Old Testament (Leviticus), and other works of antiquity. Galen (ad 130) introduced the term gonorrhea (“flow of seed”), implying interpretation of urethral exudate as semen. The causative organism was described by Neisser in 1879 and was first cultivated in 1882 by Leistikow and Löffler. Untreated infections were understood to resolve spontaneously over several weeks or months, but reinfection was recognized. Many therapies were tried, but not until the advent of the sulfonamides in the 1930s and penicillin in 1943 was truly effective treatment available. Since that time, therapy has changed radically in the face of antimicrobial resistance. We are now facing a major crisis as resistance to the last of the “single dose” third-generation cephalosporin therapies is jeopardized.1 Growth of fundamental knowledge about the organism and the host response to infection was slow for 80 years, but a surge of new information began in the 1970s and the molecular biology of the gonococcus and the pathogenesis of gonorrhea have been well elucidated. Public health control efforts have met with variable success, and gonorrhea remains the second most common reportable disease in the United States (following sexually transmitted chlamydial infection), a prime example of the influence that social, behavioral, and demographic factors can have on the epidemiology of an infectious disease despite highly effective antimicrobial therapy.
The Organism
Description
Neisseria gonorrhoeae is a non–spore-forming, gram-negative coccus that characteristically grows in pairs (diplococci) with adjacent sides flattened. It does not have flagella but can move based on extension and retraction of its type IV pilus.2 This process is known as “twitching” and is the basis of the colony types seen in piliated and nonpiliated gonococcal strains. The gonococcus closely resembles the related pathogen Neisseria meningitidis, as well as several species of nonpathogenic Neisseria. All Neisseria spp. rapidly oxidize dimethyl-p-phenylenediamine or tetramethyl-p-phenylenediamine, the basis of the oxidase test. Traditionally, gonococci are differentiated from other Neisseria by their ability to grow on selective media; to use glucose but not maltose, sucrose, or lactose; and to reduce nitrites and also by their inability to grow well at reduced temperature or on simple nutrient agar.3
Growth and Cultivation
Gonococci do not tolerate drying, and patient samples to be used for cultivation should be inoculated immediately onto an appropriate growth or transport medium. Growth is best for most strains at 35° C to 37° C, and many freshly isolated strains have a relative or absolute requirement for atmospheric carbon dioxide in concentrations of approximately 5%. All strains are strictly aerobic under usual growth conditions, but the organism grows anaerobically when nitrite is provided as an electron acceptor. Colonies appear in 24 to 48 hours, but on most media viability is rapidly lost after 48 hours because of autolysis.3
Gonococci are inhibited by many fatty acids, and it is necessary to incorporate starch or other substances that absorb fatty acids into most growth media. All strains have complex growth requirements, including requirements for several vitamins, amino acids, iron, and other factors. For clinical purposes, a satisfactory medium is chocolate agar enriched with glucose and other defined supplements. Isolation of gonococci from sites that normally contain high concentrations of the normal microbiota, especially the pharynx, rectum, and cervix, may be difficult because of overgrowth of the hardier normal microbiota—a problem that is largely overcome by use of media containing antimicrobial agents that inhibit most nonpathogenic Neisseria and other species but permit growth of most strains of N. gonorrhoeae and N. meningitidis. Chocolate agar that contains vancomycin, colistin, nystatin, and trimethoprim (modified Thayer-Martin medium) is widely used for this purpose in the United States; a similarly constituted translucent selective medium (New York City medium) is also commonly used. Specimens from sites that usually do not harbor indigenous microbiota (e.g., blood, synovial fluid, and cerebrospinal fluid) should be cultured on antibiotic-free medium.
Surface Structures
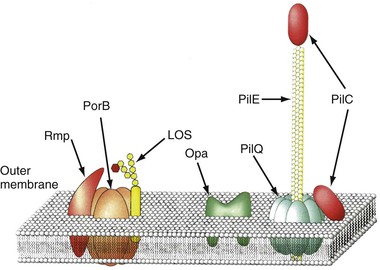
The envelope of N. gonorrhoeae is similar in basic structure to that of other gram-negative bacteria. As the interface between the gonococcus and host, the cell surface has been intensively studied (Fig. 214-1), and specific surface components have been related to adherence, tissue and cellular penetration, cytotoxicity, and evasion of host defenses both systemically and at the mucosal level.
Type IV Pili
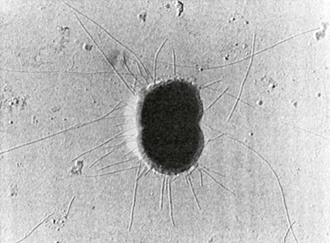
Type IV pili (fimbria) are strong flexible filaments extending from the gonococcal cell surface. They can be several microns in length and 50 to 80 angstroms in width. Pili traverse the outer membrane of the gonococcus through an integral outer membrane protein known as PilQ.4 Mature pili are composed of repeating protein subunits (pilin) with a molecular weight of 19 + 2.5 kDa.5 Through the activity of the proteins PilT and possibly PilB, gonococci can depolymerize and repolymerize the pilus strand, causing the bacteria to “twitch.” The pilin subunit has regions of considerable interstrain antigenic similarity, especially near the amino terminus, but areas of extreme antigenic variability are also present.5,6 A single strain of N. gonorrhoeae is capable of producing pili with differing antigenic compositions. This has compromised the utility of pilus-based vaccines against gonorrhea. The presence or absence of pili result in varied colonial forms, which can be distinguished when N. gonorrhoeae is grown on translucent agar.7 Fresh clinical isolates initially form colony types P+ and P++ (formerly called T1 and T2), and the organisms have numerous pili extending from the cell surface (Fig. 214-2); P– colonies (formerly T3 and T4) lack pili. Piliated gonococci are better able than organisms from P– colonies to attach to human mucosal surfaces and are more virulent in animal and organ culture models and in human inoculation experiments than nonpiliated variants. Expression of pili is a function of the pil gene complex. A spontaneous shift between P+ or P++ colonies to P– colony types, known as phase variation, occurs after 20 to 24 hours of growth in vitro. This is caused by errors in DNA replication, resulting in pilE genes that are out of frame and fail to produce functional protein.6
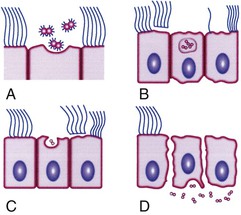
In addition to mediating attachment, pili contribute to resistance to killing by neutrophils. In the fallopian tube mucosa model (Fig. 214-3), pili facilitate attachment to nonciliated epithelial cells, which initiates a process of entry and transport through these cells into intercellular spaces near the basement membrane or directly into the subepithelial space while concurrently nearby ciliated mucosal cells lose their cilia and are sloughed.8 CD46 was considered to be the main pilin receptor, but the issue is currently uncertain and the identity of the pilus receptor is an area of active study. Other pilus-associated proteins are likely to be important to adhesion to host cells, particularly PilC.9,10 Other factors also mediate attachment, notably, opacity (Opa) proteins, lipo-oligosaccharide (LOS), and porins (Por).
Outer Membrane
Like all gram-negative bacteria, the gonococcus possesses a cell envelope composed of three distinct layers: an inner cytoplasmic membrane, a middle peptidoglycan cell wall, and an outer membrane. The outer membrane contains LOS, phospholipid, and a variety of proteins (see Fig. 214-1). Porin, formerly designated protein I, has a molecular weight of 32 to 36 kDa and is closely associated in the membrane with LOS. Porin provides channels that allow aqueous solutes to pass through the otherwise hydrophobic outer membrane and is believed to play an important role in pathogenesis. Porin is the product of a gene designated porB. Porin proteins occur in two major antigenic classes, designated PorB1A and PorB1B, each of which is composed of many distinct genetic variants. Variations in Por sequence or antigenic types form the basis for the most commonly used gonococcal typing systems.11 Strains expressing PorB1A and occasionally PorB1B are associated with genotypic resistance of N. gonorrhoeae to the bactericidal effect of normal (nonimmune) human serum and, perhaps as a direct result, with an enhanced propensity to cause bacteremia. Porin-related serum resistance is due to binding to loops on the porin protein of the complement downregulatory components C4bp or factor H.12,13 PorB1A also appears to directly promote invasion of epithelial cells, which also helps explain the propensity for bacteremic dissemination.
Opa proteins are outer membrane proteins with molecular weights of 20 to 28 kDa. They are members of a family of proteins, each produced from its own opa gene. The amino acid sequence of the Opa proteins varies somewhat, primarily because of differences in two hypervariable regions in each protein.14 Expression of Opa varies because of high-frequency variations in opa DNA that result in translational frame shifting. An individual strain of N. gonorrhoeae can express none or up to 11 Opa variants but usually not more than 3 at a time. Gonococci isolated from mucosal sites usually express Opa and their colonies are opaque, but most isolates obtained from the cervix during menstruation and isolates from normally sterile sites, such as fallopian tubes, blood, and synovial fluid, generally lack Opa and form translucent colonies. Many Opa proteins increase adherence between gonococci and to a variety of eukaryotic cells, including phagocytes.15 Certain Opa variants appear to promote invasion of epithelial cells. Two classes of Opa receptor on eukaryotic cells have been identified: heparin-related compounds and CD66, or carcinoembryonic antigen–related cell adhesion molecules (CEACAMs).16,17 Certain Opa proteins are able to bind to CEACAM receptors on B and T cells, resulting in downregulation of immune responses.18 This may help account for the poor immune response to natural infection.
Reduction-modifiable protein (Rmp) has a molecular weight of 30 to 31 kDa; is present in all gonococci in close association with porin and LOS; and shows little, if any, interstrain antigenic variation. Rmp can stimulate blocking antibodies that reduce serum bactericidal activity against N. gonorrhoeae, which may potentiate infection after sexual exposure to an infected partner.19 Several other outer membrane proteins have been identified, including multiple iron-repressible proteins, some of which are shared with N. meningitidis. Two of the iron-repressible proteins (85 and 110 kDa) constitute a specific receptor for human transferrin,20 and two others form a receptor for human lactoferrin.21 The transferrin receptor is required for successful experimental urethral infection, but the role of the lactoferrin receptor is unclear; it does not influence infectivity. Two additional proteins constitute a receptor for hemoglobin.22 Other proteins are expressed only during anaerobic growth.23 The ability of N. gonorrhoeae to grow anaerobically after removing available oxygen from the microenvironment contributes to its ability to survive in the cervical and vaginal microaerobic environment.24 IgA1 proteases, present in N. gonorrhoeae and N. meningitidis but not in nonpathogenic Neisseria, are assumed to protect the organism from secretory IgA antibody at mucosal surfaces, but this role has not been proven.
Gonococcal LOS is composed of lipid A and a core oligosaccharide that, in contrast to the polysaccharide of most gram-negative bacteria, lacks O-antigen side chains. Sialylation of LOS core sugars in vitro or in vivo masks epitopes on both LOS and porin and contributes to resistance to bactericidal antibodies.25 LOS possesses endotoxic activity and contributes to ciliary loss and the death of mucosal cells in the fallopian tube explant model (see Fig. 214-3). LOS core sugars undergo high-frequency phase and antigenic variation in vitro and in vivo, which may contribute to the pathogenesis of infection, including resistance to bacterial anti-LOS antibodies present in normal serum and invasion of epithelial cells.
The peptidoglycan layer of N. gonorrhoeae may also contribute to the inflammatory response. The gonococcus has lytic transglycosylases, which produce and release highly inflammatory peptidoglycan monomers.26 These peptidoglycan fragments are toxic in the fallopian tube explant system and cause complement consumption in vitro. In addition, peptidoglycan fragments have been found in the apparently sterile synovial fluid of patients with gonococcal arthritis-dermatitis syndrome.27 Gonococci produce a surface polyphosphate that may have capsule-like functions, such as creating a hydrophilic, negatively charged cell surface. However, a carbohydrate capsule analogous to that of N. meningitidis or Streptococcus pneumoniae is not produced.
Strain Typing
Studies of the clinical manifestations and epidemiology of gonorrhea have been greatly enhanced by the development of reproducible methods for typing N. gonorrhoeae. These methods are not available in clinical laboratories, however. They consist of characterization of gonococcal strains based on two primary methodologies, auxotyping and serotyping using monoclonal antibodies to variable epitopes on the porin protein. In the future, with the introduction of rapid and inexpensive whole-genome sequencing, strains will be compared using these methods.
Genetics
Plasmids
Many gonococci possess a 24.5-mDa conjugative plasmid and can thereby conjugally transfer other non–self-transferable plasmids with high efficiency; chromosomal genes are not mobilized. Many gonococci carry a plasmid (Pcr) that specifies production of a TEM-1 type of β-lactamase (penicillinase). The two most common Pcr plasmids have molecular weights of 3.2 and 4.4 mDa and are closely related to each other and to similar plasmids found in certain Haemophilus spp., including Haemophilus ducreyi. In fact, it is suspected that gonococci first acquired Pcr plasmids from H. ducreyi.28 Pcr plasmids are commonly mobilized to other gonococci by the conjugative plasmid.
Gonococci with plasmid-mediated high-level resistance to tetracycline, with minimal inhibitory concentrations (MICs) of 16 mg/L or greater, carry the 24.5-mDa conjugative plasmid into which the tetM transposon has been inserted.29 The tetM determinant also confers tetracycline resistance to a variety of other bacteria, including some Streptococcus and Mycoplasma spp. and various genital organisms such as Gardnerella vaginalis and Ureaplasma urealyticum. Because of its location on the conjugative plasmid, high-level tetracycline resistance is readily transferred among gonococci. The tetM determinant functions by encoding a protein that protects ribosomes from the effect of tetracycline. Finally, all gonococci contain a small (2.6 mDa) cryptic plasmid of unknown function.
Chromosomal Mutations and Transformation
Mutations in biosynthetic pathways are common, presumably reflecting the ready availability in vivo of essential nutrients such as amino acids, purines, and pyrimidines at infected mucosal sites. Nevertheless, N. gonorrhoeae is not highly mutable in that it lacks error-prone repair systems and is relatively resistant to external mutagenic stimuli such as ultraviolet light. Instead, gonococci have evolved efficient systems for phase and antigenic variation of surface components (pili, Opa, and LOS) that do not depend on such mutagenic pathways.
Gonococci also use transfer of naked DNA between cells (transformation) to promote genetic variability. The piliated variants of virtually all clinical isolates of N. gonorrhoeae are highly competent in transformation, but loss of the ability to express pili is always accompanied by a dramatic reduction in transformation competence. Uptake of transforming DNA is limited to homologous (i.e., gonococcal) DNA, which reflects recognition of a unique nucleotide sequence by a surface receptor.30 No bacteriophages have been found in N. gonorrhoeae.
Chromosomal resistance of N. gonorrhoeae to β-lactam antibiotics and the tetracyclines results from interactions between a series of individual mutations, some of which (e.g., the mtr determinant) alter the net accumulation of antimicrobial agents inside the cell. The mtr locus has been shown to be an efflux pump similar to other membrane transporters.31 The penA locus alters penicillin-binding protein 2 to reduce its affinity for penicillin.32 For epidemiologic purposes, chromosomal resistance is defined when the MIC is such that clinical failures are common with the maximum practical therapeutic dose, which corresponds to MICs of 2 mg/L or greater for both tetracycline and penicillin G.33 Clinically significant resistance to the fluoroquinolones, indicated by MICs of ciprofloxacin of 1 mg/L or higher (up to 16 mg/L), result from the additive effects of multiple chromosomal mutations involving the genes gyrA and gyrB, which code for DNA gyrases, and parC and parE, which code for topoisomerases.34
Pathobiology of Gonococcal Infection
An unusual characteristic of gonococcal infection is that the pathobiologic events that result in infection in men and women are different.35
Infection of the Male Urethra
N. gonorrhoeae infection of men occurs as an acute urethritis, which develops from the concomitant inflammatory response directed at infecting gonococci. One hallmark of gonococcal disease in men is the presence of a purulent urethral discharge, which is associated with polymorphonuclear leukocyte (PMN) influx and shedding of urethral epithelial cells. Human volunteer studies indicate that there is an incubation period from the time of infection to the onset of clinical symptoms of disease; during this time, gonococci cannot be cultured from the urethra for up to 40 hours after the initiation of infection.36 In vitro infection assays and microscopic analysis of patient exudates indicate that gonococci can enter urethral epithelial cells and are released and then infected epithelial cells are shed from the mucosal surface to the urethral lumen.37 Experimental infection of men has also demonstrated that high concentrations of the chemokine interleukin (IL)-8 and cytokines IL-6 and tumor necrosis factor-α (TNF-α) are present within the urethral lumen with progressive gonococcal infection.38 Recent evidence demonstrates that LOS elicits TNF-α, IL-1β, IL-6, and IL-8 secretion from urethral epithelial cells.39 Release of cytokines and chemokines from the urethral epithelium may, therefore, initiate the inflammatory response associated with gonococcal urethritis by triggering PMN influx. PMN influx in conjunction with cytokine release from the urethral epithelium subsequently potentiates the clinical symptoms associated with disease. Unless intercepted by effective antimicrobial therapy, this process is cyclic during the course of infection with extension into the upper male genital tract. The acquired immune response in humans is ineffective in slowing disease progression or preventing reinfection.
Microscopic examination of urethral exudates from men documented to have culture-proven gonorrhea indicates that gonococci are found within PMNs and urethral epithelial cells.40 The interaction of gonococci with PMNs is dependent on the presence of Opa, but it does not require pili. Opa proteins can be divided into two broad classes represented by Opa50 (i.e., proteins that recognize host cell heparin sulfate proteoglycans [HSPGs]) and Opa52 (i.e., proteins that recognize members of the CEACAMs41). Experimental infection of men with Opa− gonococci results in a shift to an Opa+ phenotype.38 An Opa+ phenotype is also prevalent in clinical isolates obtained from men with naturally acquired gonococcal infection. Primary male urethral epithelial cells do not express CEACAMs, and an N. gonorrhoeae strain FA1090Δ Opa mutant is not impaired in its ability to cause infection in a human experimental model. This would suggest that the role of Opa proteins in gonococcal urethritis in men resides in their ability to facilitate a gonococcal-PMN interaction. (Thus, generating such inflammation may be adaptive for the gonococcus).
CEACAMs can serve as co-receptors for other cell surface receptors (e. g., integrins) present on professional phagocytic cells. Engagement of CEACAMs sends a priming signal within PMNs that activates adhesion receptors without triggering a respiratory burst or the release of inflammatory mediators. An Opa-CEACAM interaction may, therefore, enhance gonococcal survival within these cells.
Porin also contributes to the intracellular survival of gonococci within PMNs. Gonococcal porin is unique among gram-negative bacterial porins in its ability to translocate to and insert into a targeted host cell membrane. Within a host cell membrane, porin forms an anion-selective, voltage-gated channel that is modulated through its interaction with adenosine triphosphate or guanosine triphosphate.42 Insertion of porin into the PMN membrane inhibits degranulation by causing a change in membrane potential without triggering the respiratory burst within these cells.43 Further modulation of host cell function attributed to porin is its ability to inhibit phagosome maturation44 and to downregulate cell surface receptors important to immune function (e.g., FcγRII, FcγRIII, and complement receptors 1 [CR1] and 3 [CR3]).45 A single, primary, receptor has not been identified that uniquely modulates the interaction of the gonococcus with PMNs; however, this association occurs independently of CR3.46 Fcγ-, CEACAMs, HSPGs, and integrin receptors present on the PMN cell surface may all play a role in gonococcal adherence and/or its internalization.
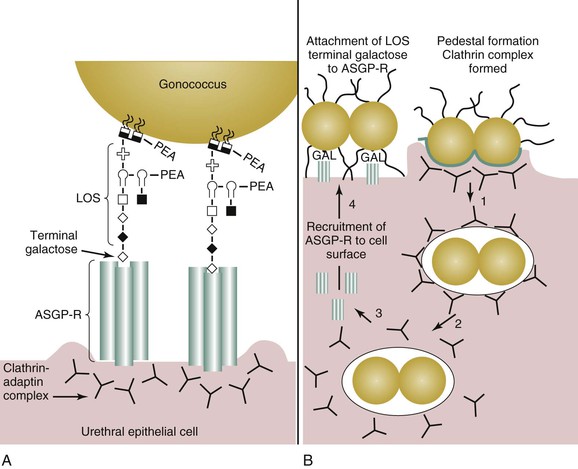
The initial site of gonococcal disease in men is the urethral epithelial cell (Fig. 214-4A). Disease occurs as a sequential process in which an initial interaction occurs between gonococcal pilus and the urethral epithelium. Pilus expression is required for efficient infection of the urethral epithelium. An intimate association between the urethral epithelium and the gonococcus (see Fig. 214-4B) is achieved through the interaction of the asialyloglycoprotein receptor (ASGP-R) and gonococcal LOS,47 a major constituent of the gonococcus cell membrane. Engagement of the ASGP-R by the gonococcus results in pedestal formation beneath the bacterium. Endocytosis ensues primarily because of actin-48 and clathrin-dependent37 processes. Endocytosis mediated by the ASGP-R results in endosomal fusion and acidification, which results in clathrin-coat disassembly and uncoupling of the ASGP-R–ligand complex. After gonococcal internalization, ASGP-R is recycled to the urethral cell surface, where it is available to bind more gonococci. Small proportions of infecting gonococci enter urethral epithelial cells by a macropinocytic mechanism, although membrane ruffling is not observed.49 The intracellular fate of the gonococcus is unclear. The gonococcus does induce antiapoptotic events that prolong the life of the epithelial cell.50,51
Adherence of the gonococcus to the ASGP-R is dependent on the presence of an exposed galactose on the terminal lacto-N-neotetraose (LNnT) moiety on LOS. This moiety mimics human paragloboside and provides one means by which the gonococcus escapes immune recognition. Additionally, the LNnT epitope can serve as a sialic acid acceptor. The presence of sialic acid on gonococcal LOS confers (unstable) resistance to the bactericidal action of normal human serum (i.e., serum resistance). The importance of the LNnT moiety to gonococcal pathogenesis in men can be inferred from human experimental infection studies and from clinical data obtained from men with naturally acquired gonorrhea, which demonstrate that LNnT is selected for in vivo.52,53 Gonococci bearing the LNnT moiety on their LOS exhibit enhanced infectivity in human volunteer studies.54 It is thought that serum-resistance conferred by LOS sialylation allows a greater proportion of gonococci to survive the harsh microenvironment of the urethral lumen during disease. Consequently, a lower infectious dose is required to establish disease because a greater proportion of the infection inoculum survives and proliferates.
Sialylation of gonococcal LOS occurs when it is intracellular. LOS sialylation is mediated by gonococcus-encoded sialyltransferase55–60 present in the gonococcal outer membrane. The gonococcus lacks the ability to synthesize cytosine 5′-monophosphate N-acetylneuraminic acid (CMP-NANA) and must parasitize this substrate from its human host.61,62 Sialylation of the LNnT epitope impairs the ability of gonococci to invade primary urethral epithelial cells and epithelial cell lines, to cause disease in human volunteers, and to be phagocytized by neutrophils. Gonococcal infectivity is restored with sialic acid removal by neuraminidase or by the replication of gonococci within the lumen of the urethra in the absence of host-derived CMP-NANA. Within the lower female genital tract, sialylated gonococci may become modified to enhance disease transmission to men. That is, neuraminidases produced by the vaginal microbiota (e.g., G. vaginalis63) may remove sialic acid from sialylated gonococci. Cervical epithelia also produce neuraminidase64; however, the specificity of this enzyme to cleave endogenous or exogenous substrates exhibits cyclic variability. The level of sialic acid found within the microenvironment of the cervix also exhibits cyclic variation. Neuraminidase and the ASGP-R are also present on human sperm.65 Sialylated gonococci in proximity to sperm cells may become desialylated through the action of neuraminidase present on these cells. Subsequent gonococcal adherence to the ASGP-R on sperm can then, in turn, facilitate disease transmission.65
Infection of the Lower Female Genital Tract
The majority of gonococci transmitted from men to their partners have sialylated LOS. However, the presence or absence of sialic acid on LOS does not influence the interaction of the gonococcus with the cervical epithelium.
In contrast to the overt inflammatory response generated with gonococcal infection of the male urethra, 50% to 80% of women with lower genital tract N. gonorrhoeae infection are asymptomatic and 70% to 90% of women with disseminated infection lack signs of genital tract involvement. Analysis of cervical secretions obtained from uninfected women and from women infected with the gonococcus reveal that an antibody response is not generated with uncomplicated infection. These findings are consistent with the ability of the gonococcus to evade and to subvert host immune function.66
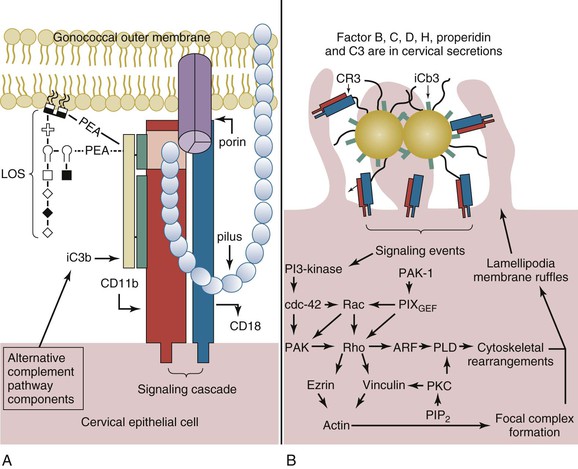
Within the lower female genital tract, the cervical epithelium provides a source of alternative pathway (AP) complement (C′) activity, albeit at a level only comparable to approximately 10% of that observed for human serum. These components are produced by the cervical epithelia. Within minutes of infection of cervical epithelia, C′ protein C3b is deposited on the lipid A portion of gonococcal LOS67 and is rapidly inactivated to iC3b (Fig. 214-5A).68 These data are supported by the predominance of iC3b (in comparison to C3b) on the surface of clinically isolated gonococci.69,70 The affinity of C′ factor H (fH) for sialylated LOS13 and for porin of a PI.A isotype13 may augment C3b inactivation. However, C3b inactivation occurs in a kinetically similar manner on gonococci of either a PI.A or a PI.B isotype and on sialylated gonococci or on gonococci that are not sialylated.68
CR3 serves as the primary receptor for N. gonorrhoeae adherence to and invasion of the ectocervix and endocervix (see Fig. 214-5A).68 Binding of gonococcal pilus to the I-domain of CR3 probably allows the gonococcus to overcome the electrostatic repulsion between its own cell surface and that of the cervical cell. The twitching action of the gonococcal pilus with reduction in pilus length may act to juxtapose the gonococcus at the cervical cell surface where C′ concentrations would be expected to allow efficient opsonization for the subsequent intimate adherence of iC3b on the organism surface and gonococcal porin to the I-domain. Thus, binding of the gonococcus requires the cooperative action of iC3b bound to the gonococcal surface in conjunction with gonococcal porin and pilus.68 Engagement of CR3 results in a complex signaling cascade in which a vinculin- and ezrin-enriched focal complex formation occurs before membrane ruffle formation.71 A signal transduction cascade that is dependent upon the activation of wortmannin-sensitive kinases (i.e., phosphatidylinositol 3-kinase or mitogen-activated protein kinases)71 and Rho GTPases68 initiates ruffling (see Fig. 214-5B). Gonococci are then internalized within macropinosomes.71
On infection of cervical epithelia, gonococci release a phospholipase D homologue that gains access to the cervical intracellular environment nonspecifically through macropinocytosis of gonococci.72 Gonococcal phospholipase D (NgPLD) appears to promote infection of the cervical epithelium in several ways. Data indicate that this secreted gonococcal protein augments signaling events that trigger CR3 mobilization to the cervical cell surface.72 This ensures gonococcal receptor availability and, consequently, efficient targeting to and association with the cervical cell surface. NgPLD also modulates cervical cell signal transduction events leading to membrane ruffling. Mutant gonococci that lack functional NgPLD activity do not elicit membrane ruffling, and they are impaired in their ability to associate with and to invade primary human cervical cells.72
As with invasion of male urethral epithelial cells, the intracellular fate of gonococci within the cervical epithelium is unclear. Ligand binding to the I-domain of CR3 does not invoke a proinflammatory response in professional phagocytic cells. Consequently, the ability of gonococci to subvert cervical cell signal transduction cascades and the complement system in such a manner to allow a cooperative mechanism of CR3-mediated adherence to and invasion of the cervical epithelium may also enhance its survival within the lower female genital tract. Gonococcal invasion in the absence of a respiratory burst increases the number of gonococci that survive intracellularly, whereas inactivation of the C′ system enhances gonococcal survival extracellularly. Consequently, subversion of host cell signal transduction and the C′ system by the gonococcus within the lower female genital tract allows this bacterium to obtain a carrier-like state. Ascending infection of the uterus and fallopian tubes may occur as a consequence of hormonal changes that alter the mucosal epithelia, molecules available for gonococcal use, and/or virulence factors expressed by the gonococcus. In this regard, menses is associated with an increased risk to women for gonococcal PID and disseminated infection. C3 production by the cervical epithelium exhibits cyclic variability, and the highest levels of C3 are detected during menses. A correlation can also be made between the presence or absence of Opa and the site of gonococcal isolation. Opa− (or transparent) gonococci predominate in the fallopian tubes and in the cervix at the time of menses. Conversely, Opa+ gonococci predominate in the male urethra and the cervix at the time of ovulation.
Infection of the Upper Female Genital Tract
Approximately 45% of women with gonococcal cervical infection will develop an ascending infection,73 the prerequisite to PID. Ascent to the upper female genital tract may be facilitated by the ability of gonococci to exhibit twitching motility in conjunction with hormonal changes, which influence the expression of C′ and molecules serving as gonococcal receptors within the female genital tract. Human expression of CR3 progressively decreases in an ascending manner from the ectocervix to the fallopian tubes.68 Conversely, expression of the lutropin receptor (LHr) increases in an ascending manner from the endometrium to the fallopian tubes, and expression is upregulated during menses.73,74 The LHr serves as a receptor for gonococcal invasion of fallopian tube epithelia.75 The interaction of the gonococcus with the LHr increases the invasive character of the gonococcus for the fallopian tube epithelia and, therefore, is said to occur in a contact-inducible manner.75,76 Human chorionic gonadotropin (hCG), a ligand for LHr, can competitively inhibit the LHr-gonococcus interaction. These data suggest that gonococci possess a surface molecule that mimics hCG. Recently, Spence and co-workers76 reported that this hCG-like molecule is the ribosomal protein L12. L12 is shown to facilitate gonococcal transcytosis through the fallopian tube epithelia. The LHr is also present on the human uterus, placenta, decidua, and fetal membranes.77 Hypothetically, a gonococcus-LHr interaction occurring on decidua and placental membranes could result in severe complications of disease and may, in part, contribute to the increased risk for spontaneous abortion associated with N. gonorrhoeae infection.
Gonococcal adherence via the LHr occurs selectively on nonciliated cells8,75; however, it is the ciliated cells of the fallopian tube epithelia that are subsequently shed.78 If the infection is left untreated, complete loss of ciliary action can occur. Cytotoxicity of ciliated cells is attributed to gonococcal peptidoglycan79 and LOS80,81 either directly or indirectly through the induction of increased production of the inflammatory cytokine TNF.82 The loss of ciliated cells within the fallopian tube provides the gonococcus access to subepithelial tissues. Access to subepithelial tissue is also obtained with invasion of nonciliated cells, after which gonococci are transcytosed to the basal lateral surface of these cells and released. In Hec1B cells, the gonococcal protein L12 mediates transcytosis to the basal lateral surface76; however, this has yet to be demonstrated in a fallopian tube organ model. Sialylation of intracellular gonococci (before their exocytosis) might prime these organisms for disseminated infection by the increased serum resistance observed with sialylation.
Epidemiology
Incidence
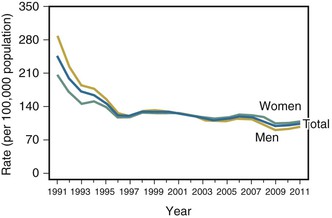
Many industrialized countries but few developing ones possess reporting systems that permit reliable estimates of the incidence of gonorrhea. The number of reported cases in the United States increased from approximately 250,000 cases in the early 1960s to a high of 1.01 million cases in 1978. The peak incidence of reported infection in modern times, 468 cases per 100,000 population, occurred in 1975 (Fig. 214-6). The incidence then declined rapidly, largely the result of systematic public health prevention measures implemented in the 1970s. The decline ceased in the mid-1990s, and the incidence remained relatively stable at 128 to 130 cases per 100,000 from 1998 through 2006. The rate may be declining once again, with 321,849 reported cases (104.2 cases per 100,000) in 2011.33 These statistics likely represent a significant underestimate of the true burden of disease; in 2000, for example, the true incidence was estimated to be approximately 718,000 incident cases, roughly double the reported rate.83
The incidence of gonorrhea is substantially lower in all countries of western Europe than in the United States, but high and rising rates have been documented in eastern Europe. In the past, the highest incidences of gonorrhea and its complications have occurred in developing countries, and this likely remains true in some areas of the world, with particularly devastating consequences for women and their reproductive health.84 However, extensive use of antibiotic regimens for syndromic management of genital complaints, including urethral and vaginal discharge and PID, has apparently effected a decline in gonorrhea prevalence in many countries.85
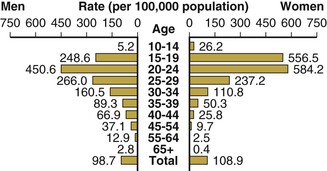
In the United States, the highest attack rates occur in 15- to 24-year-old women and men, but after adjustment for sexual experience, the highest rates are seen in sexually active 15- to 19-year-old women (Fig. 214-7).33,86 According to the population-based National Health and Nutrition Examination Survey, from 1999 to 2008, the prevalence remained higher among non-Hispanic blacks relative to whites.87 The Ad Health study of young adults showed similar results. Among 12,548 adults aged 18 to 26 years, the prevalence of gonorrhea was 0.43% (95% confidence interval [CI], 0.29% to 0.63%), and it was strikingly higher in blacks than in whites (2.13%; 95% CI, 1.46% to 3.10%).88
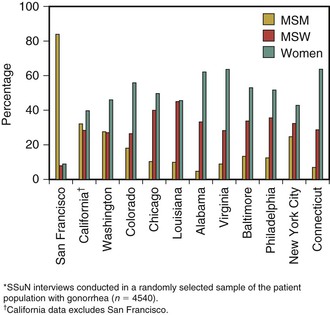
Overall, more cases of gonorrhea are reported in men than in women, likely reflecting both a greater ease of diagnosis in men and a substantially higher rate of infection in men who have sex with men (MSM) than in heterosexual men and women. The incidence of gonorrhea among MSM in industrialized countries has been relentlessly increasing in the past several years, presumably the result of behavioral disinhibition in response to improved therapy and survival of people with human immunodeficiency virus (HIV) infection.89 These trends are evidenced in data from the Centers for Disease Control and Prevention’s (CDC) STD Surveillance Network (SSuN). In 2011, the proportion of MSM with gonorrhea and chlamydia at sexually transmitted disease (STD) clinics varied by site (Fig. 214-8). The median site-specific gonorrhea prevalence was 14.5% (range by site, 2.8% to 21.0%). In Seattle, Washington, the minimum incidence of gonorrhea in MSM more than tripled from 1995, when there were at least 209 cases per 100,000 MSM in the population, to 2002 and 2003, when there were 725 and 645 cases per 100,000, respectively.90 During the same years, the rate of reported infection in the remainder of the Seattle population varied between 74 and 97 cases per 100,000. In San Francisco, among MSM attending two STD clinics in 2003, prevalence of gonorrhea by anatomic site was rectal, 6.9%; urethral, 6.0%; and pharyngeal, 9.2%. Approximately 85% of rectal infections were asymptomatic. HIV-positive men were significantly more likely to have gonorrhea. Because 64% of infections were at nonurethral sites, these infections would have been missed and not treated had only urethral screening been performed.91 A more recent analysis in the same clinic population reported that 83.8% of chlamydial and gonococcal infections would have been missed by urethral screening, compared with 9.8% by screening the rectum and pharynx.92
The rate of gonorrhea in African-American populations in the United States is almost 25 times higher than that in whites or people of Asian ancestry; Hispanics and Native Americans experience intermediate rates (Table 214-1). Only a small portion of these differences can be explained by greater attendance of nonwhite populations at public clinics, where case reporting is more complete than in private health facilities.93 Race and ethnicity are demographic markers of increased risk, not factors that directly denote a high risk for gonorrhea or other STDs. Other markers of gonorrhea risk in the United States include lower socioeconomic attainment, lesser education, residence in the southeastern part of the country, being unmarried, and illicit drug use. Contrary to popular perceptions, the population-based incidence of gonorrhea is as high in many rural settings in the United States as in urban ones. Differing incidence rates between population subgroups are related less to variations in numbers of sex partners than to complex and poorly understood differences in sex partner networks, as well as access to health care and related societal factors. A detailed analysis of increasing gonorrhea incidence in California from 2003 to 2005 raised the importance of contact with a recently incarcerated partner as a major risk and highlighted the relatively understudied contribution of this infection in correctional facilities settings.94 The demographic predictors of gonorrhea throughout the world are qualitatively similar to those in the United States.
TABLE 214-1
Reported Cases and Rates of Gonorrhea According to Race and Ethnicity, United States, 2011
| RACIAL/ETHNIC GROUP | REPORTED CASES | CASES PER 100,000 POPULATION | RATE RATIO |
| White, non-Hispanic | 50,361 | 25.2 | Referent |
| Black, non-Hispanic | 168,515 | 427.3 | 17.0 |
| Hispanic | 27,176 | 53.8 | 2.1 |
| Native American/Native Alaskan | 2,972 | 115.7 | 4.6 |
| Asian/Pacific Islander | 2,431 | 15.1 | 0.6 |
| Total | 321,849 | 104.2 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree