Fred M. Gordin, C. Robert Horsburgh Jr.
Mycobacterium avium Complex
Mycobacterium avium complex (MAC) comprises two closely related organisms: M. avium and Mycobacterium intracellulare. Four subspecies of M. avium have been described, of which subsp. hominissuis is the pathogen of humans. Three major disease syndromes are produced by MAC in humans: pulmonary disease, usually in adults whose systemic immunity is intact; disseminated disease, usually in patients with advanced human immunodeficiency virus (HIV) infection; and cervical lymphadenitis. Also, but rarely, MAC can cause disease in other sites, such as cutaneous disease. The frequency of MAC pulmonary and lymph node disease seems to be increasing, particularly in developed countries, but neither condition is reportable, and increases may be due to improved culture and radiographic techniques. Occurrence of disseminated MAC disease increased precipitously with the HIV pandemic but has declined subsequently with the introduction of effective antiretroviral therapy.
Epidemiology
Reservoir and Route of Acquisition
MAC organisms are common in many environmental sites and are thought to be acquired by inhalation or ingestion. Person-to-person spread has not been observed. Environmental sites harboring MAC are diverse, including water, soil, and animals.1,2 MAC has been found to colonize natural water sources, indoor water systems, pools, and hot tubs.3–6 Specific sites from which patients acquire MAC are identified rarely, but exposure to recirculating hot water systems has been identified as one route of acquisition of MAC in patients with acquired immunodeficiency syndrome (AIDS).6 Less than 15% of cases can be traced to this source, however, suggesting that other environmental reservoirs may be important as well. Increased risk for disseminated MAC among patients with AIDS has also been associated with exposure to swimming pools and other water sources,7 whereas MAC infection of the skin can occur after hot tub use.8 Aerosols of fresh and salt water may contain MAC, and these have been proposed as vehicles leading to transmission of MAC respiratory disease, though in a case-controlled study, aerosolized fluids were not associated with the acquisition of MAC.9 The frequent occurrence of MAC in milk (even after pasteurization) and the preponderance of cases of cervical lymphadenitis in children younger than 3 years old have led some investigators to speculate that oral exposure to organisms in milk is the route of infection for this clinical presentation.10
Pulmonary Disease
MAC pulmonary disease is seen in most developed countries. In the United States11 and in Japan,12 there are approximately 1.3 cases per 100,000 persons, whereas in France there are 0.2 case per 100,000 persons,13 and in Switzerland, there are 0.9 case per 100,000 persons.14 Prevalence in the United States appears to be increasing and shows substantial geographic variability.15–19 One study of four centers found the overall prevalence of MAC to be at a rate of 5.4 per 100,000 persons,16 though there is no national database to accurately evaluate the true occurrence of MAC in the United States. Most estimates are that disease due to MAC occurs at a higher incidence and prevalence than tuberculosis in the United States. The average age of patients with MAC pulmonary disease in the United States is around 60 years, and most patients are women.16–18 Younger persons also seem to be at risk for focal MAC pulmonary disease, however.20,21 Specific risk factors for MAC pulmonary disease include chronic obstructive pulmonary disease, a history of prior hospitalization for pneumonia, and use of steroids and other immunosuppressive agents.9,22 These factors, even taken together, do not explain the overall increase in cases of MAC over the past decade.23 Chronic bronchiectasis is associated with MAC but is likely the result of the disease rather than a predisposition. MAC pulmonary disease can occur in HIV-infected persons without dissemination, although the risk of subsequent dissemination is high.24–26 One report has identified isolates from residential bathrooms that matched isolates of patients with pulmonary disease, suggesting acquisition from this source.27 MAC hypersensitivity pneumonitis has also been linked to exposure to pigeons and exotic birds, as well as hot tubs.28 Reports have identified MAC in the sputum of patients with cystic fibrosis, and a causal role has been proposed for this organism in the destruction of pulmonary tissue seen in cystic fibrosis patients.29,30 MAC also may cause pulmonary disease in patients with pulmonary alveolar proteinosis.31
Disseminated Disease
Disseminated MAC disease was extremely rare before 1980,32 but then the heightened susceptibility of AIDS patients to this disease led to a marked increase in the number of cases. In 1994, an estimated 37,000 cases of disseminated MAC disease were seen in patients with AIDS, making this the most common clinical manifestation of MAC and the most common bacterial disease among patients with AIDS. Since then, with the introduction of preventive antibiotic regimens and effective antiretroviral therapy, the number of patients with MAC has declined substantially.33–35 Disseminated MAC disease can also be seen in children with primary immunodeficiency diseases, such as IFNγR1 or IL-12βR1 deficiency, and in patients with hairy cell leukemia.36–38
The greatest risk for MAC in patients with AIDS is in patients with severe depression of the CD4+ cell count: disseminated MAC is seen rarely in patients with greater than 100 CD4+ cells/mm3, and the median CD4+ cell count among patients with disseminated MAC and AIDS is 10 cells/mm3.39,40 The risk for MAC increases as the CD4+ count declines,40 and the prior occurrence of another opportunistic condition increases the risk for MAC at any given CD4+ cell level.41 Early in the HIV epidemic, similar risks for MAC in HIV-infected patients were seen when patients were compared by age, race, sex, or HIV transmission risk.42 Children with AIDS have a risk for MAC similar to that of adults, and rates of MAC have also fallen dramatically in this age group as HIV treatment has improved.43,44
Rates of disseminated MAC disease among patients with AIDS are higher in the southern United States compared with patients in the northern United States or Canada; these differences may be due to decreased environmental exposure to MAC in the north during the winter.45 Disseminated MAC has been reported with a frequency of 10% to 25% of AIDS patients in Europe, North America, and Australia, but the disease is less common in developing countries, particularly in Africa, where less than 1% of patients with AIDS are affected.46 These differences may be due to several factors, including a smaller proportion of AIDS patients with extremely low CD4+ cell counts, protection from MAC by prior exposure to Mycobacterium tuberculosis,47 or fewer exposures to MAC in piped water systems.
Lymphadenitis
An estimated 300 cases of culture-confirmed MAC lymphadenitis occur in the United States each year.11 This number is likely to be an underestimate, however, because many cases of lymphadenitis are not cultured or fail to grow an organism. MAC cervical adenitis is largely a disease of children, with most cases occurring in children younger than age 3 years, on the basis of reports from Europe, North America, and Australia. A report estimated the incidence of MAC lymphadenitis in children in the Netherlands at 51 cases per 100,000.48 The disease shows a modest female predominance, and nearly all reported cases are in whites.49 Before 1980, most nontuberculous lymphadenitis in the United States was due to Mycobacterium scrofulaceum, but in recent years, MAC has been the cause in most cases.50 MAC lymphadenitis is also seen in HIV-infected persons, particularly as a manifestation of the immune reconstitution inflammatory syndrome (IRIS)51; cervical, mediastinal, or intra-abdominal nodes may be involved.
Pathogen
Classification and Microbiology
Organism
Mycobacteria are aerobic, non-spore-forming, nonmotile bacilli. Their cell walls include mycolic acid–containing, long-chain glycolipids or glycopeptidolipids, or both, that protect these facultative intracellular parasites from lysosomal attack. The organisms grow slowly (10 to 21 days on solid media) and produce thin-translucent or domed-opaque colonies. Colonies are usually light tan in color, although some MAC strains produce a yellow pigment that increases with light exposure. MAC can be cultured on solid or liquid media; liquid media are more sensitive and yield results in a shorter time but do not allow quantitation of mycobacterial load.52 Glycolipid typing has divided MAC into 28 serovars; 1-6, 8-11, and 21 are M. avium, and 7, 12-20, and 25 are M. intracellulare.53 Pulsed-field gel electrophoresis has been able to resolve greater differences in these isolates, indicating there is considerable diversity in the strains of MAC that infect patients.54 MAC isolates can be identified as M. avium or M. intracellulare by DNA probes or polymerase chain reaction restriction analysis. In vitro susceptibility testing of MAC isolates against macrolides and azalides is clinically useful, but susceptibility testing against other antimycobacterial agents (e.g., ethambutol, rifamycins, fluoroquinolones, and aminoglycosides) has not been shown to predict clinical response and is not recommended.52
Virulence
MAC is relatively avirulent in the normal host. Serovars 1, 4, and 8 are uncommon in the environment, but they cause most cases of disseminated disease in AIDS patients.53 These serovars are thought to be associated with virulence and do appear more virulent in an animal model of infection. Possible relative virulence factors include adherence to intestinal epithelial cells, production of catalase, failure to acidify vesicles, and inhibition of phagosome-lysosome fusion.55–57 Clinical isolates from patients with disseminated disease are always of the smooth-transparent colony type, rather than the domed or opaque type. Colonies that are smooth and transparent are more likely to replicate in vivo; are more likely to induce the cytokines, such as tumor necrosis factor-α and interleukin-1; and usually have decreased susceptibility to antimycobacterial agents in vitro.58 An isolate from a patient with MAC disease has been shown to produce increased cell lysis and increased ability to stimulate HIV replication in vitro, relative to the abilities of an animal MAC isolate.59 MAC also seems to be able to exist symbiotically with water-borne amebas, leading to increased virulence of MAC in an animal model.60
Pathogenesis
MAC disease results from primary acquisition of the organism by either ingestion or inhalation. No cases of reactivation MAC disease have been reported.
Pulmonary Disease
MAC pulmonary disease develops after inhalation of MAC. The duration from inhalation to disease is unknown but presumably occurs over many months to years. More than one distinct MAC organism can be recovered from some patients,61 suggesting that disease, super-infection, or colonization may occur concomitantly. Tissue lesions are usually localized and appear grossly as well-circumscribed nodules. Granulomatous pleuritis, bronchitis, vasculitis, and interstitial pneumonia have also been reported.62 The histologic features vary from poorly formed to well-formed granulomas. Giant cells are seen frequently, and in rare cases, there is central caseating necrosis and cavitation. Thoracic lymph node involvement is uncommon.
Disseminated Disease
Infection is acquired through inhalation or ingestion of MAC, followed by localized disease in the lung or gut. Dissemination ensues from either location over several months.24 In patients with AIDS, 80% to 90% of infections are acquired by ingestion. Most disseminated disease is due to a single MAC strain, but multiple distinct isolates have been recovered from 15% of patients.54 The organisms penetrate the gut wall, possibly through Peyer’s patches, and subsequently are phagocytized by macrophages and other reticuloendothelial cells.63,64 Histologically, epithelial cells show only mild inflammatory changes, and ulceration is uncommon. Sheets of foamy macrophages are present in the lamina propria; these massively infected cells may expand the intestinal villi, giving an appearance similar to Whipple’s disease. On acid-fast staining, the cells are packed with bacilli.
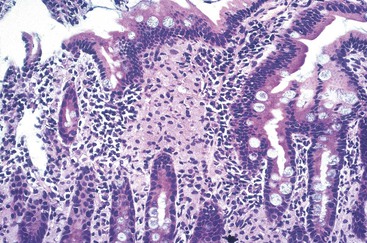
The resulting thickening of the bowel wall (Fig. 253-1) can lead to intussusception, gastrointestinal hemorrhage, or obstruction, but these are rare. Mesenteric adenopathy ensues (Fig. 253-2); poorly formed granulomas, abscesses, and necrosis with neutrophilic inflammation are seen in these nodes65,66; the cells are filled with acid-fast bacilli. Granulomas with giant cells, epithelioid macrophages, and caseating necrosis can be seen but are less common. Subsequently, hematologic dissemination occurs.67 Any organ can be seeded secondarily, but the most common sites are liver, spleen, and bone marrow (Fig. 253-3).65,66 The histologic picture in these organs is similar to that in the lymph nodes. The burden of organisms in the blood is variable, ranging from 1 to greater than 105 colony-forming units/mL.25,68 Higher levels of bacteremia likely represent a longer duration of dissemination and signal a poor prognosis. Untreated disseminated MAC disease leads to death by inanition. Decreased caloric intake and increased metabolic demand seem to play a role in this process.
Entry of MAC into the bloodstream leads to elevated serum levels of tumor necrosis factor-α and interleukin-6, which likely are responsible for the predominant symptoms of fever, night sweats, and cachexia.69,70 The mechanism of the severe anemia seen in disseminated MAC disease is not well understood because bone marrow involvement can be minimal. Erythropoietin levels are variable, and clinical response to exogenous erythropoietin is unpredictable.71
A unique pathophysiologic abnormality seen with disseminated MAC disease is marked elevation of serum alkaline phosphatase, which is seen in roughly 5% of patients. Serum enzyme levels may reach 20 to 40 times the normal level, with little elevation of transaminases, bilirubin, or other parameters of hepatic function; nonetheless, fractionation shows it to be of hepatic origin. Patients have little symptomatic discomfort, and the histologic picture in the liver does not show marked abnormality, suggesting interference with enzyme metabolism rather than hepatic tissue destruction.
Lymphadenitis
MAC cervical and abdominal lymphadenitis is likely acquired through ingestion of MAC, whereas thoracic lymphadenitis presumably occurs subsequent to inhalation. Lesions reveal granulomas, usually without caseation. Ulceration and fistula formation are frequent complications, particularly when nodes have been incised or aspirated. In the immunologically normal host, acid-fast bacilli can be seen in macrophages and giant cells, but they often are single, and dissemination of disease does not occur. In HIV-infected patients without antiretroviral therapy, there is little granulomatous response, and unrestrained mycobacterial replication leads to macrophages filled with acid-fast bacilli with eventual dissemination. When antiretroviral therapy is instituted, a vigorous granulomatous response results in elimination of bacilli from the tissues.
Host Immunity
Pulmonary Disease
The fact that many cases of MAC pulmonary disease have occurred in persons with a history of smoking or chronic lung disease, or both, suggests that impaired pulmonary clearance mechanisms may predispose patients to MAC. No specific clearance defects have been identified, however. Patients with MAC lung disease develop antibody and delayed hypersensitivity to MAC antigens, and humoral and cell-mediated immunity remain intact.72 The host immune response consists of granuloma formation with ingestion and intracellular killing of MAC by macrophages.
Disseminated Disease
Persons with inherited defects in the interferon (IFN)-γ signaling pathway are also exquisitely susceptible to disseminated MAC disease, confirming the importance of this cytokine in host defense against MAC. Sites of defects so far identified include the IFN-γ receptor ligand binding chain, the IFN-γ signal transducing chain, and defective IL-12-mediated modulation of IFN-γ production.73–76 Exogenously administered IFN-γ overcomes these defects and may lead to clinical improvement.77
In patients with AIDS, macrophage phagocytosis of MAC is unimpaired, but intracellular killing does not occur, and organisms multiply unimpeded within macrophages. Macrophages from patients with AIDS can respond normally to cytokines,78 although cytokine production by T cells is severely impaired in such patients, leading to failure of macrophage activation to eliminate intracellular MAC.79 MAC-specific T cells develop during disseminated disease in AIDS patients, but these cells do not appear to be able to control the pathogen.67 Cytotoxic CD4+ cells are important in inhibiting intracellular replication of MAC, but their function is also impaired in HIV infection.80
Humoral factors may also play a role in disseminated MAC disease. Antibodies against MAC are produced in response to disease in normal hosts but not in patients with AIDS.39 Although these antibodies are not known to have a role in protection against MAC disease, they increase MAC killing in vitro.81 Conversely, MAC growth can be stimulated by high serum levels of triglycerides and by the iron overload seen in patients with AIDS.
Lymphadenitis
Patients with MAC lymphadenitis also develop antibodies and delayed hypersensitivity to MAC antigens, indicating intact humoral and cell-mediated immunity. The histologic response usually consists of noncaseating granuloma formation; few acid-fast bacilli are seen in tissue sections.
Clinical Manifestations
Pulmonary Disease
The clinical presentation of pulmonary MAC disease is nonspecific and can be confused with other mycobacterial infections and chronic pulmonary diseases. The classic presentation of pulmonary MAC is one of a subacute-to-chronic illness occurring in individuals with a prior history of underlying pulmonary pathology due to smoking, bronchiectasis, cancer, silicosis, prior tuberculosis, or other diseases.21,82 Most of these individuals are middle-aged to older men, predominately white, and frequently with a history of heavy smoking and heavy alcohol consumption. The clinical picture in this population is one of a chronic disease, with the predominant symptoms being productive cough (occurring in >80% of patients), weight loss or weakness (in approximately half), and fever or night sweats (each in 10% to 20% of patients).13,82 A study in Japan of 634 persons with pulmonary MAC found mortality due to MAC to occur in 5.4% of persons at 5 years and 15.7% at 10 years.83 Presentation of pulmonary MAC has been reported to result in death within 2 years of diagnosis in 15% of individuals. Pulmonary MAC alone, without disseminated disease, may also occur in persons with HIV infection.26
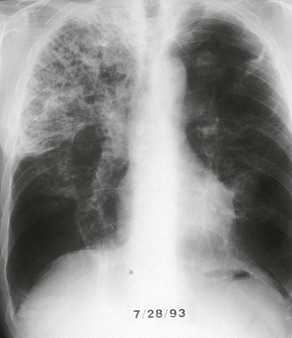
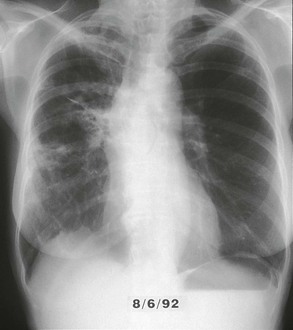
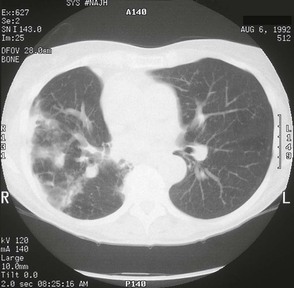
The chest radiograph in this chronic form of pulmonary MAC typically shows upper lobe fibronodular and cavitary disease, which may be associated with pleural thickening (Fig. 253-4). Rates of cavitation tend to be higher than with tuberculosis: cavitary disease is reported in 60% to 90% of patients with MAC82 compared with approximately 50% of patients with tuberculosis. The cavities in patients with MAC are also more likely to be thin walled than in tuberculosis and may be quite large, with most in the 2- to 4-cm range.84 MAC pulmonary disease is bilateral in almost half of patients. Pleural effusions are uncommon. Other features that may be identified on chest radiographs in patients with pulmonary MAC are pectus excavatum and scoliosis. Studies have reported that 11% to 27% of individuals with MAC have pectus excavatum compared with 2.4% in the general population, and about 50% of patients with MAC had scoliosis compared with 19% in the general population.20,85 Computed tomography (CT) scans of the chest may add some useful clinical information. In a series comparing patients with pulmonary MAC with patients with pulmonary tuberculosis, nodules and consolidation were found equally in both groups; bronchiectasis was found significantly more often, however, in patients with MAC (94% vs. 27%).86 The CT finding of bronchiectasis with multiple nodular infiltrates (Fig. 253-5) is particularly suggestive of MAC pulmonary disease. Tree-in-bud opacities are seen often, but these are not specific for MAC.
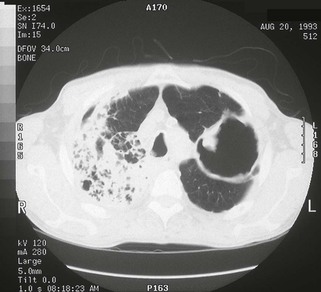
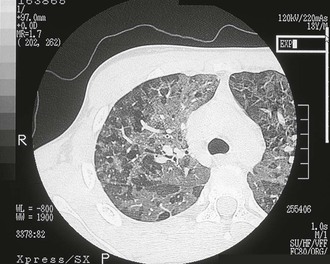
Pulmonary MAC also has been recognized with increasing frequency in middle-aged to elderly women with no preexisting lung disease.21,87,88 This syndrome, sometimes referred to as “the Lady Windermere syndrome,” was named for the principal character in Oscar Wilde’s play. This syndrome presents with a more indolent clinical picture and with fewer chest radiograph abnormalities. Patients with this syndrome usually present with chronic cough, but other constitutional symptoms, such as weight loss and fever, are uncommon. Mild scoliosis and pectus excavatum are common. The chest radiograph in these patients shows less lung involvement than in patients with predisposing lung disease, and changes may occur only over years of follow-up.84,87,88 Discrete pulmonary nodules may be seen often in the middle lobe or lingular regions, although other areas of the lungs may be involved (Fig. 253-6). Cavities have been reported in only 25% of these patients. High-resolution CT (HRCT) scans can be important as a means of detecting micronodules (<5 mm) and evidence of bronchiectasis in this population (Fig. 253-7).84,86,89
Patients with cystic fibrosis are frequently colonized with nontuberculosis mycobacteria, but the clinical importance of MAC in this population is not clear. In one multicenter prevalence study of 986 patients with cystic fibrosis, 13% had at least one of three sputum samples positive for nontuberculosis mycobacteria, most (72%) with MAC.90 Overall, patients with cystic fibrosis and MAC had similar pulmonary function tests compared with patients without MAC.91 Serial HRCT (high-resolution CT) scans provided important information because patients with MAC and progression of HRCT abnormalities were more likely to have clinical decline than other cystic fibrosis patients with MAC. In cystic fibrosis patients, HRCT abnormalities suggestive of progressive MAC were progression of areas of cystic or cavitary disease, subsegmental or larger areas of consolidation, pulmonary nodules, and tree-in-bud opacities; these changes are not specific, however, for MAC.91 Isolated MAC pleurisy has also been reported.92
Another pattern of MAC pulmonary disease, known as “hot-tub lung disease,”93–95 occurs in persons exposed to pools of heated water containing MAC. Patients with this condition are presumed to have inhaled aerosolized MAC, resulting in a hypersensitivity pneumonitis. These patients present with mild-to-moderate dyspnea and dry cough, with or without fever. Chest radiographs and CT scans show patterns similar to those seen in other hypersensitivity pneumonitides, with a variety of radiologic patterns, including bilateral alveolar infiltrates, centrilobular nodules, and “ground-glass” opacities (Fig. 253-8).93–95
Disseminated Disease
Disseminated MAC occurs almost exclusively in persons with advanced HIV disease. In a large natural history study of patients with HIV infection, MAC bacteremia developed at a median CD4+ cell count of 13 cells/mm3, and the median survival after diagnosis was only 134 days.40 It can be difficult to separate the clinical and laboratory features directly attributable to MAC from abnormalities attributable to advanced HIV disease. Most patients with disseminated MAC have high fever, weight loss, night sweats, or severe anemia (hematocrit <25%).96–98 Other features associated with MAC include abdominal pain, diarrhea, intra-abdominal lymphadenopathy, hepatosplenomegaly, and an elevated serum alkaline phosphatase level. The clinical features directly attributable to the onset of disseminated MAC have been described by evaluating patients at risk, with prospective monthly blood cultures for MAC.99 At the time blood cultures became positive, patients with MAC had more weight loss, fever, anemia, abdominal pain, or elevated alkaline phosphatase than patients who did not develop MAC. The onset of these clinical changes occurred within 2 months of the first positive blood culture for MAC.99
In patients with disseminated MAC, other organ-specific localizing signs and symptoms may be present as a manifestation of the involvement of these organ systems. A comprehensive autopsy series of 44 patients with AIDS and disseminated MAC showed the most common organs involved to be the spleen, lymph nodes, liver, intestines, colon, bone marrow, and, less commonly, lungs, adrenals, stomach, and central nervous system.100 Patients may present with clinical manifestations of disease referable to any of these body systems. Patients with AIDS and disseminated MAC may have concurrent pulmonary disease, but this is not common. Although MAC isolated from respiratory specimens may be a harbinger of disseminated MAC,24 parenchymal lung involvement occurs in less than 10% of patients with disseminated MAC.26 When parenchymal lung disease does occur in this population, the chest radiograph may reveal alveolar infiltrates, nodules, or cavitary disease.
Local manifestations of disseminated MAC may occur in AIDS patients with severe immune suppression who have been started on antiretroviral therapy; these patients can develop local symptoms as a result of an inflammatory reaction to MAC antigens as the cell-mediated immune response is restored. This phenomenon is called the IRIS or a “paradoxical reaction” (Figs. 253-9 to 253-11).51,101–104 Most often, patients exhibit painful lymphadenopathy, occurring within 1 to 12 weeks of initiating antiretroviral therapy; pulmonary-thoracic disease, abdominal pain, and hepatosplenomegaly also have been reported to occur in 25% to 30% of patients.102–104 Patients with immune reconstitution syndrome differ from other patients with disseminated MAC: fever may be present, but other constitutional symptoms (e.g., weight loss, night sweats) are usually absent, and blood cultures usually do not grow MAC. Biopsy may be required to establish an accurate diagnosis and to exclude other processes.
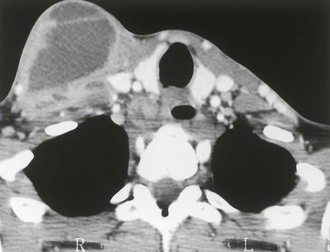
Disseminated infection with MAC rarely occurs in patients without AIDS. In one review of 37 patients with disseminated MAC but without AIDS, most patients had either received steroids or had an underlying hematopoietic malignancy.32 The clinical features of disseminated MAC disease in this population were similar to the features in persons with disseminated MAC and AIDS: fever, weight loss, and local pain occurred in 32% to 54% of patients; night sweats occurred in 14%; anemia occurred in 75%; and lymphadenopathy or hepatosplenomegaly occurred in more than 40%.
Lymphadenitis
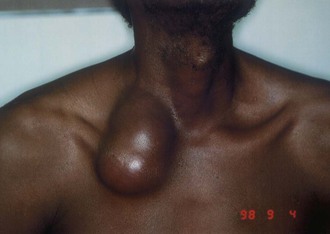
Cervicofacial lymphadenitis is the most common manifestation of MAC in children, and more than 80% of patients with MAC cervical lymphadenitis are between 1 and 5 years old.48–50,105,106,107 The disease can also occur in adults, but tuberculosis as a cause of lymphadenopathy is more common than MAC in adults.106,107 The clinical presentation is usually painless or minimally painful unilateral enlargement of a node in the submandibular or high jugular region (Fig. 253-12).48–50,105 Fever is uncommon. Bilateral disease occurs in less than 10% of individuals, and multiple nodes are involved in less than 20% of children. Nodes are most often firm but not fluctuant. Node size may vary from 1 to 7 cm in diameter, with the mean size in one series reported as 2.5 × 3 cm.105,107
Other Sites
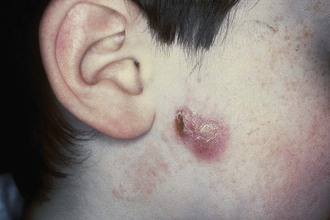
Cutaneous disease due to MAC is uncommon and not differentiated easily from other chronic skin lesions. Lesions may be ulcers, nodules, or plaques. Cases of cutaneous MAC have been reported in immunocompetent and immunosuppressed hosts.8,108–110 Most often, cutaneous MAC is due to direct inoculation of the skin by trauma, surgery, or injection. Local swelling, erythema, and tenderness may be present for months to years. The lesions are indolent, with little or no lymph node reaction or systemic symptoms. MAC is also a rare cause of renal disease, prostatitis, peritonitis, corneal ulceration, mastoiditis, mastitis, osteomyelitis, endocarditis, septic arthritis, and synovitis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree