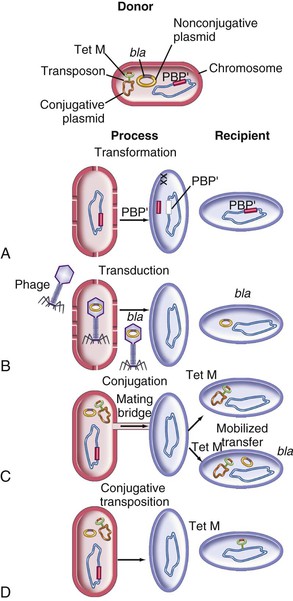
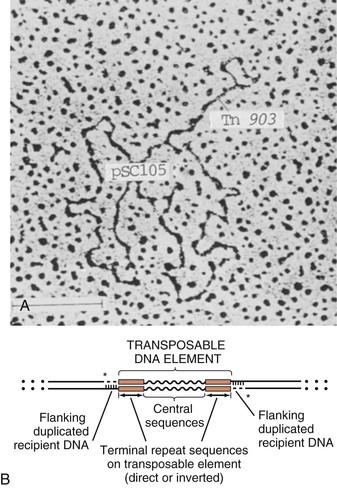
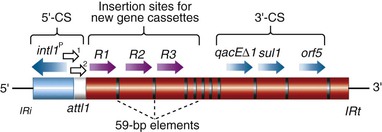
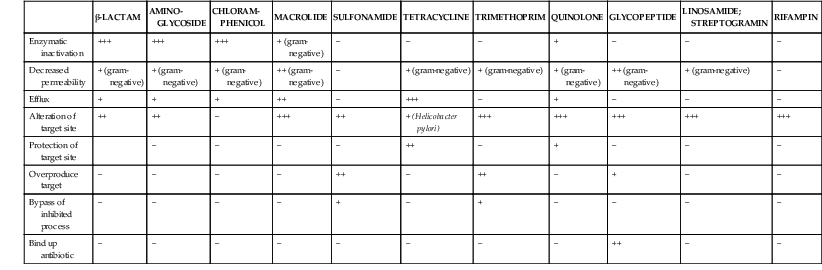
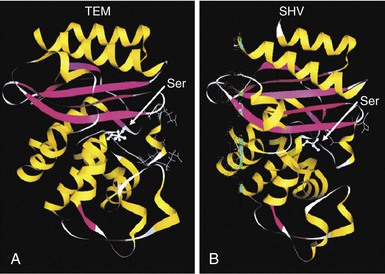
Steven M. Opal, Aurora Pop-Vicas Genetic variability is essential for microbial evolution to occur. The fitness of a microorganism depends on its capacity to adapt to changing environmental conditions.1 Antimicrobial agents exert strong selective pressures on bacterial populations, favoring organisms that are capable of resisting them.1,2 Genetic variability may occur by a variety of mechanisms. Point mutations may occur in a nucleotide base pair, which is referred to as microevolutionary change. These mutations may alter enzyme substrate specificity or the target site of an antimicrobial agent, interfering with its activity. Point mutations at crucial locations on “old” β-lactamase genes (e.g., genes for Temoneira-1 [TEM-1], sulfhydryl variable-1 [SHV-1]) are primarily responsible for the remarkable array of newly recognized extended-spectrum β-lactamases (ESBLs).3,4 A second level of genomic variability in bacteria is referred to as a macroevolutionary change and results in whole-scale rearrangements of large segments of DNA as a single event. These rearrangements may include inversions, duplications, insertions, deletions, or transposition of large sequences of DNA from one location of a bacterial chromosome or plasmid to another. These large-scale rearrangements of entire segments of the bacterial genome are frequently generated by specialized genetic elements called integrons and transposons or insertion sequences, which have the capacity to insert, rearrange, and move independently from the rest of the bacterial genome.2 A third level of genetic variability in bacteria is created by the acquisition of large segments of foreign DNA carried by plasmids, bacteriophages, naked sequences of DNA, or specialized transposable genetic elements known as integrative and conjugative elements (ICE) from other bacteria.2 These events are termed lateral or horizontal gene transfer and are now known to be a frequent event, especially for naturally competent bacteria that can take up exogenous DNA from the environment. Inheritance of foreign DNA further contributes to the organism’s genetic variability and its capacity to respond to selection pressures imposed by antimicrobial agents.3 These mechanisms endow bacteria with the seemingly unlimited capacity to develop resistance to any antimicrobial agent (Fig. 18-1). Examples of plasmid-mediated carbapenemase-producing Klebsiella pneumoniae,5 vancomycin-resistant and daptomycin-resistant Staphylococcus aureus,6,7 multidrug-resistant Yersinia pestis,8 and transferable quinolone resistance in enterobacteria9 attest to the capacity of microorganisms to adapt to environmental stresses such as antibiotic exposure. When an antibiotic-resistance gene evolves, this gene can spread between bacteria by transformation, transduction, conjugation, or transposition. Favored clones of bacteria may proliferate in the microbiota of patients who receive antibiotics.10 Antibiotic-resistance genes existed well before the introduction of antimicrobials for treatment for human infections and can be found within bacteria in Arctic region permafrost samples untouched by human hands for over 30,000 years.10 Antibiotics are often synthesized by antibiotic-producing bacteria when they are about to enter the late static growth phase and are about to become dormant or sporulate. Antibiotic-producing bacteria are resistant to the antibiotics they produce. Therefore, antibiotics left in the microenvironment by dormant bacteria are avoided as potentially toxic to competitors yet serve as a ready source of carbon (food) for the next generation of the antibiotic-producing bacterial strain once the growth phase begins again. Environmental levels of multiple classes of antimicrobial agents are now so common in soil and water samples that multiple bacterial genera have strains that subsist entirely upon antibiotics as their sole carbon source.11 These bacteria express remarkably high levels of multiple antibiotic resistance to a wide array of antibiotic classes. Aquatic environments are particularly rich with bacterial populations replete with antibiotic-resistance genes.12 Such environmental bacterial strains likely provide potential human pathogens with a source of novel antibiotic-resistance genes.11,12 Selection pressures placed on microbial populations by antibiotics favor the expansion of strains that have the capacity to resist the inhibitory effects of antibiotics. These resistant populations proliferate and spread antibiotic-resistance genes vertically to subsequent generations and horizontally to susceptible strains of related bacteria, or even between species or different genera.13 Although some antibiotic-resistance genes place a metabolic “burden” on bacteria, many microorganisms evolved strategies to limit this cost by repressing gene expression when not needed or by phase variation. This allows favorable, but sometimes “costly,” antibiotic-resistance genes to be held in reserve in the absence of antibiotic selection pressure yet express their resistance potential upon reexposure to antibiotics.14 Continous exposure to foreign DNA within microbial communities is so commonplace that many bacteria have evolved systems to defend their genomes from exogenous DNA, phages, and plasmid insertion. This is accomplished by at least two mechanisms: (1) species and strain-specific DNA modifying enzymes (e.g., methylation of selected sequences of host DNA into specific patterns) and restriction enzymes that survey cellular host DNA and degrade foreign DNA that lacks appropriate DNA modification seqences and (2) a type of adaptive defense system against foreign DNA known as CRISPR (clustered regularly spaced short palindromic repeats).15 CRISPRs are detectable in nearly 50% of all bacterial genomes, and this genetic element protects their genomes from attack by foreign DNA during transformation, phage invasion, or plasmid insertion. The mechanism of protection is mediated by inserting small sequences of the mobilized invading DNA between palindromic repeats within the CRISPR. Upon reexposure to similar DNA sequences from phage or invading bacteria, the existing sequence within the CRISPR is transcribed into a small RNA (known as crRNA) that associates with CRISPR-associated nucleases and prevents integration of the targeted foreign DNA. Maintaining the fidelity of the host genome, while permiting limited variation by microevolutionary and macroevolutionary changes, allows pathogens to strike a balance between genomic stability and plasticity in rapidly changing microenvironments. Recent evidence in the enterococci indicates that deletion of CRISPR elements is inversely related to multiple antibiotic-resistance development. CRISPR-deficient strains have been selected for, and are particularly well-adapted for, health care–associated infections. These strains have significantly larger genomes as a result of insertion of large sequences of DNA, including genes that mediate multiple antibiotic resistance.16 Extrachromosomal elements were present in bacteria before the advent of antibiotics.10,13 The introduction of antibiotics into clinical medicine in the 20th century created selection pressures, however, that favor the dissemination of resistance genes via mobile genetic elements.2,3,13 Plasmids are particularly well adapted to serve as agents of genetic exchange and resistance-gene dissemination.1,3 Plasmids are autonomously replicating genetic elements that generally consist of covalently closed, circular, double-stranded DNA molecules ranging from less than 10 kilobase (kb) pairs to greater than 400 kb. They are extremely common in bacteria.13 Although multiple copies of a specific plasmid, or multiple different plasmids, or both may be found in a single bacterial cell, closely related plasmids often cannot coexist in the same cell. This observation led to a classification scheme of plasmids based on incompatibility (Inc) groups.1,4 Plasmids may determine a wide range of functions besides antibiotic resistance, including virulence and metabolic capacities. All plasmids possess an origin of replication for DNA polymerase to bind and replicate plasmid DNA. Plasmids must also retain a set of genes that facilitate their stable maintenance in host bacteria. The transfer of plasmid DNA between bacterial species is a complex process, and the genes needed for transfer (tra genes) make conjugative plasmids larger than nonconjugative ones. Some small plasmids may be able to transfer to other bacteria via the use of the conjugation apparatus provided by co-resident conjugative plasmids or even conjugative transposons. Many plasmid-encoded functions enable bacterial strains to persist in the environment by resisting noxious agents, such as heavy metals. Mercury released from dental fillings may increase the number of antibiotic-resistant bacteria in the mouth.17 Compounds such as hexachlorophene and quarternary ammonium compounds are used as topical bacteriostatic agents, and plasmid-mediated resistance to these agents has increased significantly.2 Transposons can translocate as a unit from one area of the bacterial chromosome to another or between the chromosome and plasmid or bacteriophage DNA. Transposable genetic elements possess a specialized system of recombination that is independent of the generalized recombination system that classically permits recombination of largely homologous sequences of DNA by crossover events (the recA system of bacteria). The recA-independent recombination system (“transposase”) of transposable elements usually occurs in a random fashion between nonhomologous sequences of DNA and results in whole-scale modifications of large sequences of DNA as a single event (Fig. 18-2).2,5 There are two types of transposable genetic elements, called transposons and insertion sequences, that have similar characteristics. Evidence from whole-genome sequencing projects indicates that bacterial chromosomes are replete with transposable elements.18 These mobile sequences probably play an important physiologic role in genetic variation and evolution in prokaryotic organisms. Transposons differ from insertion sequences in that they encode functional genes that mediate a recognizable phenotypic characteristic, such as an antibiotic-resistance marker. Either element can translocate as an independent unit. Both elements are usually flanked on either end by short identical sequences of DNA in reverse order (inverted repeats). These inverted-repeat DNA termini are essential to the transposition process. Transposons (Tn) and insertion sequences (IS) are incapable of autonomous self-replication and must exist on a replicon, such as the chromosome, bacteriophage, or plasmid, to be replicated and maintained in a bacterial population. Some transposons have the capability to move from one bacterium to another without being fixed within a plasmid or bacteriophage. These elements are referred to as conjugative transposons, or integrative and conjugative elements (ICE). The ubiquitous transposable element Tn916 and its derivatives are examples of conjugative transposons and have been found primarily in aerobic and anaerobic gram-positive organisms, although they can also exist in gram-negative bacteria.2,19,20 Transposition usually results in the localized replication of the transposable element from the original donor sequence of DNA and the insertion of a copy of the transposable element into the recipient sequence of DNA (replicative transposition).1,2 Transposition, similar to point mutation, is a continuous, ongoing process in bacterial populations. An example of this phenomenon is the spread of a tetracycline-resistance transposon among Neisseria gonorrhoeae, Mycoplasma hominis, and Ureaplasma urealyticum.21,22 An important variant of transposition is “one-ended” transposition, where only one end of the transposon is responsible for asymmetrical replication. This type of element is highly efficient in mobilizing resistance genes adjacent to its insertion site. These elements play a prominent role, along with ICEs and integrons, in the evolution of large regions of the chromosome where multiple resistance genes accumulate into one set of resistance gene cassettes known as resistance genomic islands.2 Some of these genomic islands are enormous in size and scope. For example, the AbaR1 genomic island of Acinetobacter baumannii is 86 kb long and contains 46 different antimicrobial resistance genes to a wide swath of antimicrobials, antiseptics, and heavy metals.23 Once genomic resistance islands form, they often persist and gain new genes over time. These islands serve as convenient depots for insertions of new DNA without the risk of insertion into a critical metabolic or structural gene of the bacterial genome. The “fitness cost” of acquiring new resistance genes is minimized by depositing genes in genomic resistance islands.2 However, the metabolic cost of retaining all these accessory, antibiotic-resistance genes by host bacteria remains. The extra metabolic burden is only worth it in the presence of repeated environmental antibiotic exposure as might exist in hospitals and special care areas, such as the critical care unit, where antibiotic selection pressures favor multidrug-resistant organisms. Antibiotic-resistance genes, especially those existing on plasmids, are in fact frequently disposed of when bacteria are removed from such environments and maintained in antibiotic-free media. Transposons also are essential in the evolution of resistance plasmids that contain multiple antibiotic-resistance determinants.19 The recent spread of carbepenem resistance from metallo-β-lactamases among the Enterobacteriaceae in some European hospitals was facilitated by transposition of resistance genes on integrons to separate plasmids from different bacterial genera of gram-negative bacilli.24 High-level vancomycin resistance (vanA) in enterococci is mediated by a composite transposon that encodes a series of genes needed to express vancomycin resistance.25 Single transposons may encode multiple antibiotic-resistance determinants within their inverted-repeat termini as well.2 Genetic exchange of antibiotic-resistance genes occurs between bacteria of widely disparate species and different genera.26 Identical aminoglycoside-resistance genes occur in streptococci and Campylobacter,27 and enterococci apparently have acquired aminoglycoside28 and β-lactam29 resistance from staphylococci. Given the highly variable environmental selection pressures created by the clinical, agricultural, and industrial use of antibiotics and the plasticity of bacterial genomes, the ongoing evolution of multiresistant species seems inevitable.29,30 The structural genes that mediate antibiotic resistance often are closely linked and may exist in tandem along the bacterial chromosome or plasmid. Genetic analysis of sequences of DNA adjacent to antibiotic-resistance genes has revealed that unique integration units often exist near promoter sites.31 These integration elements, called integrons,32 function as recombinational “hot spots” for site-specific recombination events between largely nonhomologous sequences of DNA. Integrons facilitate the lateral transfer and integration of antibiotic-resistance genes from mobile gene cassettes. The integron provides its own unique integrase function33 that facilitates recA-independent recombination and a specialized attachment and integration site consisting of a variable length (57 to 141 base pairs [bp]) but often a 59-bp spacer sequence of highly conserved DNA. This 59-bp element is preserved at the 3′ end of inserted antibiotic-resistance genes.34,35 Although these integration elements differ structurally and functionally from transposons,36 they seem to be widespread in bacterial populations and play an important role in the dissemination of antibiotic-resistance genes.2,21,37,38 Integrons do not transpose independently as a specific unit structure from one sequence of DNA to another. This capability of autonomous movement of large sequences of DNA is reserved primarily for transposons, insertion sequence elements, and bacteriophages. They may become flanked, however, by transposable elements and become integrated into an existing transposon. The principal role of integrons is to provide a convenient insertion site for antibiotic-resistance genes from foreign DNA sources. There are five classes of integrons that encode antibiotic-resistance genes, with type 1 integrons being the most common in pathogenic microorganisms.38 A schematic representation of a class 1 integron is shown in Figure 18-3. Integrons also serve as expression cassettes for antibiotic-resistance genes in that an efficient promoter site is provided in close proximity to the 5′ end of the newly inserted DNA sequence. The frequency of transcription of integrated cassettes of antibiotic-resistance genes depends on the proximity of the gene to the promoter at the 5′ upstream end of the integron. The level of expression of a resistance gene diminishes as the distance between the promoter and the specific antibiotic-resistance gene cassette increases.33 Numerous clusters of different antibiotic-resistance genes have been identified that have evolved through specific insertions into common integrons.34 Integrons have been found to possess five or more antibiotic-resistance genes lined up in a tandem sequence along a single, functioning integron.39 Complex integrons have recently been described that are typified by a common open reading frame (orf 513) linked to the 3′ end of a typical integron, followed by a series of inserted genes (often expanded-spectrum β-lactamases) after a duplication of the 3′ end of the integron. This common 3′ end of type 1 integrons encodes genes for resistance to quarternary ammonium compounds (qacE1), sulfonamide resistance (sul1), and an open reading frame of unknown function (orf5). These complex integrons can be mobilized and spread by adjacent insertion sequences to disseminate among bacterial populations.40 At least eight distinctive mechanisms of antibiotic resistance have been described in bacteria (Table 18-1). Examples of each of these mechanisms are described in the following paragraphs. Resistance to β-lactam antibiotics occurs primarily through production of β-lactamases, enzymes that inactivate these antibiotics by splitting the amide bond of the β-lactam ring. β-Lactamases have likely coevolved with bacteria as mechanisms of resistance against natural antibiotics over time, and the selective pressure exerted by the widespread use of antimicrobial therapy in modern medicine may have accelerated their development and spread. β-Lactamases are encoded either by chromosomal genes or by transferable genes located on plasmids and transposons. In addition, β-lactamase genes (bla) frequently reside on integrons, which often carry multiple resistance determinants. If mobilized by transposable elements, integrons can facilitate further dissemination of multidrug resistance among different bacterial species.41 β-Lactamases can be classified according to their amino-acid structure into four molecular classes, A through D (Table 18-2), as first suggested by Ambler. Alternatively, the Bush-Jacoby-Medeiros system classifies the enzymes according to their substrate profile and susceptibility to β-lactamase inhibitors, such as clavulanic acid, into several functional groups (Table 18-3).42 Class A, C, and D β-lactamases hydrolyze the β-lactam ring through a serine residue at their active site, whereas class B enyzmes are metallo-β-lactamases that use zinc (Zn)2+ to break the amide bond (Fig. 18-4). TABLE 18-3 Bush-Jacoby-Medeiros Functional Classification Scheme for β-Lactamases * B, both; C, chromosomal; P, plasmid. † New groups; derived from Bush K, Jacoby GA, Medeiros AA. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211-1233. AER-1, Aeromonas-1; CARB-3, carbenicillin-3; FEC-1, isolated from feces; IMI-1, imipenem hydrolyzing; IMP-1, imipenem-1; IRT-2, inhibitor-resistant TEM-2; KPC, K. pneumoniae carbapenemase; MIR-1, Miriam Hospital–1; NDM-1, New Delhi metallo-β-lactamase-1; NMC-A, not metalloenzyme carbapenamase-A; OXA-1, oxacillin-1; PSE-1, Pseudomonas-specific enzyme; SAR-2, southern Africa–related enzyme; SHV-1, sulfhydryl variable-1; TEM-3, Temoneira-3. The first β-lactamase was described as a “penicillinase” capable of hydrolyzing penicillin in Escherichia coli in 1940, about the same time as the first clinical use of penicillin was reported in the literature.43 The next years witnessed the rapid spread of plasmid-encoded penicillin resistance among the majority of S. aureus clinical isolates.44,45 Among gram-negative organisms, the rise in ampicillin resistance in the 1960s was ascribed to the emergence of TEM-1, a plasmid-encoded β-lactamase named after a Greek patient, Temoniera, in whom the first isolate was recovered. The family of TEM β-lactamases disseminated worldwide through various Enterobacteriaceae, as well as Pseudomonas aeruginosa, Haemophilus influenzae, and N. gonorrhoeae.46,47 Similarly, both chromosomally encoded, as well as plasmid-mediated SHV-type β-lactamases, with a molecular structure related to TEM enzymes, became widely prevalent among E. coli and K. pneumoniae isolates. The pharmaceutical industry’s development of third-generation cephalosporins, initially stable to the action of TEM- and SHV-type β-lactamases, was soon followed by the emergence and global spread of ESBL, capable of hydrolyzing monobactam and broad-spectrum cephalosporins.3,45,46,47,48 In addition, increasing reports of carbapenemase emergence and spread have raised concern over the currently limited antimicrobial arsenal against infections with multidrug-resistant gram-negative bacteria.49,50 TEM-1 is the most common β-lactamase in gram-negative bacteria, and it can hydrolyze penicillins and narrow-spectrum cephalosporins in Enterobacteriaceae, N. gonorrhoeae, and H. influenzae.45 The extended-spectrum of activity for TEM-derived ESBLs is obtained through changes in a single or a few amino acids that change the configuration of the enzyme at its active site, making it more accessible to the bulky R1 oxymino side chains of third-generation cephalosporins (cefotaxime, cefpodoxime, ceftazidime, ceftriaxone), and monobactam (aztreonam).51 The first TEM-derived ESBL, TEM-3, was reported in 1988.52 There are now more than 200 TEM-derived ESBLs (see Lahey Clinic [Burlington, MA] website, www.lahey.org/Studies/temtable.asp). These enzymes are found primarily in E. coli and K. pneumoniae isolates but also in other Enterobacteriaceae, such as Enterobacter aerogenes, Morganella morganii, Proteus spp., and Salmonella spp.45 The majority of TEM-derived ESBLs remain susceptible to inhibition by clavulanic acid, although inhibitor-resistant TEM variants have also been described.53 The SHV-1 β-lactamase has a biochemical structure similar to that of TEM-1 (68% of amino acids are shared54), and its ESBL derivatives are also produced by point mutations (one or more amino-acid substitutions) at its active site. SHV-type β-lactamases are found primarily in K. pneumoniae strains. A three-dimensional image of TEM-type and SHV-type ESBLs is provided in Figure 18-5. Cefotaxime-M (CTX-M) β-lactamases are not evolutionarily related to SHV and TEM families because they are thought to have been acquired by plasmids from the chromosomal ampicillin C (AmpC) enzymes of Kluyvera spp., environmental gram-negative rods of low pathogenic potential.55,56 In general, the CTX-M family hydrolyzes cefotaxime and ceftriaxone better than ceftazidime, and they are inhibited more by tazobactam than by clavulanic acid,45,54 although point mutations leading to increased activity against ceftazidime can occur. CTX-M enzymes have disseminated rapidly and are now among the most prevalent ESBLs worldwide.57 Recent reports of community-acquired bloodstream infections with multiresistant, CTX-M Escherichia coli isolates from Spain and Israel have raised significant public health concerns.58,59 The ST131 (O25:H4) clone associated with the CTX-M-15 enzymes has emerged as an important multidrug-resistant pathogen and may have been responsible for the majority of infections with multidrug-resistant E. coli infections in Europe and the United States since 2007.60 Oxacillin (OXA)-type β-lactamases are also plasmid derived and hydrolyze oxacillin and its derivatives very effectively; they are poorly inhibited by clavulanic acid.42,45 OXA-derived ESBLs have been described mainly in P. aeruginosa, in which they confer high-level resistance to oxymino-β-lactams.45 AmpC β-lactamases are primarily chromosomial enzymes that confer resistance to penicillins, narrow-spectrum cephalosporins, oxymino-β-lactams, and cephamycins and are not susceptible to β-lactamase inhibitors such as clavulanic acid (molecular class C, functional group 1).61 Cefepime and aztreonam are usually poor substrates, although modulation by point mutations at the R2 loop of the active site have been responsible for variants with increased ability to hydrolyze cefepime. AmpC production in gram-negative bacilli is normally repressed. However, a transient increase in production (10- to 100-fold) can occur in the presence of β-lactam antibiotics in the following species that possess inducible AmpC enzymes: Enterobacter, Citrobacter freundii, Serratia, M. morganii, Providencia, and P. aeruginosa.62 AmpC β-lactamase production returns to low levels again after antibiotic exposure is discontinued, unless spontaneous mutations occur in the ampD locus of the gene, leading to permanent hyperproduction (derepression) in these species. Third-generation cephalosporin use in Enterobacter spp. infections can therefore select for the overgrowth of these stably derepressed mutants, leading to the emergence of antibiotic resistance during treatment.61,63 More than 20 plasmid-mediated AmpC enzymes, derived from chromosomally encoded genes in Enterobacteriaceae or Aeromonas spp., have been described in E. coli, K. pneumoniae, Salmonella enterica, and Proteus mirabilis. β-Lactam resistance attributable to this system appears to be increasing and confers a resistance phenotype similar to that of Enterobacter spp.47 Carbapenemases confer the largest antibiotic-resistance spectrum because they can hydrolyze not only carbapenems but also broad-spectrum penicillins, oxymino-cephalosporins, and cephamycins. The K. pneumoniae carbapenemase (KPC) enzymes are currently the most important class A serine carbapenemases. Since initially reported from K. pneumoniae isolates in several northeastern U.S. outbreaks,64–67 KPCs have been found worldwide in multiple other gram-negative species, such as E. coli, Citrobacter, Enterobacter, Salmonella, Serratia, and P. aeruginosa.5 Class B metallo-β-lactamases (MBLs) use a Zn2+ cation for hydrolysis of the β-lactam ring; are susceptible to ion chelators, such as ethylenediaminetetraacetic acid (EDTA); and resistant to clavulanic acid, tazobactam, and sulbtactam. They confer resistance to all β-lactam antibiotics except monobactams. Chromosomally encoded MBLs are primarily found in environmental isolates of Aeromonas, Chryseobacterium, and Stenotrophomonas spp. and are of usually low pathogenic potential.68 Most clinically important MBLs belong to five different families (imipenem [IMP], Verona integron-encoded metallo-β-lactamase [VIM], Sao Paulo metallo-β-lactamase [SPM], German imipenemase [GIM], and Seoul imipenemase [SIM]), typically transmitted by mobile gene elements inserted into integrons and spread through P. aeruginosa, Acinetobacter, other gram-negative nonfermenters, and enteric bacterial pathogens.24,69 The New Delhi metallo-β-lactamase–1 (NDM-1) has received the most attention recently. Originally described in a K. pneumoniae isolate from India in 2008, NDM-1 enzymes have since been reported in the United States, the United Kingdom, and a number of other countries, primarily in connection with travel to India or Pakistan.70,71 These enzymes confer resistance to all β-lactams except aztreonam. However, most metallo-β-lactamases reside on mobile gene cassettes inserted into integrons that harbor additional antibiotic-resistance genes to other antimicrobial classes; this multidrug resistance can be transferred to other species via transposons and plasmids, severely limiting therapeutic options in serious infections.72,73 Finally, class D carbapenemases have been described among four subfamilies of OXA-type β-lactamases (OXA-23, OXA-24, OXA-58, and OXA-146), primarily in A. baumanii. In the latter, the intrinsically weaker carbapenemase activity is augmented by coupling β-lactamase production with an additional resistance mechanism, such as decreased membrane permeability or increased active efflux.74 Among gram-positive bacteria, staphylococci are the major pathogens that produce β-lactamase. Staphylococcal β-lactamases preferentially hydrolyze penicillins. Most are inducible and are excreted extracellularly.3 The genes that determine staphylococcal β-lactamases usually are carried on small plasmids or transposons. Larger plasmids encoding β-lactamase and other resistances also exist and can transfer by conjugation, not only between strains of S. aureus but also between S. aureus and Staphylococcus epidermidis.75 Enterococci produce a plasmid-determined β-lactamase that seems to be of staphylococcal origin.76 Since the appearance of the first strain in Texas in 1981, β-lactamase–producing enterococci have been found throughout the United States and in South America.77 The genes often coexist with genes that determine high-level resistance to gentamicin and may occur on transposons and on plasmids. These transposons are similar to staphylococcal β-lactamase transposons and may be derived from them.78 β-Lactamases also contribute to the resistance of anaerobic bacteria to β-lactam antibiotics.79,80 The β-lactamases of fusobacteria and clostridia are principally penicillinases.81,82 The β-lactamases produced by Bacteroides fragilis are predominantly cephalosporinases, some of which have been found to hydrolyze cefoxitin and imipenem and may be transferable.83,84 Most of the cephalosporinases are inhibited by clavulanate, sulbactam, or tazobactam. Some isolates of Bacteroides spp. produce carbapenemases, metalloenzymes inhibited by EDTA but not clavulanate, that confer resistance to imipenem. The level of antibiotic resistance mediated by a particular β-lactamase in a population of bacteria is determined by at least five variables. The efficiency of the β-lactamase in hydrolyzing an antibiotic depends on (1) its rate of hydrolysis and (2) its affinity for the antibiotic. Other variables are (3) the amount of β-lactamase produced by the bacterial cell, (4) the susceptibility of the target protein (penicillin-binding protein [PBP]) to the antibiotic, and (5) the rate of diffusion of the antibiotic into the periplasm of the cell. Within the bacterial cell, β-lactamases contribute to antibiotic resistance in several ways. The simplest model is that of penicillinase-producing staphylococci, in which the bacteria, on exposure to penicillin, begin to produce β-lactamase, which they excrete extracellularly. Two events then take place concurrently: (1) penicillin lyses bacteria and (2) β-lactamase hydrolyzes penicillin. If viable bacterial cells remain after the level of penicillin has declined to less than the minimal inhibitory concentration (MIC), regrowth of bacteria occurs.39 Another model is exemplified by gram-negative bacteria, which (1) produce a β-lactamase that remains trapped in the periplasmic space and (2) have no barrier to antibiotic penetration. An example is H. influenzae strains that produce the TEM-1 β-lactamase.85 In this model and the first one discussed, a marked inoculum effect occurs in that the MIC for a large inoculum (106 organisms/mL) may be 1000-fold greater than that for a small inoculum (102 organisms/mL). Lysis of the organism by ampicillin releases the trapped β-lactamase into the microenvironment, providing partial protection to adjacent bacteria residing in the same location. If a high inoculum exists before ampicillin exposure occurs, the release of all the periplasmic β-lactamase enzymes in a confined space might be sufficient to protect some of the remaining viable bacteria of the original population of microorganisms. However, the low level of resistance of single cells makes it possible for ampicillin to cure some infections caused by β-lactamase–producing strains of H. influenzae when the initial inoculum of infecting bacteria is low. Another model is exemplified by ampicillin resistance of E. coli strains that produce the TEM-1 β-lactamase. These bacteria have a barrier to entry of β-lactam molecules (the outer membrane), and they produce a β-lactamase that remains localized to the periplasmic space. In this model, the kinetics are more complicated. The enzyme is situated strategically between the barrier to antibiotic penetration (outer membrane) and the antibiotic targets (PBPs on the cytoplasmic membrane). In this position, the enzyme can destroy antibiotic molecules sequentially as they make their way through the barrier, analogous to a sharpshooter with abundant ammunition who aims at targets passing through a single entry point. As a consequence, high levels of resistance occur with single bacterial cells, in contrast to the previous example.39 Variations on this model occur when the amount of β-lactamase produced increases with exposure to a β-lactam (induction), as occurs in Enterobacter and Pseudomonas spp. High levels of β-lactamase are produced only after a period of exposure to the inducing antibiotic, and resistance may be expressed late. When Enterobacter strains are exposed to two β-lactam antibiotics, one of which is a potent inducer (e.g., cefamandole), antagonism between the two antibiotics may result.82 Table 18-4 lists mechanisms of resistance to β-lactam antibiotics. Often these mechanisms work in concert and may accumulate in a single patient. An example is a 19-month-old child with aplastic anemia who over 3 months had nine blood isolates of E. coli, all derived from a common ancestor, despite multiple courses of antibiotics, including ceftazidime.86 The first isolate produced a TEM-1 β-lactamase but was susceptible to ceftazidime (MIC, 0.25 µg/mL). A subsequent isolate became resistant (MIC of ceftazidime, 32 µg/mL) by acquiring a new plasmid-determined β-lactamase (SHV-1) linked to an efficient promoter and turning off production of an outer membrane porin. An even higher level of resistance (MIC of ceftazidime ≥128 µg/mL) occurred when the SHV-1 β-lactamase mutated to form the ESBL, SHV-8, which hydrolyzes ceftazidime much more rapidly. By turning off porin production to slow the rate of entry of ceftazidime into the periplasmic space and producing an extended-spectrum, ceftazidime-inactivating β-lactamase, the infecting E. coli used two mechanisms synergistically to achieve a high level of resistance to ceftazidime.86 TABLE 18-4 Mechanisms of Resistance to β-Lactam Antibiotics PBP, penicillin-binding protein. Among aerobic bacteria, aminoglycoside resistance is most commonly due to enzymatic inactivation through aminoglycoside-modifying enzymes. These may be coded by genes on plasmids or chromosomes. Several aminoglycoside-modifying enzymes have been shown to be carried on transposons.87 Aminoglycoside-modifying enzymes confer antibiotic resistance through three general reactions: N-acetylation, O-nucleotidylation, and O-phosphorylation. For each of these general reactions, there are several different enzymes that attack a specific amino or hydroxyl group. The nomenclature for these enzymes lists the molecular site where the modification occurs after the type of enzymatic activity. An aminoglycoside acetyltransferase (AAC) that acts at the 3′ site is designated AAC(3′) (Table 18-5). There may be more than one enzyme that catalyzes the same reaction, however, and Roman numerals may be necessary (e.g., AAC[3′]-IV). TABLE 18-5 Aminoglycoside-Modifying Enzymes A, amikacin; AAC, aminoglycoside acetyltransferase; ANT, aminoglycoside nucleotidyltransferase; APH, aminoglycoside phosphotransferase; cr, ciprofloxacin resistance; Ar, arbekacin, E, Enterobacteriaceae; Ent, enterococci, *FQ, fluoroquinolone (acetylates the piperazine ring in some fluroquinolones), G, gentamicin; K, kanamycin; PR, Providencia-Proteus; PS, pseudomonads; SA, staphylococci; SR, streptococci; T, tobramycin. Enzymatic aminoglycoside resistance is achieved by modification of the antibiotic in the process of transport across the cytoplasmic membrane.88 Resistance to a particular aminoglycoside is a function of two different rates—that of drug uptake versus that of drug inactivation. An important factor in determining the level of resistance is the affinity of the modifying enzyme for the antibiotic. If an enzyme has a high affinity for the specific aminoglycoside, drug inactivation can occur at very low concentrations of the enzyme. The differences in the worldwide distribution of aminoglycoside-modifying enzymes may be partially a function of antibiotic selection pressures and may have had profound implications on the choice of antibiotics used at specific medical centers. Aminoglycoside phosphotransferase (APH)(3′) and APH(3″) are distributed widely among gram-positive and gram-negative species worldwide and have led to decreased use of kanamycin and streptomycin. The gene for aminoglycoside nucleotidyltransferase (ANT)(2″) has been associated with multiple nosocomial outbreaks in the 1990s across the United States. The gene for aminoglycoside acetyltransferase AAC(6′)-I has been found to be more prevalent in enteric bacteria and in staphylococci in East Asia.89 The AAC(3′) group of enzymes have been responsible for outbreaks of antibiotic resistance in South America, western Europe, and the United States. Although each outbreak of aminoglycoside-resistant Enterobacteriaceae has its own pattern, the most typical manner of spread has been the appearance of a plasmid-carrying, aminoglycoside-resistant strain of K. pneumoniae, usually carrying the ANT(2″) gene, with subsequent dissemination to other strains of the species and further spread later to other species and genera of Enterobacteriaceae.88 Major increases in plasmid-mediated aminoglycoside resistance have been noted among enterococci,90 initially in the developing world91 but increasingly in the United States and Europe.92,93 Their clinical impact is exacerbated by the frequent co-transmission of β-lactamases, resulting in a loss of synergy when combination therapy is used for serious enterococcal infections. S. aureus and S. epidermidis have become increasingly resistant to aminoglycosides because of the interspecies and intraspecies dissemination of plasmid-mediated, aminoglycoside-modifying enzymes.94 The two most interesting developments in aminoglycoside-modifying ennzymes have been the discovery of bifunctional enzymes. The first example is the AAC(6′)APH(2″) enzyme that has two functioning active sites, one for acetylation and the other for phosphorylation of aminoglycosides. This bifunctional enzyme probably arose from a fusion event of the genes for these two enzymes. This enzyme is now widespread in staphylococci and enterococci, frequently residing on a common transposon Tn4001 found on the chromosome and on transferable plasmids. The gene aac(6′)aph(2″) accounts for most of the high level gentamicin and arbekacin resistance observed in methicillin-resistant S. aureus (MRSA) and enterococcal isolates in many countries worldwide.95,96 Recently, another major discovery with aminoglycoside-modifying enzymes finds evidence of variant enzymes that can modify the structure of an entirely different class of antimicrobial agent. The first bifunctional enzyme that can modify aminoglycosides and a fluoroquinolone (ciprofloxacin) was described in 2006.97 This enzyme, designated as AAC(6′)–Ib-cr, not only acetylates kanamycin, gentamicin, and tobramycin, but also acetylates the piperazinyl side group of ciprofloxacin. This acetylated quinolone is fourfold less active than the parent compound and may lead to clinically significant resistance in enteric bacteria, particularly in strains that possess other mechanisms for diminished quinolone activity. This enzyme has two important mutations in the gene for the basic AAC(6′) enzyme (W102R and D179Y) and 12 unique base pairs at its 5′ end that are essential to alter substrate specificity and allow ciprofloxacin acetylation function for this enzyme.98 The widespread use of ciprofloxacin and other fluoroquinolones over the past 2 decades has changed the selection pressures on bacterial populations promoting quinolone-resistance development.98
Molecular Mechanisms of Antibiotic Resistance in Bacteria
Molecular Genetics of Antibiotic Resistance
Plasmids
Transposable Genetic Elements
DNA Integration Elements
Mechanisms of Antibiotic Resistance
Enzymatic Inhibition
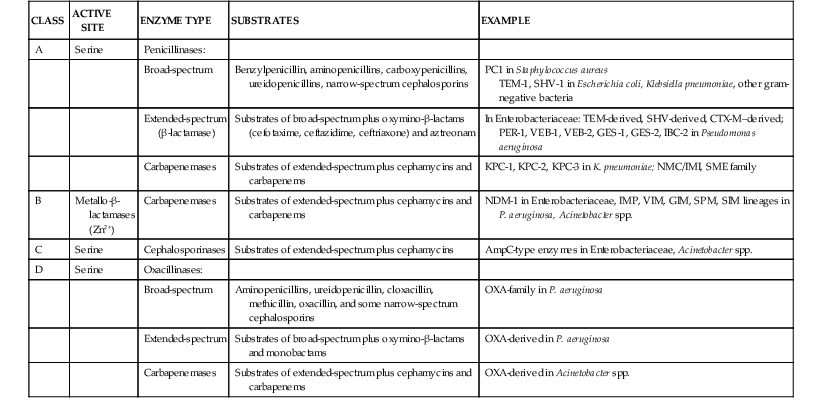
β-Lactamases
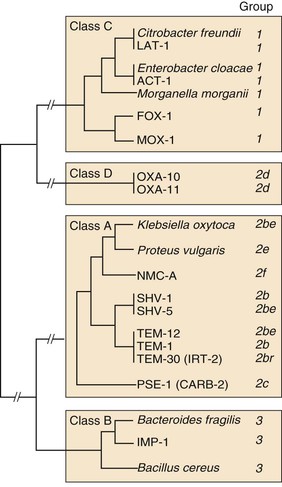
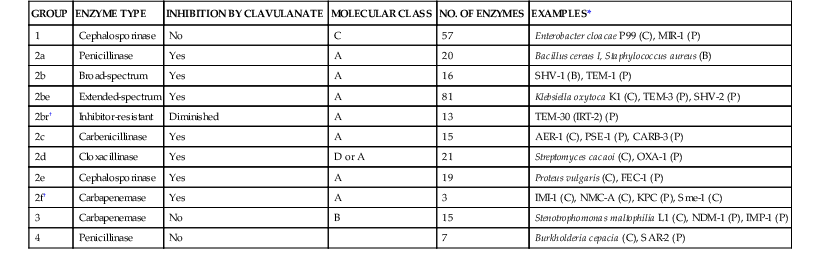
GROUP
ENZYME TYPE
INHIBITION BY CLAVULANATE
MOLECULAR CLASS
NO. OF ENZYMES
EXAMPLES*
1
Cephalosporinase
No
C
57
Enterobacter cloacae P99 (C), MIR-1 (P)
2a
Penicillinase
Yes
A
20
Bacillus cereus I, Staphylococcus aureus (B)
2b
Broad-spectrum
Yes
A
16
SHV-1 (B), TEM-1 (P)
2be
Extended-spectrum
Yes
A
81
Klebsiella oxytoca K1 (C), TEM-3 (P), SHV-2 (P)
2br†
Inhibitor-resistant
Diminished
A
13
TEM-30 (IRT-2) (P)
2c
Carbenicillinase
Yes
A
15
AER-1 (C), PSE-1 (P), CARB-3 (P)
2d
Cloxacillinase
Yes
D or A
21
Streptomyces cacaoi (C), OXA-1 (P)
2e
Cephalosporinase
Yes
A
19
Proteus vulgaris (C), FEC-1 (P)
2f†
Carbapenemase
Yes
A
3
IMI-1 (C), NMC-A (C), KPC (P), Sme-1 (C)
3
Carbapenemase
No
B
15
Stenotrophomonas maltophilia L1 (C), NDM-1 (P), IMP-1 (P)
4
Penicillinase
No
7
Burkholderia cepacia (C), SAR-2 (P)
Extended-Spectrum β-Lactamases
TEM-Derived.
SHV-Derived.
CTX-M–Derived.
OXA-Derived.
AmpC Enzymes.
Carbapenemases.
Gram-Positive Bacteria
Anaerobic Bacteria
Contribution of β-Lactamases to β-Lactam Antibiotic Resistance

Aminoglycoside Resistance–Modifying Enzymes
ENZYMES
USUAL ANTIBIOTICS MODIFIED
COMMON GENERA
Phosphorylation
APH(2″)
K, T, G
SA, SR
APH(3′)-I
K
E, PS, SA, SR
APH(3′)-III
K , ±A
E, PS, SA, SR
Acetylation
AAC(2′)
G
PR
AAC(3′)-I
±T, G
E, PS
AAC(3′)-III, -IV, or -V
K, T, G
E, PS
AAC(6′)
K, T, (A)
E, PS, SA
Adenylation
ANT(2″)
K, T, G
E, PS
ANT(4′)
K, T, A
SA
Bifunctional Enzymes
AAC(6′)APH(2″)
G, Ar
SA, Ent
AAC(6′)-Ib cr
G, K, T, FQ*
E
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Molecular Mechanisms of Antibiotic Resistance in Bacteria
18