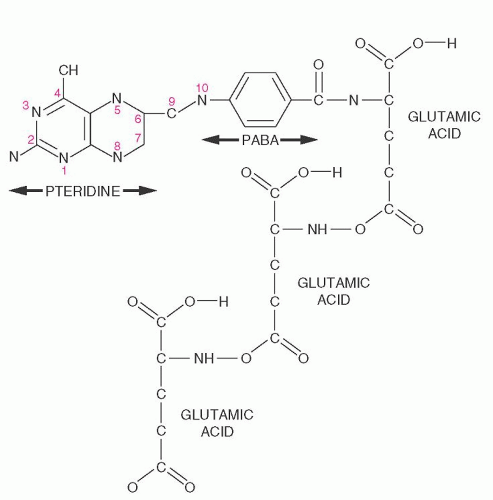
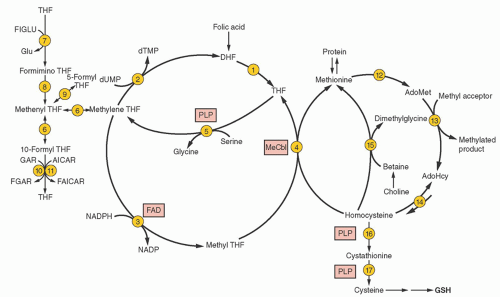
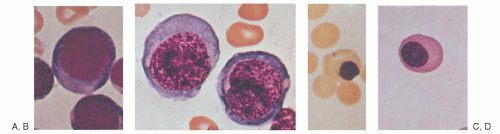
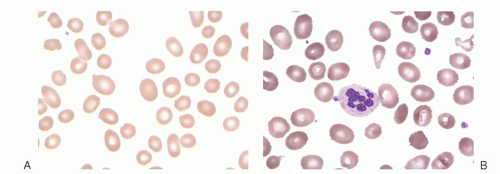
purines (reactions 10 and 11), and pyrimidines (reaction 2). By indirectly contributing to S-adenosylmethionine (AdoMet) generation, folate is linked to important methylations, including methylation of DNA cytosine (Fig. 36.2, reaction 13). Cellular folate metabolism and its compartments are reviewed elsewhere.18, 19
by inhibiting both methyleneTHF reductase (reaction 3) and betaine hydroxymethyltransferase (an alternate remethylator of homocysteine [reaction 15] confined to liver and perhaps kidney and lens), and by activating cystathionine β-synthase (reaction 16).29
pH and operating at higher concentrations of folate, reduced folate carrier assumes major responsibility for folate uptake from the blood by the liver, kidney, leukocytes, placenta, and parts of the brain.
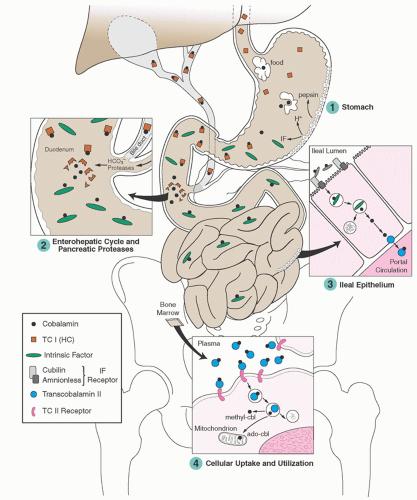
Holoprotein proportions can vary widely in some disorders, and the reason is not always known.76, 78 Desialylated TC I is cleared nonspecifically by hepatic receptors for asialoglycoproteins,79 whereby some of its cobalamin is cleared in bile.
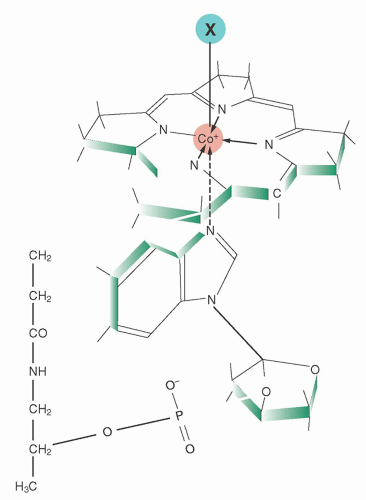
the vast human microbiome in the gut and elsewhere, as well as withholding unusable analogs from human cells.80 The subject requires careful study. Several minor cobalamin-binding proteins and complexes have also been described in plasma, but their roles are unknown.56, 79, 81, 82, 83
TABLE 36.1 COBALAMIN-BINDING PROTEINS | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||
associated with broad malnutrition and with alcohol abuse. In contrast, the evolution of cobalamin deficiency is usually measured in years, and it tends to be a purer deficiency state because malabsorption is often restricted to cobalamin alone.
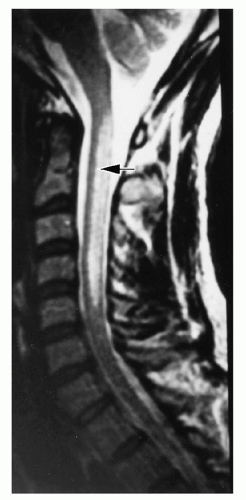
demyelination.118, 119 The classic myelopathic syndrome is subacute combined degeneration, in which posterior and lateral column damage predominates; dorsal, pyramidal, and spinocerebellar tracts are affected. The earliest changes appear in the cervical or thoracic spine and can be detected by magnetic resonance imaging (MRI) as hyperintensity on T2-weighted images (Fig. 36.7). Larger, more heavily myelinated fibers tend to be affected most often.120
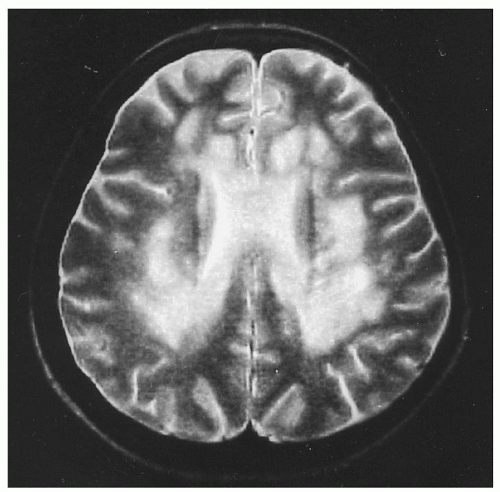
only in retrospect after treatment. MRI reveals focal and diffuse changes in the brain138 (Fig. 36.8). Tensor diffusion imaging also shows white matter changes in adjacent areas that seem normal on MRI.139 Nevertheless, despite low cobalamin levels in 10% to 20% of patients with chronic dementias,95, 140 the low levels usually reflect only subclinical cobalamin deficiency (SCCD) and appear unrelated to chronic dementias, which rarely improve with cobalamin therapy.141 In very young children, however, some cobalamin deficiencies can cause developmental delay, lethargy, cerebral atrophy, and seizures.142
showed in 1985 that most of them reflected mild biochemical insufficiency that responded to cobalamin and, equally important, rarely involved IF-related malabsorption.94, 95, 96, 180 None of the subjects had cobalamin-related anemia although bone marrow cells displayed reversible metabolic defects. Similarly, none had clinical neurologic findings although some,96, 141, 181 but not all,182 displayed mild, reversible electrophysiologic changes.
TABLE 36.2 COMPARISON BETWEEN CLINICAL AND SUBCLINICAL COBALAMIN DEFICIENCY STATES | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
and 25 mg pyridoxine) slowed brain atrophy by 30%204 and reduced cognitive decline.205 Its important features were that the subjects were mildly cognitively impaired at baseline (which predicts likely progression, but avoids the irreversibility of dementia), the responders were hyperhomocysteinemic, and it is not clear which of the three vitamins was the beneficial one.
patients with myelofibrosis or chronic myelogenous leukemia. It is unclear whether iron deficiency can cause hypersegmentation.230 Neutrophil segmentation is normally greater in blacks than in whites.228
TABLE 36.3 CAUSES OF MACROCYTOSIS, DEFINED AS MEAN CORPUSCULAR VOLUME (MCV) GREATER THAN 97 FLa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
or holo-TC II).190 Metabolic tests are essential in the diagnosis of inborn errors of metabolism, in which vitamin levels are often normal. Metabolites are also ideal for monitoring response of deficiency to therapy because their levels do not change unless the therapy was effective, whereas vitamin levels (serum cobalamin, holo-TC II, or folate) rise upon vitamin entry into the bloodstream regardless of efficacy. Metabolite improvement is also delayed for several days, allowing a post-therapy window of time for diagnostic reassessment if needed.
TABLE 36.4 BIOCHEMICAL TESTS FOR THE DIAGNOSIS AND DIFFERENTIATION OF CLINICALLY RELEVANT COBALAMIN AND FOLATE DEFICIENCIES | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree