Measles
William J. Moss
Martin O. Ota
INTRODUCTION
Measles is one of the most important infectious diseases of humans and has caused millions of deaths since its emergence as a zoonotic disease thousands of years ago. For infectious disease epidemiologists, measles has served as a model of an acute infectious disease, particularly for understanding the nature of epidemics. Kenneth Maxcy, the second chair of the Department of Epidemiology at the Johns Hopkins University School of Public Health, wrote in 1948, “The simplest of all infectious diseases is measles.”1 Despite the apparent simplicity, much has been learned about measles in the half-century since Maxcy’s chapter was written. Detailed investigations of the virology, immunology, and transmission dynamics have shown measles to be a much more complex disease than Maxcy’s statement indicates, and many questions remain unanswered. But this ignorance has not impeded enormous progress in global measles control.
DISEASE BURDEN
The disease burden caused by measles has decreased because of a number of factors. Measles mortality declined in developed countries in association with economic development, improved nutritional status, and supportive care, particularly antibiotic therapy to control secondary bacterial pneumonia. Remarkable progress in reducing measles incidence and mortality has been, and continues to be, made in resourcepoor countries as a consequence of increasing measles vaccine coverage, provision of a second opportunity for measles vaccination through supplementary immunization activities (SIA), and efforts by the World Health Organization (WHO), the United Nations Children’s Fund (UNICEF), and their partners to target endemic countries for accelerated and sustained measles mortality reduction. Specifically, this targeted strategy aims to achieve greater than 90% measles vaccination coverage in every district and to ensure that all children receive a second dose of measles vaccine. Provision of vitamin A through polio and measles SIA has contributed further to the reduction in measles mortality.
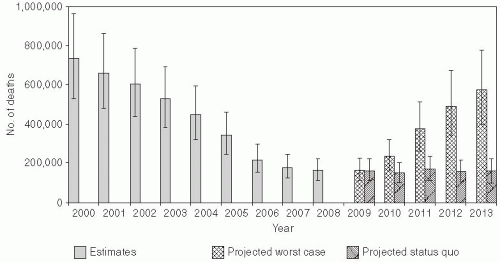
In 2003, the World Health Assembly endorsed a resolution urging member countries to reduce the number of deaths attributed to measles by 50% compared with 1999 estimates by the end of 2005. This global public health target was met, with estimated measles mortality reduced by 60% from an estimated 873,000 deaths in 1999 (uncertainty bounds: 634,000-1,140,000) to 345,000 deaths in 2005 (uncertainty bounds: 247,000-458,000) (Figure 16-1).2 Further reductions in global measles mortality were achieved by 2008, during which there were an estimated 164,000 deaths due to measles (uncertainty bounds: 115,000-222,000) (Figure 16-1).3 These achievements attest to the enormous publichealth significance of measles vaccination. Prior to this effort, an estimated 30 million cases of measles occurred each year, with more than 1 million deaths. The revised global goal, as stated in the Global Immunization Vision and Strategy 2006-2015 by WHO and UNICEF,4 is to reduce global measles deaths by 90% by 2015 compared to the estimated 733,000 deaths in 2000 (uncertainty bounds: 530,000-959,000).
The WHO Region of the Americas has eliminated measles, and four of the five remaining WHO regions have set measles elimination targets of or before 2020. In the Americas, intensive vaccination and surveillance efforts interrupted endemic measles virus transmission.5 Progress in reducing measles incidence and mortality in sub-Saharan Africa6 led to the proposal to eliminate measles in the WHO
African region by 2020.7 Ironically, measles remains a public health problem in Europe.8
African region by 2020.7 Ironically, measles remains a public health problem in Europe.8
Even if achieved, numerous outbreaks highlight the challenges of sustaining measles elimination. Despite very high levels of measles vaccine coverage and population immunity, clustering of susceptible persons can lead to measles outbreaks.9 Of greater concern are recent large measles outbreaks in countries of southern and eastern Africa,10 reflecting the ease with which measles virus can reenter communities and cause large outbreaks if high levels of population immunity are not sustained. WHO projected that the number of measles deaths could reach 1.7 million between 2009 and 2013 if high-risk countries are unable to maintain current recommended strategies for measles control.3
Estimating Measles Mortality
Accurate assessment of the number of deaths attributable to measles is important in assessing progress in measles control. In the pre-vaccine era, almost all persons acquired measles, and the number of measles cases could be approximated by the number of births. Deaths due to measles could be estimated by multiplying the number of cases by age-specific case fatality proportions. This relatively simple method of counting measles cases and deaths (assuming the number of births and the case-fatality proportion could be accurately estimated) was no longer of use after the introduction of measles vaccination. More sophisticated methods of estimating measles mortality were needed.
The first obstacle in estimating the global number of measles deaths is defining those deaths attributable to measles. The difficulty arises from the fact that much of measles mortality is delayed until after resolution of the rash and is due to secondary infections that arise as a consequence of the prolonged state of immune suppression. A common method is to attribute a death to measles if it occurs within one month of the onset of rash.
The ideal method for estimating measles mortality is through accurate disease surveillance and registration of deaths. In the absence of accurate disease surveillance, measles mortality models were developed to estimate the global burden of measles and to monitor the progress of control programs. WHO used two different methods to estimate the global burden of measles in 2000.11 In countries with an estimated measles vaccine coverage of greater than 80% and intensive case-based surveillance, measles incidence was estimated by adjusting the reported number of cases by a factor to correct for underreporting. This correction factor reflects the notification efficiency and was estimated to be between 5% and 40%. For countries with moderate to poor measles control, the number of children immune to measles in each country was estimated by multiplying the number of births, the estimated measles vaccine coverage, and the estimated vaccine effectiveness (the proportion of vaccinated children who develop protective immunity). All remaining susceptible children were assumed to develop measles, thereby providing an estimate of measles incidence. For both
methods, the number of measles deaths was determined by multiplying the number of measles cases by a country- and age-specific case-fatality proportion. An advantage of this approach is that adjustments can be made for changes in vaccine coverage. A major weakness is the absence of reliable data to estimate several parameters, particularly the casefatality proportions.12
methods, the number of measles deaths was determined by multiplying the number of measles cases by a country- and age-specific case-fatality proportion. An advantage of this approach is that adjustments can be made for changes in vaccine coverage. A major weakness is the absence of reliable data to estimate several parameters, particularly the casefatality proportions.12
Using a different approach, the proportion of all mortality caused by measles was derived from community-based studies in those countries with the highest mortality rates for children younger than age 5 years. These studies measured the specific cause of death in children younger than 5 years of age.13 A meta-regression model was used to relate characteristics of the study population to the proportional mortality outcomes. From this model, the proportional distribution of child deaths by major cause, including measles, was estimated for the selected countries. The number of measles deaths was then calculated by multiplying the total number of deaths in children younger than 5 years of age (approximately 10 million) by the measlesspecific proportional mortality. Using this method, measles was estimated to cause 1% of deaths in children younger than 5 years of age (uncertainty bounds: 1%-9%), compared to 5% of deaths as estimated by WHO.14 These methods highlight the difficulties in accurately estimating measles mortality and in tracking the progress of control programs.15
BIOLOGIC CHARACTERISTICS OF THE MEASLES VIRUS
Measles virus is the causative agent of measles and was first isolated from the blood of David Edmonston in 1954 by John Enders and Thomas Peebles.16 The development of vaccines against measles soon followed. Measles virus is a spherical, nonsegmented, single-stranded, negative-sense RNA virus and a member of the Morbillivirus genus in the family of Paramyxoviridae. Other members of the Morbillivirus genus, although not pathogenic to humans, are rinderpest virus and canine distemper virus. Rinderpest virus was eradicated in 2011—only the second pathogen eradicated by human intervention—and was the most closely related morbillivirus to measles. Rinderpest was a historically important pathogen of cattle and swine. It was responsible for devastating epidemics resulting in significant famines as animals necessary for feed, as a direct source of meat or milk, and for labor, such as plowing fields, were killed. Measles was originally a zoonotic infection, arising from cross-species transmission from animals to humans by an ancestor morbillivirus.
Although RNA viruses have high mutation rates, measles virus is considered to be an antigenically monotypic virus, meaning that the surface proteins responsible for inducing protective immunity have retained their antigenic structure across time and space. The public health significance is that measles vaccines developed decades ago from a single measles virus strain remain protective worldwide. The virus is killed by ultraviolet light and heat, and attenuated measles vaccine viruses retain these characteristics, necessitating a cold chain for transporting and storing measles vaccines.
Measles Virus Genes and Proteins
The measles virus RNA genome consists of approximately 16,000 nucleotides and is enclosed in a lipid-containing envelope derived from the host cell. The genome encodes eight proteins, two of which (V and C) are nonstructural proteins that are expressed from the phosphoprotein (P) gene. Of the six structural proteins, P, large protein (L), and nucleoprotein (N) form the nucleocapsid housing the viral RNA. The hemagglutinin protein (H), fusion protein (F), and matrix protein (M), together with lipids from the host cell membrane, form the viral envelope. This relatively simple combination of proteins and ribonucleic acid has evolved to be one of the most highly infectious, directly transmitted agents known, and it remains the cause of millions of deaths.
In the epidemiology and control of measles, the two surface proteins F and H are most important. The H protein interacts with F to mediate fusion of the viral envelope with the host cell membrane.17 The primary function of the H protein is to bind to the host cellular receptors for measles virus, the best known of which are CD46 and CD150 (SLAM). CD46 is a complement regulatory molecule expressed on all nucleated cells in humans. SLAM— an acronym for signaling lymphocyte activation molecule—is expressed on activated T and B lymphocytes and antigen-presenting cells. The distribution of these host proteins determines the cell types that can be infected by measles virus; in other words, they define the virus’s tissue tropism. Wildtype measles virus enters cells primarily through the cellular receptor SLAM, and most vaccine strains bind to CD46, although wild-type measles virus may use both CD46 and SLAM as receptors during acute infection.18 CD147/EMMPRIN
(extracellular matrix metalloproteinase inducer) was recently identified as a measles virus receptor on epithelial cells.19
(extracellular matrix metalloproteinase inducer) was recently identified as a measles virus receptor on epithelial cells.19
PATHOGENESIS
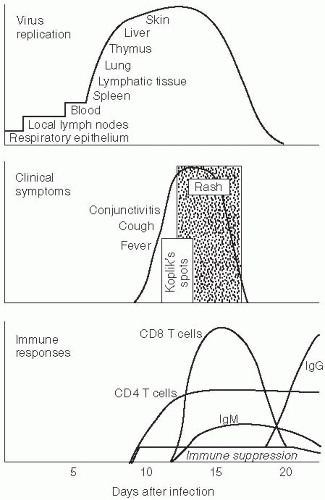
Respiratory droplets from infected persons transmit infection by carrying measles virus to epithelial cells of the respiratory tract of susceptible hosts. During the 10- to 14-day incubation period between infection and the onset of clinical signs and symptoms, the virus replicates and spreads within the infected host (Figure 16-2). Initial viral replication occurs in epithelial cells at the portal of entry in the upper respiratory tract, and the virus then spreads to local lymphatic tissue. Replication in local lymph nodes is followed by viremia (the presence of virus in the blood) and the dissemination of measles virus to many organs, including lymph nodes, skin, kidney, gastrointestinal tract, and liver, where the virus replicates in epithelial and endothelial cells as well as monocytes and macrophages.
Although measles virus infection is clinically inapparent during the incubation period, the virus is actively replicating and the host immune responses are developing. Evidence of these processes can be detected. During the incubation period, the number of circulating lymphocytes is reduced (lymphopenia). Measles virus can also be isolated from the nasopharynx and blood during the later part of the incubation period and in the several-day prodromal period prior to the onset of rash when levels of viremia are highest. The prodrome ends with the appearance of the measles rash. This rash results from measles virus-specific cellular immune responses and marks the beginning of viral clearance from blood and tissue. Clearance of infectious virus from the blood and other tissues occurs within the first week after the appearance of the rash, although measles virus RNA can be detected in body fluids of some children for at least one month using a polymerase chain reaction (PCR)-based assay.20
Immune Responses to Measles Virus
Host immune responses to measles virus are essential for viral clearance, clinical recovery, and the establishment of long-term immunity (Figure 16-2). Early nonspecific (innate) immune responses occur during the prodromal phase of the illness. These innate immune responses contribute to the control of measles virus replication before the onset of more specific (adaptive) immune responses.21 The adaptive immune responses consist of measles virusspecific humoral (antibody) and cellular responses. The protective efficacy of antibodies to measles virus is illustrated by the immunity conferred to infants from passively acquired maternal antibodies and the protection of exposed, susceptible individuals following administration of anti-measles virus immune globulin.22
The first measles virus-specific antibodies produced after infection are immunoglobulin M (IgM) subtype. The IgM antibody response is typically absent following reexposure or revaccination and serves as a marker of primary infection. Immunoglobulin A (IgA) antibodies to measles virus are found in mucosal secretions. The most abundant and most rapidly produced antibodies are against the nucleoprotein (N), and the absence of antibodies to N is the most accurate indicator of seronegativity to measles virus. Although not as
abundant, antibodies to H and F proteins contribute to virus neutralization and are sufficient to provide protection against measles virus infection. The H protein elicits strong immune responses, and the lifelong immunity that follows infection is attributed to neutralizing antibodies against H.
abundant, antibodies to H and F proteins contribute to virus neutralization and are sufficient to provide protection against measles virus infection. The H protein elicits strong immune responses, and the lifelong immunity that follows infection is attributed to neutralizing antibodies against H.
Evidence for the importance of cellular immunity to measles virus is demonstrated by the ability of children with agammaglobulinemia (congenital inability to produce antibodies) to fully recover from measles, whereas children with severe defects in T-lymphocyte function often develop severe or fatal disease.23 CD4+ T lymphocytes are activated in response to measles virus infection and secrete cytokines capable of modulating the humoral and cellular immune responses.24 The initial predominant Th1 response (characterized by interferon-gamma [IFN-γ]) is essential for viral clearance, and the later Th2 response (characterized by interleukin-4 [IL-4]) promotes the development of measles virus-specific antibodies.
The duration of protective immunity following wild-type measles virus infection is generally thought to be lifelong.25 The immunologic mechanisms involved in sustaining high levels of neutralizing antibody to measles virus are not completely understood, although general principles of immunologic memory probably govern this process. Immunologic memory to measles virus includes both continued production of measles virus-specific antibodies and the circulation of measles virusspecific CD4+ and CD8+ T lymphocytes.26 Although immune protection is determined by measurement of anti-measles virus antibodies, long-lasting cellular immune response almost certainly plays an important role in protection from infection. Observations of the measles epidemic on the isolated Faroe Islands in 1846 demonstrated the long-term protective immunity conferred by measles. In this population, measles was reintroduced to the island decades after the last occurrence. Adults exposed earlier were protected despite having no exposure to measles between epidemics. Thus reexposure to measles virus was not necessary to maintain this long-term protection.27
Measles vaccine also induces both humoral and cellular immune responses. Antibodies first appear between 12 and 15 days after vaccination and peak at 21 to 28 days. IgM antibodies appear transiently in blood, IgA antibodies are predominant in mucosal secretions, and immunoglobulin G (IgG) antibodies persist in blood for years. Vaccination also induces measles virus-specific T lymphocytes. Although both humoral and cellular responses can be induced by measles vaccine, as is frequently the case with vaccine-induced responses they are of lower magnitude and shorter duration compared to those following wild-type measles virus infection.
Measles Virus-Associated Immune Suppression
The intense immune responses induced by measles virus infection are paradoxically associated with depressed responses to unrelated (non-measles virus) antigens, lasting for several weeks to months beyond resolution of the acute illness.28 This state of immune suppression enhances susceptibility to secondary bacterial and viral infections causing pneumonia and diarrhea, and is responsible for much of the measles-related morbidity and mortality. Delayed-type hypersensitivity (DTH) responses to recall antigens, such as tuberculin, are suppressed29 and cellular and humoral responses to new antigens are impaired following measles.30 Reactivation of tuberculosis and remission of autoimmune diseases have been described and are attributed to this state of immune suppression.
Abnormalities of both the innate and adaptive immune responses have been described following measles virus infection. Transient lymphopenia with a reduction in CD4+ and CD8+ T lymphocytes occurs in children. Functional abnormalities of immune cells have also been detected, including decreased lymphocyte proliferative responses.31 Dendritic cells—a key antigen-presenting cell—mature poorly, lose the ability to stimulate proliferative responses in lymphocytes, and undergo cell death when infected with measles virus in vitro.32 The dominant Th2 response in children recovering from measles can inhibit Th1 responses and increase susceptibility to intracellular pathogens.28 Engagement of CD46 and CD3 on monocytes has been shown to induce production of high levels of IL-10 and transforming growth factor (TGF)-β, an immunomodulatory and immunosuppressive cytokine profile characteristic of regulatory T cells.33 The role of these cytokines in the immune suppression following measles is supported by in vivo evidence of elevated levels of IL-10 in the plasma of children after measles virus infection.34
Clinical Disease and Complications
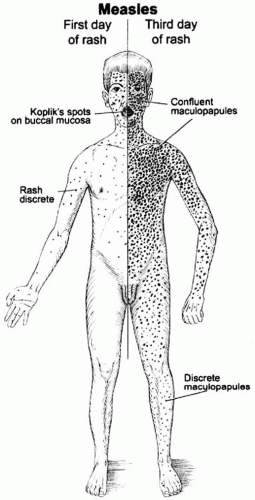
Clinically apparent measles begins with a prodrome characterized by fever, cough, coryza (runny nose), and conjunctivitis (Figure 16-2). Koplik’s spots—small white lesions on the buccal mucosa inside the mouth— may be visible during the prodrome and allow the
astute clinician to diagnose measles prior to the onset of rash. The prodromal symptoms intensify several days before the onset of rash. The characteristic erythematous and maculopapular rash appears first on the face and behind the ears, and then spreads in a centrifugal fashion to the trunk and extremities (Figure 16-3). The rash lasts for 3 to 4 days and fades in the same manner as it appeared. Malnourished children may develop a deeply pigmented rash that desquamates or peels during recovery.
astute clinician to diagnose measles prior to the onset of rash. The prodromal symptoms intensify several days before the onset of rash. The characteristic erythematous and maculopapular rash appears first on the face and behind the ears, and then spreads in a centrifugal fashion to the trunk and extremities (Figure 16-3). The rash lasts for 3 to 4 days and fades in the same manner as it appeared. Malnourished children may develop a deeply pigmented rash that desquamates or peels during recovery.
In uncomplicated measles, clinical recovery begins soon after appearance of the rash. Unfortunately, complications occur in as many as 40% of measles cases, and the risk of complication is increased by extremes of age and malnutrition.35 Complications of measles have been described in almost every organ system. The respiratory tract is a frequent site of complication, with pneumonia accounting for most measles-associated deaths.36 Pneumonia is caused by secondary viral or bacterial infections or by measles virus itself. Pathologically, measles virus infection of the lung is characterized by multinucleated giant cells, which form when measles virus proteins on the cell surface allow cells to fuse. Other respiratory complications include laryngotracheobronchitis (croup) and otitis media (ear infection). Mouth ulcers, or stomatitis, may hinder children from eating or drinking. Many children with measles develop diarrhea, which further contributes to malnutrition. Eye disease (keratoconjunctivitis) is common after measles, particularly in children with vitamin A deficiency, and was a frequent cause of blindness in the past.
Because the rash of measles is a consequence of the cellular immune response, persons with impaired cellular immunity, such as those with acquired immunodeficiency syndrome (AIDS), may not develop the characteristic measles rash. These persons have a high case-fatality rate and frequently develop a giant-cell pneumonitis caused by measles virus. T-lymphocyte defects due to causes other than human immunodeficiency virus (HIV) infection, such as cancer chemotherapy, also are associated with increased severity of measles.
Rare but serious complications of measles involve the central nervous system. Post-measles encephalomyelitis complicates approximately 1 in 1000 cases, mainly in older children and adults. Encephalomyelitis occurs within two weeks of the onset of rash and is characterized by fever, seizures, and a variety of neurologic abnormalities. The finding of periventricular demyelination, the induction of immune responses to myelin basic protein, and the absence of measles virus in the brain suggest that post-measles encephalomyelitis is an autoimmune disorder triggered by measles virus infection.
Other central nervous system complications that occur months to years after acute infection are measles inclusion body encephalitis (MIBE) and subacute sclerosing panencephalitis (SSPE). In contrast to postmeasles encephalomyelitis, MIBE and SSPE are caused by persistent measles virus infection. MIBE is a rare but fatal complication that affects individuals with defective cellular immunity; it typically occurs months after infection. SSPE is a slow progressive disease characterized by seizures and progressive deterioration of cognitive and motor functions, followed by death that occurs 5-15 years after measles virus infection. It most often occurs in persons infected with measles virus before 2 years of age.
LABORATORY DIAGNOSIS OF MEASLES
The characteristic clinical features of measles are of sufficient sensitivity and specificity to have high predictive value in regions where measles is endemic. However, laboratory diagnosis is necessary where measles virus transmission rates are low or in immunocompromised persons who might not have the characteristic clinical manifestations. Infection with rubella, parvovirus B19, human herpes virus 6, and dengue viruses may mimic measles. Detection of IgM antibodies to measles virus by enzyme immunoassay (EIA) is the standard method of diagnosing acute measles (Figure 16-2).37 Alternatively, seroconversion using IgG-specific EIA or virus neutralization assays can be used to diagnose acute measles based on testing serum or plasma obtained during the acute and convalescent phases.
Measles virus can be isolated in tissue culture from white blood cells, respiratory tract secretions and urine, although the ability to isolate measles virus diminishes quickly after rash onset. Amplification and detection of measles virus RNA by reverse transcription-polymerase chain reaction (RT-PCR) from blood, urine, and nasal discharge is highly sensitive in detecting measles virus RNA and allows sequencing of the measles virus genome for molecular epidemiologic studies.
EPIDEMIOLOGIC CHARACTERISTICS
Mode of Transmission
Measles virus is transmitted primarily by respiratory droplets small enough to traverse several feet but too large to remain suspended in the air for long periods of time. The symptoms induced during the prodrome, particularly sneezing and coughing, enhance transmission. Measles virus also may be transmitted by the airborne route, suspended on small particles for a prolonged time. Direct contact with infected secretions can transmit measles virus, but the virus does not survive long on fomites, as it is quickly killed by heat and ultraviolet radiation.
Reservoir
Humans are the only reservoir for measles virus, a crucial fact for the potential eradication of measles. Nonhuman primates may be infected with measles virus and develop an illness similar to measles in humans, with rash, coryza, and conjunctivitis. However, populations of wild monkeys are not of sufficient size to maintain measles virus transmission.
Incubation Period
The incubation period for measles—that is, the time from infection to clinical disease—is approximately 10 days to the onset of fever and 14 days to the onset of rash, with a median of 12.5 days (95% confidence interval: 11.8-13.3 days).38 The incubation period may be shorter in infants or following a large inoculum of virus, and it may be longer in adults. During this seemingly quiescent period, the virus is rapidly replicating and infecting target tissues as described earlier. The incubation period is best measured during outbreaks in which the time of exposure to the index case can be precisely determined. The first accurate measurement of the incubation period for measles was by the Danish physician Peter Panum during the measles outbreak on the sparsely populated Faroe Islands in 1846.
Infectious Period
The infectious period for measles is more difficult to measure than the incubation period, as it requires careful observation of the contacts of exposed persons prior to the onset of rash. Generally, persons with measles are infectious for several days before and after the onset of rash, when titers of measles virus in the blood and body fluids are highest (Figure 16-2). As with many other acute viral infections (severe acute respiratory syndrome [SARS] coronavirus being a notable exception*), the fact that measles virus is contagious prior to the onset of recognizable disease hinders the effectiveness of quarantine measures. Detection of measles virus in body fluids by a variety of means, including identification of multinucleated giant cells in nasal secretions or the use of RT-PCR, suggests the potential for prolonged infectious periods in persons immunocompromised by severe malnutrition or HIV infection.20, 39 However, whether the measles virus detected by these methods is infectious over this prolonged time is unclear.
Infectivity
Measles virus is one of the most highly contagious infectious agents, and outbreaks can occur in populations in which fewer than 10% of all persons are susceptible. Chains of transmission commonly occur among household contacts, school-age children, and healthcare workers. The contagiousness of measles virus is best expressed by the basic reproductive number R0, which represents the mean number of secondary cases that arise if an infectious case is introduced into a completely susceptible population.40 R0 can be empirically measured, although the introduction of
measles virus into a completely susceptible population is rare. In the 1951 measles epidemic in Greenland, the index case attended a community dance during the infectious period resulting in an R0 of 2000.41 R0 may also be estimated from the average age of infection (A), the life expectancy (L), and the duration of protection from maternally acquired antibodies (M) using the following equation:
measles virus into a completely susceptible population is rare. In the 1951 measles epidemic in Greenland, the index case attended a community dance during the infectious period resulting in an R0 of 2000.41 R0 may also be estimated from the average age of infection (A), the life expectancy (L), and the duration of protection from maternally acquired antibodies (M) using the following equation:
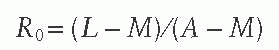
The estimated R0 for measles virus is 12-18,42 compared to only 5-7 for smallpox virus and 2-3 for SARS coronavirus. The high infectivity of the measles virus implies that a high level of population immunity is required to interrupt measles virus transmission, as described later in this chapter. Previously vaccinated children who acquire measles are thought to be less infectious than unvaccinated index cases.
Maternally Acquired Anti-measles Antibodies
Young infants in the first months of life are protected against measles by maternally acquired IgG antibodies. An active transport mechanism in the placenta is responsible for the transfer of IgG antibodies from the maternal circulation to the fetus starting at about 28 weeks’ gestation and continuing until birth.43 Three factors determine the degree and duration of protection in the newborn: (1) the level of maternal anti-measles antibodies; (2) the efficiency of placental transfer; and (3) the rate of catabolism in the child. Although providing passive immunity to young infants, maternally acquired antibodies can interfere with the immune responses to the attenuated measles vaccine by inhibiting replication of vaccine virus. In general, maternally acquired antibodies are no longer present in the majority of children by 9 months of age, the time of routine measles vaccination in many countries. The half-life of maternal anti-measles antibodies was estimated to be 48 days in the United States and Finland, but it is shorter in developing countries. Women with vaccine-induced immunity tend to have lower anti-measles virus antibody concentrations than women with naturally acquired immunity, and their children may be susceptible to measles at an earlier age.44 Infants born to HIV-infected women may have lower levels of protective maternal antibodies independent of their own HIV infection status and also may be susceptible to measles at a younger age.45
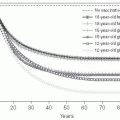
Average Age of Infection
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree