A significant number of cancer patients will develop intracranial metastases. In general, treatment of these lesions uses a combination of surgery and radiation. However, over the past several decades the specifics of this treatment paradigm have evolved significantly. This article describes the current standard of care with respect to intracranial metastases, highlighting the latest therapeutic developments.
- •
Surgery for intracranial metastases is indicated for a single brain metastasis, as well as symptomatic lesions with significant mass effect or cases in which there is diagnostic ambiguity.
- •
Evolving surgical indications include focal failures following stereotactic radiosurgery, management of adverse radiation effects, and delivery of novel therapies, such as laser thermocoagulation.
- •
For patients with brain metastases, treatment with whole-brain radiotherapy decreases the risk of intracranial recurrence, but does not improve survival and has an adverse impact on neurocognitive status.
- •
As systemic therapies evolve and survival improves, emerging management paradigms are focusing on maintaining quality of life, and thus there is an increasing interest in the use of stereotactic radiosurgery.
Introduction
Approximately 15% to 40% of patients with cancer will develop metastatic lesions to the brain. In fact, metastatic intracranial disease is 10-fold more common than primary brain tumors. Historically the presence of brain metastases was considered to be a poor prognostic indicator. However, recent studies suggest that patients with intracranial metastases are surviving longer, which is largely due to improvements in systemic therapies and is supported by more aggressive approaches to intracranial therapy. The current treatment paradigm for intracranial metastases reflects a multidisciplinary approach that combines both surgery and radiation. This article describes the current state of the art regarding the management of brain metastases.
Surgery for brain metastases
Traditional Indications: Solitary Metastases
Surgical management of intracranial metastases first gained popularity several decades ago, with the advent of computerized axial tomographic (CT) scanning. This technology improved lesion detection and enumeration, and rare patients with single intracranial metastases were referred for surgical resection. Despite the relative popularity of surgical resection during this era, the evidence favoring this approach was retrospective in nature, and many studies found no additional benefit to surgical intervention. Hence surgical management of single brain metastases remained controversial until 1990, when a seminal study by Patchell and colleagues that addressed this issue was published. In this prospective randomized trial, 48 patients with single brain metastasis were assigned to either surgical resection followed by radiotherapy or needle biopsy, followed by radiotherapy. All patients had a Karnofsky Performance Status (KPS) greater than or equal to 70. Patients who underwent surgical resection had improved survival, remained functionally independent for longer, and had a decreased incidence of recurrence at the original metastasis site.
In 1998, a follow-up study by the same group examined the role of whole-brain radiotherapy (WBRT) following surgical resection of a single intracranial metastasis. Ninety-five patients who had undergone complete resection of a single intracranial metastasis were randomized to either postoperative WBRT or no further treatment. This study determined that patients in the radiotherapy group had a decreased incidence of tumor recurrence anywhere in the brain, including the site of original metastasis, and were less likely to die of neurologic causes. There was no significant difference between the 2 groups with respect to length of survival or length of functional independence.
Based on the results of these 2 studies, a standard of care for patients with single, resectable intracranial metastases was established; patients with good functional status were referred for surgical resection followed by WBRT.
Traditional Indications: Other
In addition to its role in the management of single intracranial metastases, surgery established roles in other clinical scenarios. Specifically, a small percentage of patients with cancer will present with neurologic symptoms as their initial presentation of cancer. In approximately 15% of these patients no systemic disease will be detectable, and biopsy or surgical resection of an intracranial lesion will be necessary for diagnosis. In addition, in neurologically symptomatic patients with surgically accessible metastases, even in the presence of multiple intracranial lesions, surgical decompression is the fastest and most effective method by which to obtain relief of mass effect and thereby decrease the need for corticosteroids. The cessation of steroids is particularly important in cancers such as melanoma whose treatments are immunotherapy based. In patients with obstructive hydrocephalus caused by deep-seated lesions, placement of a ventriculoperitoneal shunt or performance of an endoscopic third ventriculostomy can facilitate safe delivery of radiation therapy. Endoscopic third ventriculostomy, though technically more difficult and not always possible in the setting of variable patient anatomy, carries the advantage of not needing to place a foreign body into patients who frequently become transiently immunocompromised, thus decreasing the risk for infection.
Evolving Roles for Surgery
Despite the broad nature of traditional surgical indications, in today’s clinical environment microsurgical resection of brain metastases remains a relatively unattractive treatment option, which is attributable to several factors.
- 1.
Surgical morbidity and mortality . In a patient with an average expected survival of 4 to 6 months, unless there is a need for immediate relief of mass effect via surgical decompression, radiotherapy has also been demonstrated to relieve symptomatology, albeit more slowly, in a large proportion of patients without the concomitant risks of general anesthesia, postoperative neurologic deficit, and wound healing. The difficulty in deciding who should get surgery lies in our current inability to accurately predict survival, despite numerous attempts to create sophisticated prediction models. In addition, it has been the authors’ experience that permanent neurologic deficit following surgical intervention often results in a decrease in quality of life that rapidly changes the goals of management toward hospice, especially if brain metastases are occurring late in the patient’s disease course. For this reason, all surgical interventions that are offered need to clearly carry less risk or offer more benefit than any available less-invasive treatment.
- 2.
Delay in systemic therapy . Given the increased risk of poor wound healing associated with postoperative WBRT and traditional chemotherapy, surgery has historically resulted in a delay of 4 to 6 weeks for initiation/resumption of systemic therapies. During this period it is certainly possible for systemic disease to progress. Since control of systemic disease has been well documented to be one of the strongest predictors of survival, surgery has not been considered a good option in patients in whom systemic disease is not controlled. However, the ability of newer surgical techniques to minimize postoperative delays in systemic therapy may change this balance.
- 3.
Improving imaging technologies . Magnetic resonance imaging (MRI) has supplanted CT scanning as the most sensitive and specific imaging test for the detection of brain metastases. Use of MRI has led to clinicians questioning the statistical probability of a true, single intracranial metastasis. In the CT era, approximately half of all brain metastases were thought to be single metastases. However, with the increasing use of high-resolution MRI, many fewer patients have only one intracranial metastasis detected at the time of diagnosis. Recently, several studies have demonstrated that refinements in MRI scanning technique (resolution, gadolinium dose) can lead to the detection of additional lesions in 30% to 40% of patients. This advance has led to a significant decrease in the population of patients who would satisfy traditional, single-metastasis criteria for surgical resection, as identified in 1990 by Patchell and colleagues.
- 4.
Availability of equivalent alternative therapy . Stereotactic radiosurgery (SRS) is the delivery of single-fraction, high-dose focused radiation to only the tumor without significant radiation to the surrounding brain (see later discussion). Several studies have tried to compare radiosurgery with microsurgery in terms of providing local lesional control, with somewhat conflicting results. However, it is well documented that radiosurgery results in good local control over a 6- to 8-month period, without surgical intervention. Moreover, accumulating evidence suggests that for these patients, further improvements in local, intracranial control do not improve survival, especially in the setting of widespread systemic disease.
Thus, the clear advantages of radiosurgery over surgery include (1) the ability to treat multiple lesions simultaneously, including those in surgically inaccessible areas; (2) the avoidance of anesthesia and surgical wounds; (3) the rapidity with which treatment can be scheduled and delivered; and (4) the ability to treat small (3–5 mm) asymptomatic lesions that would be difficult to find surgically. Because of the ability of SRS to control small brain metastases, the need for aggressive surgical resection has been further decreased. However, SRS is limited by lesion size and proximity to the optic apparatus, and in these situations surgery still plays a significant role, although fractionated radiation can also be considered.
The last factor to contribute to the evolving role of surgery has been the increasing use of brain MRI as part of (1) screening for medical oncology clinical trials, (2) comprehensive staging at the time of initial diagnosis in certain malignancies with a known propensity for developing central nervous system (CNS) metastases, and (3) restaging as changes in systemic management are needed to cope with disease progression. Traditionally patients have only undergone intracranial imaging for workup of neurologic findings; however, with the increasing interest in the development of targeted, disease-specific systemic agents and an increasing number of patients being enrolled in clinical trials, the role of imaging has changed. Because of the increasing availability of MRI and SRS, brain metastases can now be found and treated noninvasively before patients ever develop symptoms.
In response to many of the aforementioned changes, the role of surgery for intracranial metastases continues to change. The traditional indications with respect to establishing a tissue diagnosis and alleviating mass effect still apply, and for patients with a true, single intracranial lesion and otherwise good functional status, surgical resection remains an option well supported by clinical trial data.
However, 2 newer indications for surgery are coming into prominence: (1) for patients with single or multiple metastases in whom SRS is being used without WBRT for primary treatment of brain metastases; and (2) for patients with focal failure of SRS.
Patients in whom WBRT may potentially be deferred as the primary treatment for brain metastases include:
- 1.
Patients at the extremes of age. In the young patient, it is well documented that the longer the patient survival, the higher the risk of developing radiation-induced leukoencephalopathy and neurocognitive decline following WBRT. In the elderly patient with a more limited CNS reserve, there is also an increased risk for neurocognitive decline.
- 2.
Patients with histologies that are traditionally considered radioresistant, including melanoma, renal cell cancer, and sarcomas.
- 3.
Patients with favorable prognoses following treatment of their brain metastases, including those with Her2Neu-positive breast cancer and those with lung cancers with clearly identified gene mutations, such as mutation of endothelial growth factor receptor or ALK gene rearrangements.
- 4.
Patients who elect to avoid WBRT because the literature suggests it has the potential to cause neurocognitive decline.
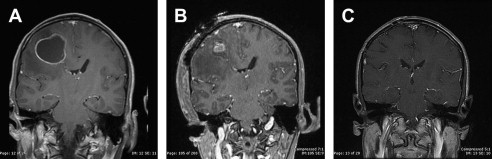
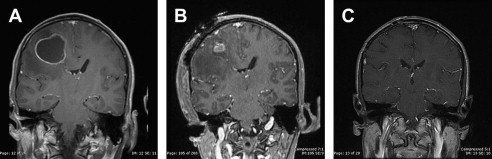
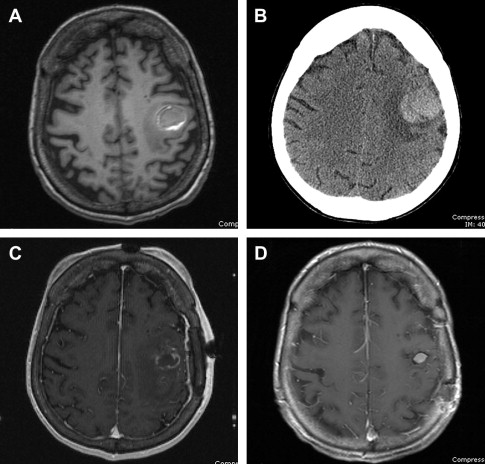
In these situations, the rate of radiosurgery failure increases with increasing lesion size. It has been the authors’ experience that lesions bordering on 2.5 to 3 cm in diameter, if surgically accessible, are better controlled with initial surgical resection to decrease lesion volume, followed by consolidative SRS ( Fig. 1 ). Several retrospective studies have demonstrated that consolidative SRS is as effective as WBRT in providing local control. Furthermore, if the majority of the tumor is removed surgically, optimal radiosurgical dose delivery to the residual tumor or resection cavity can be achieved and WBRT can be avoided.
At the authors’ institution, several factors have contributed significantly to the optimization of surgical resection of metastases, including: (1) the ability to obtain the navigation MRI in the operating room before surgery, thus decreasing time delay caused by imaging scheduling; (2) the ability to perform small craniotomies directed by intraoperative navigation; and (3) a dedicated neuroanesthesia team facilitating rapid postoperative recovery.
This facility is now capable of offering, within a single 2-day admission, rapid surgical management of the brain metastasis followed by consolidative SRS on postoperative day 2, and discharge to home following completion of the SRS procedure. This combination has been particularly successful in patients with hemorrhagic melanoma metastases, in whom the majority of the lesion being treated is in fact hematoma rather than tumor. Decompression of the hematoma, especially in functionally critical areas such as the motor and speech cortices, has facilitated a more rapid recovery of neurologic function and delivery of higher radiation doses to the remaining tumor, thus resulting in better local control ( Fig. 2 ).
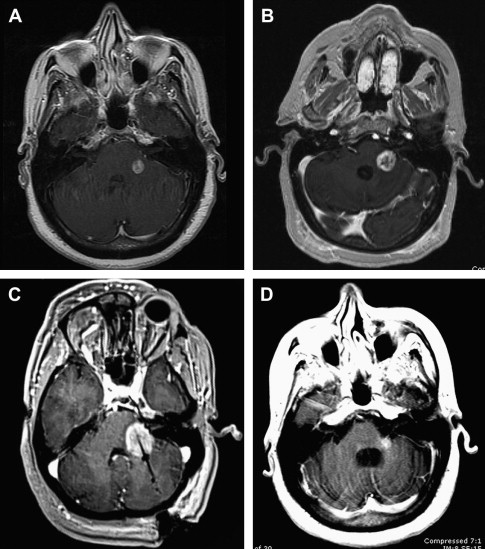
The second new indication for surgery involves the management of adverse radiation effects (ARE). Of the approximately 2500 metastatic lesions treated using SRS over the past 5 years at the authors’ institution, 40 have required subsequent post-SRS resection for symptomatology and radiographically documented lesional growth. Of these 40 lesions, approximately one-half have demonstrated histologically persistent tumor admixed with radiation changes, but the other half have demonstrated only radiation changes, without any histopathologic evidence of residual tumor. Radiographically, this condition has been termed ARE. Recent studies including histopathologic correlates suggest that immunologic processes contribute to these radiographic findings.
As the number of patients undergoing SRS increases, a significantly larger portion of lesions has been noted to increase in size on follow-up, surveillance MRI. A study at the authors’ institution demonstrates that approximately one-half of patients with intracranial metastases will have at least one of their SRS-treated lesions increase in size in the first 2 years after SRS. The differential for these imaging findings is always tumor regrowth versus ARE, but most growing lesions are due to ARE and not tumor regrowth. To make the appropriate diagnosis and treatment plan, however, a surgical biopsy is often required. Although future advancements in imaging techniques, especially with respect to perfusion MRI and 11 C-methionine positron emission tomography, may eliminate the need for invasive biopsies, such technology is not yet standardized. Moreover, as researchers seek to improve imaging technology such that noninvasive diagnoses of tumor regrowth versus ARE can be made, they will initially need tissue correlation to validate their findings.
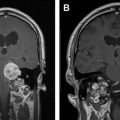
Because ARE-associated lesions can continue to grow, causing morbidity and mortality, aggressive treatment is often indicated if patients have otherwise adequate systemic control of their cancer. None of the current treatment options, including aspirin, hyperbaric oxygen therapy, vitamin E, and pentoxifylline, have been shown to be conclusively effective in treating ARE. Several studies have shown a benefit to bevacizumab, but this agent is expensive, difficult to obtain, and sometimes precludes the use of other systemic agents. For this reason, surgical management remains a good option for the management of ARE. Recently, minimally invasive therapies such as laser thermocoagulation and focused ultrasonography have been investigated. The authors’ institution has had some preliminarily positive experiences with the use of laser thermocoagulation for the treatment of ARE, but larger series will be required to confirm its effectiveness ( Fig. 3 ).
Future Role of Surgery
The role of surgical intervention for brain metastases will continue to change in the coming years. Future roles of surgery should seek to both improve the ability of oncologists to understand the intracranial disease process and provide novel methods to improve treatments. The understanding of radiographic changes in the brain following either radiation or chemotherapy requires tissue validation, and this will likely become an area of increasing interest. The introduction of the intraoperative MRI allows, for the first time, documentation of the site of biopsy as well as direct 3-dimensional coregistration with other MRI sequences such as spectroscopy. In post-SRS lesions where both ARE and tumor can coexist, this information can begin to help oncologists and neuroradiologists interpret radiographic data. Another area in which surgery may play a role will be in determining the degree of intracranial penetration of novel, targeted small-molecule therapies or in the elucidation of the mechanisms by which some systemic therapies have an effect in the brain. As systemic therapies improve and the survival from cancer continues to increase, intracranial metastases are becoming the last frontier that needs to be conquered. In this new era, surgeons will need to be increasingly focused on maintaining a patient’s postoperative quality of life. Moreover, they will also be needed to actively participate in the development of new tissue-guided treatments for brain metastases. In the future, it is expected that the vast majority of these treatment decisions will be made by multidisciplinary oncology teams in conjunction with neurosurgeons with specialized experience in the management of intracranial metastases.
Surgery for brain metastases
Traditional Indications: Solitary Metastases
Surgical management of intracranial metastases first gained popularity several decades ago, with the advent of computerized axial tomographic (CT) scanning. This technology improved lesion detection and enumeration, and rare patients with single intracranial metastases were referred for surgical resection. Despite the relative popularity of surgical resection during this era, the evidence favoring this approach was retrospective in nature, and many studies found no additional benefit to surgical intervention. Hence surgical management of single brain metastases remained controversial until 1990, when a seminal study by Patchell and colleagues that addressed this issue was published. In this prospective randomized trial, 48 patients with single brain metastasis were assigned to either surgical resection followed by radiotherapy or needle biopsy, followed by radiotherapy. All patients had a Karnofsky Performance Status (KPS) greater than or equal to 70. Patients who underwent surgical resection had improved survival, remained functionally independent for longer, and had a decreased incidence of recurrence at the original metastasis site.
In 1998, a follow-up study by the same group examined the role of whole-brain radiotherapy (WBRT) following surgical resection of a single intracranial metastasis. Ninety-five patients who had undergone complete resection of a single intracranial metastasis were randomized to either postoperative WBRT or no further treatment. This study determined that patients in the radiotherapy group had a decreased incidence of tumor recurrence anywhere in the brain, including the site of original metastasis, and were less likely to die of neurologic causes. There was no significant difference between the 2 groups with respect to length of survival or length of functional independence.
Based on the results of these 2 studies, a standard of care for patients with single, resectable intracranial metastases was established; patients with good functional status were referred for surgical resection followed by WBRT.
Traditional Indications: Other
In addition to its role in the management of single intracranial metastases, surgery established roles in other clinical scenarios. Specifically, a small percentage of patients with cancer will present with neurologic symptoms as their initial presentation of cancer. In approximately 15% of these patients no systemic disease will be detectable, and biopsy or surgical resection of an intracranial lesion will be necessary for diagnosis. In addition, in neurologically symptomatic patients with surgically accessible metastases, even in the presence of multiple intracranial lesions, surgical decompression is the fastest and most effective method by which to obtain relief of mass effect and thereby decrease the need for corticosteroids. The cessation of steroids is particularly important in cancers such as melanoma whose treatments are immunotherapy based. In patients with obstructive hydrocephalus caused by deep-seated lesions, placement of a ventriculoperitoneal shunt or performance of an endoscopic third ventriculostomy can facilitate safe delivery of radiation therapy. Endoscopic third ventriculostomy, though technically more difficult and not always possible in the setting of variable patient anatomy, carries the advantage of not needing to place a foreign body into patients who frequently become transiently immunocompromised, thus decreasing the risk for infection.
Evolving Roles for Surgery
Despite the broad nature of traditional surgical indications, in today’s clinical environment microsurgical resection of brain metastases remains a relatively unattractive treatment option, which is attributable to several factors.
- 1.
Surgical morbidity and mortality . In a patient with an average expected survival of 4 to 6 months, unless there is a need for immediate relief of mass effect via surgical decompression, radiotherapy has also been demonstrated to relieve symptomatology, albeit more slowly, in a large proportion of patients without the concomitant risks of general anesthesia, postoperative neurologic deficit, and wound healing. The difficulty in deciding who should get surgery lies in our current inability to accurately predict survival, despite numerous attempts to create sophisticated prediction models. In addition, it has been the authors’ experience that permanent neurologic deficit following surgical intervention often results in a decrease in quality of life that rapidly changes the goals of management toward hospice, especially if brain metastases are occurring late in the patient’s disease course. For this reason, all surgical interventions that are offered need to clearly carry less risk or offer more benefit than any available less-invasive treatment.
- 2.
Delay in systemic therapy . Given the increased risk of poor wound healing associated with postoperative WBRT and traditional chemotherapy, surgery has historically resulted in a delay of 4 to 6 weeks for initiation/resumption of systemic therapies. During this period it is certainly possible for systemic disease to progress. Since control of systemic disease has been well documented to be one of the strongest predictors of survival, surgery has not been considered a good option in patients in whom systemic disease is not controlled. However, the ability of newer surgical techniques to minimize postoperative delays in systemic therapy may change this balance.
- 3.
Improving imaging technologies . Magnetic resonance imaging (MRI) has supplanted CT scanning as the most sensitive and specific imaging test for the detection of brain metastases. Use of MRI has led to clinicians questioning the statistical probability of a true, single intracranial metastasis. In the CT era, approximately half of all brain metastases were thought to be single metastases. However, with the increasing use of high-resolution MRI, many fewer patients have only one intracranial metastasis detected at the time of diagnosis. Recently, several studies have demonstrated that refinements in MRI scanning technique (resolution, gadolinium dose) can lead to the detection of additional lesions in 30% to 40% of patients. This advance has led to a significant decrease in the population of patients who would satisfy traditional, single-metastasis criteria for surgical resection, as identified in 1990 by Patchell and colleagues.
- 4.
Availability of equivalent alternative therapy . Stereotactic radiosurgery (SRS) is the delivery of single-fraction, high-dose focused radiation to only the tumor without significant radiation to the surrounding brain (see later discussion). Several studies have tried to compare radiosurgery with microsurgery in terms of providing local lesional control, with somewhat conflicting results. However, it is well documented that radiosurgery results in good local control over a 6- to 8-month period, without surgical intervention. Moreover, accumulating evidence suggests that for these patients, further improvements in local, intracranial control do not improve survival, especially in the setting of widespread systemic disease.
Thus, the clear advantages of radiosurgery over surgery include (1) the ability to treat multiple lesions simultaneously, including those in surgically inaccessible areas; (2) the avoidance of anesthesia and surgical wounds; (3) the rapidity with which treatment can be scheduled and delivered; and (4) the ability to treat small (3–5 mm) asymptomatic lesions that would be difficult to find surgically. Because of the ability of SRS to control small brain metastases, the need for aggressive surgical resection has been further decreased. However, SRS is limited by lesion size and proximity to the optic apparatus, and in these situations surgery still plays a significant role, although fractionated radiation can also be considered.
The last factor to contribute to the evolving role of surgery has been the increasing use of brain MRI as part of (1) screening for medical oncology clinical trials, (2) comprehensive staging at the time of initial diagnosis in certain malignancies with a known propensity for developing central nervous system (CNS) metastases, and (3) restaging as changes in systemic management are needed to cope with disease progression. Traditionally patients have only undergone intracranial imaging for workup of neurologic findings; however, with the increasing interest in the development of targeted, disease-specific systemic agents and an increasing number of patients being enrolled in clinical trials, the role of imaging has changed. Because of the increasing availability of MRI and SRS, brain metastases can now be found and treated noninvasively before patients ever develop symptoms.
In response to many of the aforementioned changes, the role of surgery for intracranial metastases continues to change. The traditional indications with respect to establishing a tissue diagnosis and alleviating mass effect still apply, and for patients with a true, single intracranial lesion and otherwise good functional status, surgical resection remains an option well supported by clinical trial data.
However, 2 newer indications for surgery are coming into prominence: (1) for patients with single or multiple metastases in whom SRS is being used without WBRT for primary treatment of brain metastases; and (2) for patients with focal failure of SRS.
Patients in whom WBRT may potentially be deferred as the primary treatment for brain metastases include:
- 1.
Patients at the extremes of age. In the young patient, it is well documented that the longer the patient survival, the higher the risk of developing radiation-induced leukoencephalopathy and neurocognitive decline following WBRT. In the elderly patient with a more limited CNS reserve, there is also an increased risk for neurocognitive decline.
- 2.
Patients with histologies that are traditionally considered radioresistant, including melanoma, renal cell cancer, and sarcomas.
- 3.
Patients with favorable prognoses following treatment of their brain metastases, including those with Her2Neu-positive breast cancer and those with lung cancers with clearly identified gene mutations, such as mutation of endothelial growth factor receptor or ALK gene rearrangements.
- 4.
Patients who elect to avoid WBRT because the literature suggests it has the potential to cause neurocognitive decline.
In these situations, the rate of radiosurgery failure increases with increasing lesion size. It has been the authors’ experience that lesions bordering on 2.5 to 3 cm in diameter, if surgically accessible, are better controlled with initial surgical resection to decrease lesion volume, followed by consolidative SRS ( Fig. 1 ). Several retrospective studies have demonstrated that consolidative SRS is as effective as WBRT in providing local control. Furthermore, if the majority of the tumor is removed surgically, optimal radiosurgical dose delivery to the residual tumor or resection cavity can be achieved and WBRT can be avoided.