Primary central nervous system lymphoma (PCNSL) is an uncommon and aggressive variant of non-Hodgkin lymphoma that involves the brain, eyes, leptomeninges, or spinal cord. Therapeutic progress has been modest, and our understanding of the molecular mechanisms that drive this disease is limited. Clinicians treating PCNSL face a challenge to balance the need to administer aggressive regimens to achieve a cure with the risks of delayed neurotoxicity after treatment. The standard treatment is a methotrexate-containing chemotherapy regimen. The timing and dose of whole-brain radiation therapy is controversial, given the significant risks of late neurotoxic effects, particularly in elderly patients.
- •
Primary central nervous system (CNS) lymphoma, an extranodal form of non-Hodgkin lymphoma, is an aggressive and uncommon malignancy involving the CNS.
- •
The goals of the diagnostic evaluation are to understand the extent of disease and to confirm that the lymphoma is localized to the CNS.
- •
Methotrexate-containing multiagent regimens are the standard treatment of this disease entity.
- •
The timing and dose of whole-brain radiation therapy is still unclear, given the significant risks of late neurotoxic effects.
Introduction
Primary central nervous system (CNS) lymphoma (PCNSL), an extranodal form of non-Hodgkin lymphoma (NHL), is an aggressive and uncommon malignancy involving the CNS. PCNSL accounts for 2.4% of primary brain tumors in the United States. Although there was nearly a 3-fold increase in the incidence between 1973 and 1984, the incidence recently has stabilized. The major risk factor for the development of PCNSL is immunodeficiency, either acquired or congenital. Infection with human immunodeficiency virus (HIV), which increases the risk of PCNSL by 3600-fold compared with the general population, largely accounted for this increased incidence.
Approximately 90% of PCNSL cases are diffuse large B-cell lymphoma (DLBCL), with the other 10% being Burkitt lymphomas, poorly characterized low-grade lymphomas and T-cell lymphomas. Despite similar histopathologic features, the prognosis of PCNSL is worse than other forms of extranodal or nodal DLBCL, and the therapeutic options in this area are unsatisfactory. This review focuses on the treatment strategies in immunocompetent patients with primary CNS lymphoma.
Pathophysiology
PCNSL is a lymphoid neoplasm that probably arises from the late germinal center or postgerminal center cells, and subsequently localizes to the CNS by unknown neurotropic mechanisms. Histologically, the primary CNS DLBCL is characterized by immunoblasts or centroblasts clustered around cerebral blood vessels; infiltration by reactive T cells often renders it challenging for pathologists to discriminate between a reactive and neoplastic process. The molecular mechanisms that drive transformation in primary CNS lymphoma remain poorly understood. One of the limitations in studying this disease process is the lack of ample tissue for molecular studies, because diagnosis is typically made with stereotactic needle biopsy. Certain molecular features are similar in both systemic and CNS DLBCL. Like systemic DLBCL, gene expression analysis has shown that PCNSLs can be classified into the 3 molecular subclasses: germinal center B-cell, activated B-cell, and type 3 large B-cell lymphoma. Common to both diseases are chromosomal translocations of the BCL6 gene, aberrant somatic hypermutation in proto-oncogenes including MYC, and PAX5, and deletions in 6q.
However, there are certain molecular features that distinguish primary CNS DLBCL from systemic DLBCL. Pathway analysis of genome-wide gene expression patterns reveals that when compared with systemic DLBCL, PCNSL is characterized by differential expression of multiple pathways related to adhesion and the extracellular matrix including the genes MUM1, CXCL13, and CHI3L1. PCNSL also has increased expression of c -Myc and Pim-1. These results are hypothesis-generating in that there may be genes responsible for the CNS tropism of PCNSL. However, functional work to further elucidate the specific role of these genes in PCNSL is needed. Understanding the molecular mechanisms of PCNSL will enable development of more rational therapeutic approaches for this disease.
Pathophysiology
PCNSL is a lymphoid neoplasm that probably arises from the late germinal center or postgerminal center cells, and subsequently localizes to the CNS by unknown neurotropic mechanisms. Histologically, the primary CNS DLBCL is characterized by immunoblasts or centroblasts clustered around cerebral blood vessels; infiltration by reactive T cells often renders it challenging for pathologists to discriminate between a reactive and neoplastic process. The molecular mechanisms that drive transformation in primary CNS lymphoma remain poorly understood. One of the limitations in studying this disease process is the lack of ample tissue for molecular studies, because diagnosis is typically made with stereotactic needle biopsy. Certain molecular features are similar in both systemic and CNS DLBCL. Like systemic DLBCL, gene expression analysis has shown that PCNSLs can be classified into the 3 molecular subclasses: germinal center B-cell, activated B-cell, and type 3 large B-cell lymphoma. Common to both diseases are chromosomal translocations of the BCL6 gene, aberrant somatic hypermutation in proto-oncogenes including MYC, and PAX5, and deletions in 6q.
However, there are certain molecular features that distinguish primary CNS DLBCL from systemic DLBCL. Pathway analysis of genome-wide gene expression patterns reveals that when compared with systemic DLBCL, PCNSL is characterized by differential expression of multiple pathways related to adhesion and the extracellular matrix including the genes MUM1, CXCL13, and CHI3L1. PCNSL also has increased expression of c -Myc and Pim-1. These results are hypothesis-generating in that there may be genes responsible for the CNS tropism of PCNSL. However, functional work to further elucidate the specific role of these genes in PCNSL is needed. Understanding the molecular mechanisms of PCNSL will enable development of more rational therapeutic approaches for this disease.
Clinical presentation
Although PCNSL can occur in all age groups, the median age at diagnosis is between 53 and 61 years, with a slight male predominance. Patients commonly present with a single mass lesion, most often supratentorial. The duration of symptoms before diagnosis is typically 2 to 3 months. In a retrospective series of 248 immunocompetent patients with PCNSL treated in 19 centers in France and Belgium, 33% presented with symptoms or signs of increased intracranial pressure, 43% with neuropsychiatric symptoms, 70% with focal neurologic deficits, 14% with seizures, and 4% with vitreous involvement. The incidence of lymphomatous meningitis in PCNSL ranges from 8% when measured by magnetic resonance imaging (MRI) to 42% when assessed by cerebrospinal fluid (CSF) cytology. Primary leptomeningeal lymphoma is observed in less than 5% to 10% of cases. Primary intramedullary spinal cord lymphoma is also rare, with fewer than 50 cases reported in the literature. Ocular involvement occurs in 10% to 20% of cases, with common symptoms being blurry vision, floaters, and eye pain. Unlike other forms of NHL, B symptoms such as fevers, weight loss, and night sweats are rare.
Diagnosis
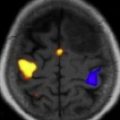
The International Primary CNS Lymphoma Collaborative Group (IPCG) has published consensus guidelines for the baseline diagnostic evaluation of patients with PCNSL. The goals of the diagnostic evaluation are to understand the extent of disease and to confirm that the lymphoma is localized to the CNS. The physical examination should include a comprehensive neurologic assessment, a thorough lymph node examination, and a testicular examination in men. Because there is no standard neuropsychological testing, at a minimum, a Mini-Mental Status Examination (MMSE) should be performed and serially monitored. Unless contraindicated, a lumbar puncture should be performed and CSF examined for cell count, cytology, flow cytometry, and immunoglobulin heavy-chain gene rearrangement studies. Imaging should include a gadolinium-enhanced cranial MRI scan ( Fig. 1 ), but contrast-enhanced cranial computed tomography (CT) can be substituted in cases in which an MRI is contraindicated. Because extraneural disease has been reported in 3.9% to 12.5% of patients with primary CNS lymphoma, CT scans of the chest, abdomen, and pelvis should be obtained, and a bone marrow biopsy and aspirate performed to rule out occult systemic disease. An ophthalmologic examination, including a slit-lamp examination, is required to exclude involvement of the optic nerve, retina, or vitreous cavity. Laboratory studies should include serum lactate dehydrogenase, a complete blood count, creatinine clearance, and HIV testing.
Prognosis
Identifying prognostic markers in PCNSL not only enables more informative discussions with individual patients, but also allows clinicians and investigators to develop risk-adjusted therapeutic approaches. Certain molecular markers have prognostic significance. Deletions in 6q are correlated with a shorter survival, whereas BCL-6 expression has a favorable prognosis. Various clinical prognostic scoring systems have been proposed to risk stratify patients with PCNSL. In a retrospective review of 338 patients with newly diagnosed PCNSL, age and performance status were the 2 variables identified in multivariate analysis, and this is consistent with other studies. Patients were divided into 3 classes: those younger than 50 years; those with a Karnofsky Performance Scale (KPS) score higher than 70 and older than 50 years; and those older than 50 years and with a KPS score less than 70. When this scoring system was applied to 3 large Radiation Therapy Oncology Group (RTOG) PCNSL trials, median overall survival was 5.2 years in class 1 (age ≤50 years), 2.1 years in class 2 (KPS ≥70, age >50 years), and 0.8 years in class 3 (KPS <70; age >50 years).
The International Extranodal Lymphoma Study Group also devised a prognostic scoring system based on data from 378 patients with PCNSL treated at 23 centers. The 5 variables associated with poor prognosis were age greater than 60 years; Eastern Cooperative Oncology Group performance status greater than 1; increased CSF protein level; increased serum lactate dehydrogenase level; and tumor involvement of the deep regions within the brain (basal ganglia, periventricular regions, brainstem, or cerebellum). Patients with 0 to 1, 2 to 3, and 4 to 5 of these unfavorable variables had 2-year overall survival rates of 80%, 48%, or 15%, respectively. Further investigation is needed to evaluate whether distinct therapeutic approaches should be used for the different risk groups.
Treatment
Background
Historically, patients with newly diagnosed PCNSL were treated with whole-brain radiation therapy (WBRT) and corticosteroids alone, with median survival reported to be between 12 and 15 months; chemotherapy was reserved for recurrent PCNSL. In 1985, Deangelis and colleagues reported on 31 patients with newly diagnosed PCNSL who were treated with a combined modality approach of intravenous methotrexate (MTX) (1 g/m 2 ), intraventricular MTX, followed by WBRT, and then high-dose cytarabine (ara-C). Sixteen patients were treated with WBRT alone because they either refused chemotherapy or had initiated radiation elsewhere. The median survival in the combined modality group was 42.5 months compared with 21.7 months in the radiation group. Although the results were not statistically significant, at that time, this was the longest median survival that had been reported for any therapeutic regimen in PCNSL. Subsequently, studies were designed to evaluate combined modality approaches. Multiple phase II studies and retrospective analyses confirmed that the combination of chemotherapy and radiation was more effective than radiation alone. However, the neurotoxicity of the combination is often significant, particularly in the elderly PCNSL patient population. Thus, regimens that defer WBRT have been developed. Over the last several years, clinicians and investigators have been faced with the challenge to balance the need to administer aggressive regimens to achieve a cure with the risks of delayed neurotoxicity after treatment.
Monitoring Response to Treatment
Although there have been numerous phase II trials in PCNSL, response criteria have not been consistent between studies, and thus, it is a challenge to compare results of various studies. For this reason, the IPCG created standard guidelines for monitoring response to therapy in the setting of clinical trials. Response criteria take into account imaging, corticosteroid dose, CSF cytology, and ophthalmologic examination ( Table 1 ). On completion of therapy, the IPCG recommended assessment of the patient every 3 months for 2 years, then every 6 months for 3 years, and then every 12 months for 5 years. At follow-up visits, the recommended testing is a history and physical examination (including an MMSE) and MRI.
| Response | Brain Imaging | Steroid Dose | Ophthalmologic Examination | CSF Cytology |
|---|---|---|---|---|
| CR | No contrast-enhancing disease | None | Normal | Negative |
| Unconfirmed CR | No contrast-enhancing disease Minimal enhancing disease | Any Any | Normal Minor RPE abnormality | Negative Negative |
| PR | 50% decrease in enhancement No contrast-enhancing disease | NA NA | Normal or minor RPE abnormality Decrease in vitreous cells or retinal infiltrate | Negative Persistent or suspicious |
| PD | 25% increase in enhancing disease Any new site of disease | NA | Recurrent or new disease | Recurrent or positive |
| SD | All scenarios not covered by responses above |
Role of Surgery
PCNSL is typically diagnosed with a stereotactic-guided biopsy. Gross total resection does not play a therapeutic role in PCNSL, a multifocal malignancy often with dissemination to the CSF and eyes. In a meta-analysis of 50 prospective and retrospective studies, the extent of resection did not affect survival. Extensive resections carry the risk of worsening neurologic deficits and treatment delays. Surgical resection should be reserved for the rare cases of impending neurologic deterioration secondary to brain herniation.
Corticosteroids
Corticosteroids can cause a rapid regression of tumor and decrease in tumor-associated edema. However, if administered before a diagnostic biopsy, the cellular morphology may be disrupted, and diagnostic inaccuracies may result. For this reason, before a diagnostic biopsy, other methods, such as the administration of mannitol, should be used for symptomatic increase of intracranial pressure. An initial response to corticosteroids portends a more favorable outcome in PCNSL. A retrospective study reported that median survival of patients with newly diagnosed PCNSL with a documented radiologic response to corticosteroids was 117.0 months compared with 5.5 months in the nonresponders. However, most patients inevitably relapse, even after an initial response to steroids, necessitating the administration of other treatments.
Radiation Therapy
WBRT as a single modality results in a median survival of 12 to 18 months, and 5-year overall survival rates ranging from 10% to 29%. Although the initial response rate is observed in more than 90% of patients undergoing WBRT, most patients relapse. The addition of chemotherapy to WBRT results in higher response rates and improved overall survival ( Table 2 ). Complete remission rates of 56% to 88% and median overall survival of 33 to 51 months have been reported with combined modality therapy. Neurotoxicity, particularly cognitive dysfunction, after combined modality treatment is a serious challenge in the treatment of patients with PCNSL (see Table 2 ). Only a limited number of studies have systematically evaluated cognitive function in this patient population, and definitions of cognitive impairment vary, rendering it difficult to compare outcomes between different studies. Among the studies that do evaluate cognitive function, there is evidence of cognitive impairment in patients who undergo chemotherapy and WBRT, and stable or improved cognitive function in those patients who received single-modality treatment with chemotherapy.
| Systemic Treatment, Reference | WBRT | Intrathecal Treatment | N | CR (%) | PR (%) | PFS (mo) | OS (mo) | Neurotoxicity (%) |
|---|---|---|---|---|---|---|---|---|
| MTX (3.5 g/m 2 ) | 30 Gy | None | 25 | 56 | 32 | 32 | 33 | 8 |
| MTX (1 g/m 2 ), ara-C | 40 Gy with 14 Gy boost | MTX | 31 | 87 | – | 40 | 42 | 32 |
| MTX (3 g/m 2 ), adriamycin, ara-C, cyclophosphamide, vincristine, methylprednisolone | 20 Gy with 30 Gy boost | MTX, ara-C, hydrocortisone | 25 | 56 | 16 | NR | 2 y OS 70% | 0 |
| MTX (3 g/m 2 ), teniposide, carmustine | 30 Gy with 10 Gy boost | ara-C, MTX | 52 | 69 | 12 | NR | 46 | 12 |
| MTX (3.5 g/m 2 ), procarbazine, vincristine, ara-C | 45 Gy in <60 y old | MTX | 57 | 56 | 33 | 129 | 51 | 30 |
| MTX (3.5 g/m 2 ) ± ara-C | Dose depended on age, response, participating center | None | 79 | MTX, 18; MTX+ ara-C, 46 | MTX, 23; MTX+ ara-C, 23 | 3 y: MTX, 21%; MTX+ ara-C, 38% | 3 y: MTX, 32%; MTX+ ara-C, 46% | MTX, 20; MTX+ ara-C, 6 |
| MTX (3.5 g/m 2 ), rituximab, procarbazine, vincristine, ara-C | 23.4 Gy if CR; 45 Gy if not CR | None | 30 | 77 | NR | 40 | 2 y OS 67% | NR |
Lower doses of whole-brain radiation are being investigated as a potential method by which to reduce the incidence of neurotoxicity. A study by Shah and colleagues evaluated the toxicity and efficacy of a lower dose of WBRT (23.4 Gy) in patients who achieved a complete response to rituximab, MTX, procarbazine, and vincristine. At a median follow-up of 37 months, the 2-year overall survival was 67% and no neurotoxicity was observed. In a subsequent phase III randomized noninferiority trial, the omission of radiation was evaluated, with 551 patients with newly diagnosed PCNSL randomized to receive high-dose MTX-based chemotherapy with or without WBRT. There was a trend toward increased median progression-free survival in the WBRT cohort compared with the chemotherapy-only cohort (18.3 months vs 11.9 months; P = .14), without a difference in median overall survival (32.4 vs 37.1 months; P = .71). However, conclusions cannot reliably be made from this study, given the large number of protocol violations (30% of patients) and the biased analyses of the protocol violations.
Although the data are limited, many centers have adopted the approach of administering chemotherapy alone, and deferring WBRT for use in relapsed patients.
Chemotherapy
In contrast to the standard treatment of systemic DLBCL, CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) does not have a role in the treatment of PCNSL. Retrospective series have reported that cyclophosphamide, doxorubicin, vincristine, and doxorubicin added to MTX results in higher toxicity without a survival benefit. A randomized trial, although underpowered, failed to show a survival benefit when CHOP was added to WBRT.
MTX is the backbone of any upfront regimens for PCNSL. Systemic high-dose MTX alone has efficacy. Therapeutic CSF concentrations of MTX can be achieved with intravenous MTX doses 3 g/m 2 or greater. The New Approaches to Brain Tumor Therapy (NABTT) CNS Consortium conducted a multicenter phase II trial of single-agent intravenous MTX (8 g/m 2 ) every 2 to 4 weeks in 25 patients with newly diagnosed PCNSL. The complete response rate was 52%, the median progression-free survival 12.8 months, and overall survival 55.4 months. Twelve patients experienced grade 3 or 4 toxicities. The clinical response reported in this study was comparable with results obtained with other more toxic combinations. Other studies of single-agent high-dose MTX have reported similar results, and low rates of neurotoxicity.
Numerous phase II studies have evaluated the addition of other cytotoxic agents to MTX ( Table 3 ). In a randomized phase II study of 79 patients with newly diagnosed PCNSL, the addition of ara-C (4 doses of 2 g/m 2 every 3 weeks) to MTX (4 courses of 3.5 g/m 2 every 3 weeks) resulted in higher radiographic responses (complete response rate = 46% in the combination arm vs 18% in the MTX-alone arm; P = .006) and a trend toward improved survival (3-year overall survival 46% vs 32%; P = .07). Toxicities were generally limited, but tended to be more common in the combination arm, with 3 toxicity-related deaths (1 liver toxicity, 2 sepsis) in the combination arm and 1 in the MTX arm (cardiac toxicity). Given the higher rates of complete responses, and trends toward improved survival in the combination arm, the combination of MTX and ara-C is a reasonable first-line regimen for newly diagnosed PCNSL, outside a clinical trial.
| Treatment, Reference | Intrathecal Treatment | N | CR (%) | PR (%) | PFS (mo) | OS (mo) | Neurotoxicity (%) |
|---|---|---|---|---|---|---|---|
| MTX Alone | |||||||
| MTX (8 g/m 2 ) | None | 31 | 65 | 35 | 17 | 30 | 0 |
| MTX (8 g/m 2 ) | None | 25 | 52 | 22 | 13 | 55 | 5 |
| MTX (8 g/m 2 ) | None | 37 | 30 | NR | 10 | 25 | 20 |
| MTX in Combination With Other Chemotherapy | |||||||
| MTX (5 g/m 2 ), ara-C, vincristine, ifosfamide, dexamethasone, cyclophosphamide | MTX, prednisolone, ara-C | 65 | 61 | 10 | 21 | 50 | 3 |
| MTX (1 g/m 2 ), lomustine, procarbazine, methylprednisolone | MTX, ara-C | 50 | 42 | 6 | 11 | 14 | 8 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree