Low-grade gliomas are uncommon tumors whose optimal management remains to be determined. Although well-designed clinical trials have been mounted to address certain aspects of postoperative radiotherapeutic management, additional studies are required to refine management based on tumor-specific and patient-specific variables. There is mounting evidence that the relative completeness of surgical resection can improve survival, and the molecular and histopathologic characterization of the glioma requires adequate samples for analysis. Current imaging and operative techniques can direct surgical resection, and the same imaging techniques can help monitor patients postoperatively and predict prognosis.
- •
Low-grade gliomas are slow-growing primary brain tumors and are most commonly diagnosed in young adults after new-onset seizures.
- •
A complete resection of a low-grade glioma may confer a survival benefit; at least one phase III trial is under way to test this.
- •
Chromosomal codeletion of 1p19q (oligodendroglial histology), isocitrate dehydrogenase (IDH) mutations, and smaller tumor size all predict an improved survival.
- •
Phase III trials have showed no survival benefit for early postoperative irradiation or for radiotherapy doses exceeding approximately 45 to 50 Gy.
- •
Clinical trial-based data guiding decisions about the use of systemic chemotherapy are lacking.
Introduction
Low-grade gliomas (LGGs) are primary brain tumors that most commonly arise in the cerebral hemispheres. According to the World Health Organization (WHO), LGGs are classified as Grade I and II tumors. Grade I LGGs are well circumscribed and include pilocytic astrocytomas, subependymomas, and dysembryoplastic neuroepithelial tumors. Grade II LGGs are far more common and represent diffusely infiltrating glial neoplasms with atypical nuclei.
The most common WHO II LGGs are astrocytomas, oligodendrogliomas, and mixed oligoastrocytic tumors. This article focuses on WHO Grade II gliomas located in the supratentorial area in adults, because they are the most common form of this disease. These gliomas are slow-growing primary brain tumors that represent approximately 15% to 25% of gliomas.
Clinical findings, epidemiology, and natural history
The typical LGG patient is a young adult between age 20 and 40 years who reaches medical attention following the new onset of seizures. In a study that compared the prevalence of seizures in glioma patients, LGGs were more likely to present with seizures (85% vs 69% and 49%, in patients with anaplastic glioma and glioblastoma, respectively). These patients classically present with modest or only slight neurologic deficits. Some patients demonstrate changes in mental status (10%), headaches (40%), and nausea. Weakness, dysarthria, and personality changes can be detected with frontal lobe tumors; difficulty with speech, vision changes, and frequent seizures may be seen in temporal lobe lesions, and sensory deficits and visual symptoms are reported in patients with parietal lobe gliomas. Visual changes are seen with involvement of the occipital lobes, and cerebellar tumors can cause hydrocephalus.
The natural history of a WHO Grade II LGG ultimately results in transformation into a high-grade tumor. This transition is the major factor in determining a patient’s survival. Whereas the timing of change to a high-grade tumor may be unpredictable, the outcome once this has occurred is nearly universally fatal. Several factors have been useful in anticipating the biological behavior in LGG patients, and these are addressed in this article.
The prognosis for LGG patients is classically determined by age, tumor size, and preoperative neurologic functional impairment. Patients younger than of 40 years with complete resection and an oligodendroglioma generally have a more favorable prognosis. The cumulative 5-, 10-, 15-, and 20-year survival rates from the Surveillance, Epidemiology, and End Results (SEER) database among all individuals initially diagnosed with a supratentorial low-grade glioma at the end of the twentieth century were 59.9%, 42.6%, 31.9%, and 26.0%, respectively. Patients diagnosed with histologically mixed tumors or oligodendrogliomas enjoy longer survival than patients diagnosed with astrocytomas. Histopathologic classification of LGGs is historically based on morphologic findings. This method has been inadequate to provide optimal guidance for prognosis, because of ambiguity in interpretation of biopsy findings. Immunohistochemical markers and analyses of tumor genetic and epigenetic changes have proved to be of prognostic value and often help guide treatment strategies.
It is now well recognized that LGGs containing deletions of chromosomal arms 1p and 19q have a more favorable prognosis. Tumors that share these deletions have a more indolent clinical course regardless of treatment methods. Codeletion of 1p/19q is highly correlated with oligodendroglioma histopathology and is observed in only 7% of fibrillary astrocytomas. Isocitrate dehydrogenase active-site mutations have been recently demonstrated in a majority of WHO Grade II gliomas. These mutations have been found to be an independent favorable prognostic factor.
Immunohistochemical assessment of the proliferation index (PI) with Ki-67/MIB-1 markers also provides diagnostic and prognostic value. LGGs usually have a PI of less than 4%, and the prognosis is poorer for patients whose tumors have a high PI in comparison with those with a low PI.
Alkylating agents (eg, temozolomide [TMZ] and the nitrosoureas carmustine [BCNU] and lomustine [CCNU]) inhibit growth primary through DNA alkylation. A method of intrinsic resistance of most gliomas is the expression of O 6 -methylguanine DNA methyltransferase (MGMT), which is a DNA repair enzyme that cleaves alkyl groups. MGMT levels in tumors are now used as a marker of chemotherapeutic response to TMZ in LGG and have been shown to correlate with progression-free survival (PFS).
Clinical findings, epidemiology, and natural history
The typical LGG patient is a young adult between age 20 and 40 years who reaches medical attention following the new onset of seizures. In a study that compared the prevalence of seizures in glioma patients, LGGs were more likely to present with seizures (85% vs 69% and 49%, in patients with anaplastic glioma and glioblastoma, respectively). These patients classically present with modest or only slight neurologic deficits. Some patients demonstrate changes in mental status (10%), headaches (40%), and nausea. Weakness, dysarthria, and personality changes can be detected with frontal lobe tumors; difficulty with speech, vision changes, and frequent seizures may be seen in temporal lobe lesions, and sensory deficits and visual symptoms are reported in patients with parietal lobe gliomas. Visual changes are seen with involvement of the occipital lobes, and cerebellar tumors can cause hydrocephalus.
The natural history of a WHO Grade II LGG ultimately results in transformation into a high-grade tumor. This transition is the major factor in determining a patient’s survival. Whereas the timing of change to a high-grade tumor may be unpredictable, the outcome once this has occurred is nearly universally fatal. Several factors have been useful in anticipating the biological behavior in LGG patients, and these are addressed in this article.
The prognosis for LGG patients is classically determined by age, tumor size, and preoperative neurologic functional impairment. Patients younger than of 40 years with complete resection and an oligodendroglioma generally have a more favorable prognosis. The cumulative 5-, 10-, 15-, and 20-year survival rates from the Surveillance, Epidemiology, and End Results (SEER) database among all individuals initially diagnosed with a supratentorial low-grade glioma at the end of the twentieth century were 59.9%, 42.6%, 31.9%, and 26.0%, respectively. Patients diagnosed with histologically mixed tumors or oligodendrogliomas enjoy longer survival than patients diagnosed with astrocytomas. Histopathologic classification of LGGs is historically based on morphologic findings. This method has been inadequate to provide optimal guidance for prognosis, because of ambiguity in interpretation of biopsy findings. Immunohistochemical markers and analyses of tumor genetic and epigenetic changes have proved to be of prognostic value and often help guide treatment strategies.
It is now well recognized that LGGs containing deletions of chromosomal arms 1p and 19q have a more favorable prognosis. Tumors that share these deletions have a more indolent clinical course regardless of treatment methods. Codeletion of 1p/19q is highly correlated with oligodendroglioma histopathology and is observed in only 7% of fibrillary astrocytomas. Isocitrate dehydrogenase active-site mutations have been recently demonstrated in a majority of WHO Grade II gliomas. These mutations have been found to be an independent favorable prognostic factor.
Immunohistochemical assessment of the proliferation index (PI) with Ki-67/MIB-1 markers also provides diagnostic and prognostic value. LGGs usually have a PI of less than 4%, and the prognosis is poorer for patients whose tumors have a high PI in comparison with those with a low PI.
Alkylating agents (eg, temozolomide [TMZ] and the nitrosoureas carmustine [BCNU] and lomustine [CCNU]) inhibit growth primary through DNA alkylation. A method of intrinsic resistance of most gliomas is the expression of O 6 -methylguanine DNA methyltransferase (MGMT), which is a DNA repair enzyme that cleaves alkyl groups. MGMT levels in tumors are now used as a marker of chemotherapeutic response to TMZ in LGG and have been shown to correlate with progression-free survival (PFS).
Molecular pathogenesis of low-grade glioma
Until recently, it was commonly held that most LGGs harbored either a tp53 mutation (astrocytoma) or loss of chromosomal arms 1p and 19q (oligodendroglioma). The recent identification of isocitrate dehydrogenase (IDH) mutations early in LGG molecular pathogenesis has changed the general understanding; and indeed, IDH mutations appear to arise before subsequent genetic alterations, and are present in the vast majority of secondary glioblastomas. Of interest is the nature of the mutations: they change the molecular substrate of the IDH enzyme. It normally catalyzes the conversion of isocitrate to α-ketoglutarate, but when mutated, catalyzes the conversion of α-ketoglutarate to 2-hydroxyglutarate (2HG). 2HG is an oncometabolite whose accumulation is now linked to the formation and malignant progression of gliomas. The mechanistic link between 2HG accumulation and the development of malignancy is still uncertain, but interference with the respiratory chain and the induction of redox stress via hypoxia-inducible factor 1α has been identified as a plausible mechanism.
Recently, the possibility of oncogene addiction in gliomas has been broached. If this is found to be true for subsets of LGGs, it would have significant implications for targeting the critical molecular pathways maintaining the aberrant phenotype.
Imaging of low-grade glioma
LGGs may be difficult to delineate on computed tomography (CT) scan. Most LGGs do not enhance with contrast administration, and although there may be evidence of calcification in some tumors (oligodendrogliomas), most LGGs appear only as areas of low density with indistinct margins. For this reason, magnetic resonance imaging (MRI) has supplanted CT imaging for establishing the anatomic distribution of the tumor, as well as for surgical planning and monitoring.
Most LGGs have a classic appearance on MRI of the brain ( Fig. 1 ). The typical findings are of an infiltrative, nonenhancing mass lesion that arises in white matter and often extends into the cortex. These tumors are hypointense on T1 spin-echo sequences, and hyperintense on T2 and proton-density sequences, with varying degrees of white matter infiltration and edema around the central part of the tumor. As already stated, unlike high-grade lesions LGGs generally do not enhance with contrast administration, although up to 25% of oligodendrogliomas may have partial contrast enhancement.
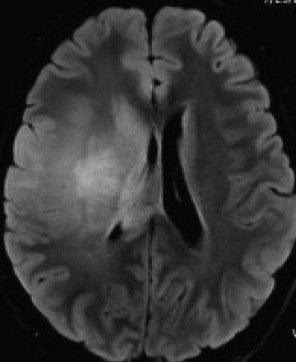
It is generally found that the acquisition of contrast enhancement in a previously nonenhancing LGG is evidence for transition to a high-grade lesion, and even if a contrast-enhancing tumor is histopathologically low grade, the behavior has often become that of a more aggressive glioma. Because of their relatively slow growth rate they are less likely higher-grade tumors to cause vasogenic edema. Fluid-attenuated inversion recovery (FLAIR) or T2-weighted MRI sequences are optimal for revealing the contours of these tumors, which typically appear on these pulse sequences as hyperintense lesions.
Some oligodendrogliomas are often associated with intratumoral calcifications that can be seen on MRI and CT scans. Loss of chromosomal arms 1p and 19q has been reported to be associated with an indistinct border on T1-weighted images and mixed-intensity signal on T1 and T2 imaging. Loss of 1p and 19q was also associated with a paramagnetic susceptibility effect and with calcification, which is, as noted earlier, a common histopathologic finding in oligodendrogliomas.
LGGs are typically hypometabolic on 18 F-fluorodeoxyglucose positron emission tomography (PET) scans. The use of radiolabeled amino acids and other tracers in the diagnosis and management of patients with low-grade gliomas is still largely investigational, with only small, single-institution series providing glimpses of how metabolic imaging may be of value in the future. For example, the identification of radiolabeled fluoroethyltyrosine ( 18 F-FET) hot spots within an LGG may help to direct biopsy of a more aggressive subcomponent that will affect management decisions and alter prognosis. PET imaging is still in the developmental phase for low-grade glioma assessment, but in additional attempts to go beyond checking integrity of the blood-brain barrier (ie, the absence or presence of contrast enhancement) in assessing low-grade glioma biological activity, other advanced imaging techniques are being used to evaluate tumors.
Magnetic resonance spectroscopy (MRS) is a noninvasive MRI technology that assesses metabolite levels within lesions. LGGs demonstrate a decrease in N -acetylaspartate (NAA) and creatine levels and an increase in choline (Cho) levels, secondary to increased proliferation of cells within the tumor; this profile is increasingly prominent the higher the grade of the glioma. Responses to TMZ therapy in patients with LGGs have been reported to be detectable with MRS at 3 months. These early changes predicted responses at 14 months, and were more informative than tumor T2-weighted or FLAIR volume changes. Perhaps MRS will prove valuable in determining which patients may require changes in therapy before conventional radiographic or clinical assessments can reliably determine whether a given therapy is efficacious.
Diffusion-weighted and perfusion-weighted MRI are techniques that, respectively, evaluate the mobility of water molecules within the tumor’s interstitial space and evaluate tumor-induced neovascularization. Diffusion tensor imaging is especially important for patients with tumors near critical language, motor, and other eloquent areas, and when normal anatomic landmarks may be distorted by the presence of the tumor and associated edema. An elevated baseline relative cerebral blood volume (rCBV) within a low-grade glioma has been shown to be predictive of a shorter PFS and overall survival. Longitudinal assessment of rCBV in patients with LGGs has also been able to detect early progression to high-grade gliomas using conventional assessment techniques.
Postoperative imaging of patients with LGGs can provide prognostic information as well. In a prospective multicenter phase II study, conducted by the Radiation Therapy Oncology Group, of patients younger than 40 years with a neurosurgeon-ascertained gross total resection who had preoperative and postoperative imaging, 59% of patients were found to have less than 1 cm of residual disease, whereas 32% had 1 to 2 cm of residual disease and 9% had more than 2 cm of residual disease. The recurrence rates for these subsets were 26%, 68%, and 89%, respectively. Two-year and 5-year survival rates of the 111 patients enrolled on this trial were 99% and 93%.
Surgical management
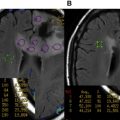
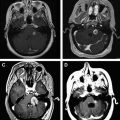
The optimal treatment for an LGG is a controversial topic, but there is growing evidence that aggressive surgical resection provides the best chances for minimizing the risk of radiographic or clinical progression and malignant transformation. There have been no randomized controlled trials evaluating whether aggressive surgery provides a better outcome than less aggressive surgery that establishes the diagnosis and avoids risking operative injury, and it is doubtful that any study will ever document that this is the case. The most recent evidence-based review of treatment options suggests that if a patient is to receive treatment for a presumed LGG and surgical resection is not considered possible, a biopsy should still be obtained to confirm the tissue diagnosis ( Figs. 2 and 3 ).
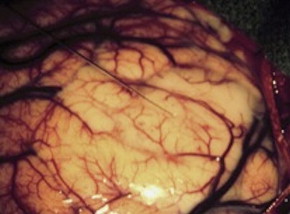
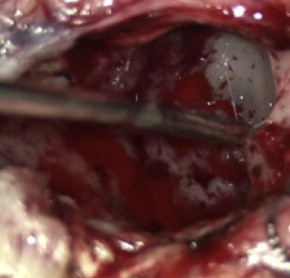
Biopsy of an LGG is commonly directed by stereotactic guidance. Because gliomas may contain regions of variable histopathologic findings, stereotactic biopsy of a diffusely infiltrating glioma carries a risk that any small sample may not contain the component with the greatest prognostic importance. Although most of these tumors have a homogeneous appearance on MRI, specific imaging characteristics can help guide the surgeon to target the areas of highest risk for anaplasia. A region of contrast enhancement within an otherwise nonenhancing LGG is commonly targeted, as it is suggestive of transformation to a more anaplastic tumor. Alternatively, when spectroscopic imaging is used to assist target selection, the region of the highest Cho/NAA ratio is considered to be the area of highest cellularity, and more likely to result in appropriate tumor grading from a small sample of a larger lesion. As noted earlier, dynamic 18 F-FET PET has also been reported as useful in identifying biopsy targets in LGGs.
There is abundant level-2 and level-3 evidence, but no data from randomized controlled trials, to indicate that extensive resection of an LGG is associated with improved survival when compared with subtotal resection or biopsy. In retrospective studies, extensive resection was found to have a positive impact on survival, and when preoperative and postoperative tumor volumes and extent of resection have been analyzed, patients who had 90% or more of their tumor resected were found to have a significant survival advantage.
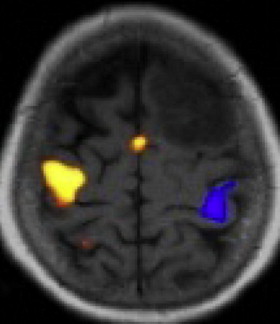
The survival benefit from aggressive resection of LGG has motivated surgeons to use stereotactic imaging, functional imaging, physiologic monitoring, and intraoperative imaging to maximize their ability to remove tumors safely while minimizing neurologic impairment. During surgical resection many LGGs are difficult to distinguish from normal white matter. The difficulty in detecting tumor margins can result in problems in deciding how much tissue to remove. Stereotactic localization (frameless or frame-based) can provide guidance to the lesion and its margins based on preoperative imaging data. These techniques have become the standard of care for resection of large tumors and lesions that reside below the cortex. When tumors arise near critical cortical and subcortical regions that serve motor, language, and vision, diffusion tensor imaging and functional MRI ( Fig. 4 ) are often obtained before surgery to illustrate the topographic relationship between these areas and the tumor.