In the United States, approximately 65,000 people are diagnosed with primary brain tumors each year, with an incidence of 19.3 cases per 100,000 person-years. These numbers represent a wide spectrum of disease, from benign to malignant, and prognosis varies widely based on disease. Treatment of primary brain tumors most often uses a combination of surgery and radiation. However, over the past several generations, technological advancements have significantly altered the treatment paradigm. This article reviews the current role of neurosurgery and radiation therapy in the management of primary brain tumors.
- •
The basic goals of any intracranial tumor surgery are to safely obtain a tissue diagnosis and, when possible, to totally remove the tumor.
- •
Recent advances in imaging techniques (functional magnetic resonance imaging, diffusion tensor imaging, intraoperative magnetic resonance imaging) have helped to maximize extent of resection and preserve neurologic function.
- •
Radiation therapy seeks to open a therapeutic window between damage to the tumor and damage to normal brain.
- •
Improvements in targeting and treatment planning technologies have allowed for more conformal radiotherapy, with sparing of normal brain tissue.
Introduction
Management of primary brain tumors requires a multidisciplinary approach that includes input from both neurosurgeons and radiation oncologists. Over the past several decades, advances in surgical and radiation techniques have improved outcomes for patients with brain tumors. In this article, the role of neurosurgery and radiation therapy in the management of the most common primary brain tumors is described. General treatment principles, tumor-specific paradigms, and ongoing and future clinical trials are discussed.
Neurosurgery
Basic Surgical Principles
Surgical approach varies widely based on tumor type, location, and size. Surgical options are also heavily influenced by the patient’s overall clinical status, including Karnofsky Performance Score, age, and the presence of medical comorbidities. The basic goals of any intracranial tumor surgery are to safely obtain a tissue diagnosis and, when possible, to totally remove the tumor. When a total removal is not possible, surgical intervention allows for decompression and relief of mass effect, with consequent improvement in neurologic status. Surgical resection can result in significant cytoreduction, thereby reducing tumor burden and enhancing the efficacy of adjuvant therapy.
Technological Advances
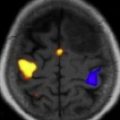
Current neurosurgical practice uses many technological advances to improve outcome. Central to this improvement are advancements in magnetic resonance imaging (MRI) technologies. Improved MRI allows for high-resolution delineation of critical anatomic, functional, and metabolic regions. Specifically, functional imaging with MRI (fMRI) has been used to identify primary cortical regions that subserve motor/sensory capacity, language, and vision ( Fig. 1 ). In addition, diffusion tensor imaging (DTI) tractography can accurately delineate the corticospinal tract, arcuate fasciculus, or optic radiations ( Fig. 2 ). This technology has significant implications for operative planning; more aggressive surgical resections can be accomplished and neurologic function preserved.
Advances in MRI have also translated to improvements in intraoperative stereotactic guidance. This allows the surgeon to precisely localize their operative position and facilitates both lesion resection and biopsy.
The development of intraoperative MRI (iMRI) has further increased the surgeon’s operative options. Use of iMRI allows for correction of brain shifts, detection of residual tumor, and earlier identification of potential complications.
Tumor-specific Management: Surgery
High-grade gliomas
Malignant gliomas have an annual incidence of approximately 5 cases per 100,000 people. Amongst malignant gliomas approximately 60% to 70% are glioblastomas, 10% to 15% are anaplastic astrocytomas, and 10% are anaplastic oligodendrogliomas or oligoastrocystomas ( Fig. 3 ).
Irrespective of disease, the surgical goals for all malignant gliomas are the same: establishing a tissue diagnosis and cytoreduction and preserving or improving neurologic status. Although no prospective, randomized trials exist, dozens of studies have retrospectively examined the effect of extent of resection on survival for patients with malignant gliomas. Most of these studies have reported that greater extent of resection independently confers a survival benefit.
Many malignant gliomas are located in eloquent regions of the brain, which are not easily amenable to surgical resection. However, advances in fMRI and DTI have improved the surgeon’s ability to operate in these areas. By combining these imaging modalities with traditional intraoperative stimulation for cortical and subcortical mapping, extent of resection can be safely maximized.
Recent improvements in technology have also aided the surgeon to achieve maximal surgical resection. Specifically, the use of 5-aminolevulinic acid (an agent that accumulates as fluorescent porphyrins in portions of the tumor that contain an impaired blood-brain barrier) has been shown to increase extent of resection. In addition, the presence of residual tumor has decreased in cases that use iMRI.
Thus, all patients with surgically accessible malignant gliomas should undergo maximal, safe surgical resection, and evolving technologies should be used as needed to achieve this goal. Adjuvant therapy consists of chemotherapy and radiation; the specifics of these treatments are based on tumor pathology.
Low-grade gliomas
Low-grade gliomas (LGGs) encompass a heterogeneous group of World Health Organization (WHO) grade I and II gliomas and account for approximately 15% of all primary brain tumors. The management of WHO grade I and II LGGs is different ( Fig. 4 ).
Most WHO grade I lesions (pilocytic astrocytoma, subependymal giant cell astrocytoma, pleomorphic xanthoastrocytoma, subependymoma) are well demarcated from surrounding brain tissue and complete surgical resection is curative. Surgical resection is the gold standard; after resection, patients should be monitored with surveillance imaging.
WHO grade II lesions (diffuse astrocytomas, oligodendrogliomas, oligoastrocytomas) are molecularly distinct from grade I lesions and have a more invasive phenotype. However, they do not have the anaplastic features of a grade III lesion and in turn have a more favorable prognosis. Surgical resection is needed to ensure proper diagnosis and subsequent treatment. In addition, multiple studies have reported that extent of resection is associated with survival in patients with grade II LGGs. Hence, the surgical goal for all patients with grade II LGGs is gross total resection; for those lesions located in eloquent areas of the brain, the technologies discussed earlier are often used to achieve this goal. Adjuvant therapy is determined based on histopathology and molecular markers.
Ependymomas
Ependymomas are glial-based tumors that arise from the ependymal lining of the ventricular system ( Fig. 5 ). They are rare intracranial tumors and occur more commonly in children than adults. Most often, intracranial ependymomas are intimately associated with the ventricles, although ependymomas may occasionally occur at sites distant from the ventricular system. Surgical resection is critical to treatment; several studies have reported that extent of resection is associated with progression-free and overall survival. Thus, if complete resection is possible, this should be achieved. In addition, surgical resection can often restore normal cerebrospinal fluid (CSF) outflow in the case of an obstructing mass lesion, and this should be attempted. Given the propensity of this tumor to disseminate via CSF, staging of the entire neuroaxis is necessary to determine a comprehensive treatment plan. Need for adjuvant therapy is determined by tumor grade (WHO II vs III) and the presence of disseminated disease.
Pineal tumors
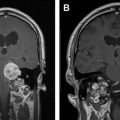
Pineal region tumors represent a heterogeneous group of lesions, ranging from benign to malignant. Overall, these are rare tumors, which occur more commonly in children than adults. Given their location deep within the brain, surgical resection of pineal region tumors is challenging ( Fig. 6 ). Before the advent of microsurgical techniques, surgical mortality was upwards of 50%. In current practice, with the use of the operative microscope and improvements in neuroanesthesia, the operative mortality has been reduced to less than 5%.
Surgical approach varies widely with disease; however, a few basic principles exist when addressing any pineal region tumor. First, these lesions often cause obstructive hydrocephalus secondary to compression of the aqueduct of Sylvius. To correct this condition, an endoscopic third ventriculostomy can be performed (preferably) or a ventriculoperitoneal shunt can be placed. Second, tissue diagnosis is required to direct further treatment; this can be achieved via endoscopic, stereotactic, or open biopsy. CSF and serum tumor markers can also be sent to aid in pathologic diagnosis. If test results show a pure germinoma, surgical resection is not required because these tumors can be effectively treated with radiation alone. For all other lesions (nongerminomatous germ cell tumors, gliomas, pineal-parenchymal tumors, dermoids/epidermoids, meningiomas), maximal, safe surgical resection should be achieved, followed by adjuvant therapy (chemotherapy/radiation) for malignant lesions and surveillance imaging for benign lesions.
Primary central nervous system lymphoma
A rare variant of non-Hodgkin lymphoma, primary central nervous system lymphoma (PCNSL) can affect any part of the neuroaxis. In contrast to most primary brain tumors, PCNSL is more sensitive to chemotherapy, corticosteroids, and radiation. Radiographically, PCNSL tends to present as an infiltrative, homogeneously enhancing lesion on MRI ( Fig. 7 ). Nonetheless, PCNSL can have highly variable imaging features and is known to mimic the appearance of many other intracranial lesions. Because of its highly infiltrative nature, complete surgical resection is not feasible. Even large lesions with symptomatic mass effect respond well to medical therapies. Therefore, surgical involvement in disease management is limited to stereotactic biopsy, to establish a histopathologic diagnosis. In addition, if there is evidence of leptomeningeal spread and a desire to deliver intrathecal chemotherapy, surgical involvement may extend to placement of an Ommaya reservoir.
Meningiomas
Meningiomas are common, predominantly benign intracranial tumors that arise from arachnoid cap cells ( Fig. 8 ). Surgical resection is the mainstay of treatment and is used for symptomatic lesions or those with rapid growth on surveillance imaging. Studies have shown that lesions greater than 2.5 cm in initial diameter and tumors with linear growth rates of more than 10% per year are most likely to cause progressive neurologic symptoms and require surgical intervention. More than 90% of meningiomas are histologically benign WHO grade I lesions; the remainder are grade II (atypical) or grade III (anaplastic). For grade I lesions, complete surgical resection, when achieved, is curative. Adjuvant therapy (primarily radiotherapy) may be used for incompletely resected lesions, WHO grade II or III lesions, lesions that are not easily amenable to surgical resection, or patients who are poor surgical candidates.
Vestibular schwannomas
Vestibular schwannomas are benign lesions arising from Schwann cells that myelinate the vestibular portion of the eighth cranial nerve ( Fig. 9 ). Because of their location in the cerebellopontine angle, surgical treatment of these lesions is technically challenging. Historically, surgery for vestibular schwannomas has been associated with high rates of morbidity and mortality. However, advances in microsurgical techniques have significantly reduced this risk. In current practice, surgical morbidity is most often related to CSF leak and postoperative cranial neuropathy (particular the facial nerve). Despite these risks, microsurgical resection is required for large lesions that exert mass effect on the brainstem. In addition, microsurgery is the most durable treatment strategy for vestibular schwannomas; less than 2% of patients require additional treatments at an average follow-up of 5 years. Nonetheless, for small lesions without associated mass effect, radiotherapy (particularly stereotactic radiosurgery [SRS]) plays an increasingly importantly role in management, as discussed later.
Pituitary tumors
Most pituitary tumors are benign and are generally classified as nonsecreting or secreting ( Fig. 10 ). Surgical resection is the primary treatment of all pituitary adenomas, with the exception of prolactin-secreting tumors, which respond well to medical management. Current surgical practice relies heavily on advancements in both the surgical endoscope and neuronavigation. For secreting adenomas, large reviews have consistently reported hormonal-remission rates of more than 70% after surgical intervention. With respect to nonsecreting macroadenomas, if gross-total resection is achieved, surgical intervention is curative. Nonetheless, although surgery is required to reduce mass effect from large pituitary lesions (which often compress the optic chiasm), gross-total resection is not always possible. For example, if there is tumor infiltration of the cavernous sinus, it is not advisable to resect these portions of the tumor. In the event of residual tumor (based on either MRI or hormonal studies), SRS is commonly used. Early studies suggest that SRS provides adequate tumor control and hormonal stabilization; however, the long-term durability of this strategy is not yet known.
Brain metastases
A complete discussion of brain metastases is beyond the scope of this review; however, there are a few well-established surgical principles ( Fig. 11 ). In the 1990s, a series of pivotal studies by Patchell and colleagues determined that patients with good functional status and solitary intracranial metastases should undergo surgical resection. In addition, surgery may be required to (1) alleviate mass effect from large, symptomatic lesions, (2) treat hydrocephalus via placement of a ventriculoperitoneal shunt, or (3) establish a tissue diagnosis if a primary cannot be found.
Neurosurgery
Basic Surgical Principles
Surgical approach varies widely based on tumor type, location, and size. Surgical options are also heavily influenced by the patient’s overall clinical status, including Karnofsky Performance Score, age, and the presence of medical comorbidities. The basic goals of any intracranial tumor surgery are to safely obtain a tissue diagnosis and, when possible, to totally remove the tumor. When a total removal is not possible, surgical intervention allows for decompression and relief of mass effect, with consequent improvement in neurologic status. Surgical resection can result in significant cytoreduction, thereby reducing tumor burden and enhancing the efficacy of adjuvant therapy.
Technological Advances
Current neurosurgical practice uses many technological advances to improve outcome. Central to this improvement are advancements in magnetic resonance imaging (MRI) technologies. Improved MRI allows for high-resolution delineation of critical anatomic, functional, and metabolic regions. Specifically, functional imaging with MRI (fMRI) has been used to identify primary cortical regions that subserve motor/sensory capacity, language, and vision ( Fig. 1 ). In addition, diffusion tensor imaging (DTI) tractography can accurately delineate the corticospinal tract, arcuate fasciculus, or optic radiations ( Fig. 2 ). This technology has significant implications for operative planning; more aggressive surgical resections can be accomplished and neurologic function preserved.
Advances in MRI have also translated to improvements in intraoperative stereotactic guidance. This allows the surgeon to precisely localize their operative position and facilitates both lesion resection and biopsy.
The development of intraoperative MRI (iMRI) has further increased the surgeon’s operative options. Use of iMRI allows for correction of brain shifts, detection of residual tumor, and earlier identification of potential complications.
Tumor-specific Management: Surgery
High-grade gliomas
Malignant gliomas have an annual incidence of approximately 5 cases per 100,000 people. Amongst malignant gliomas approximately 60% to 70% are glioblastomas, 10% to 15% are anaplastic astrocytomas, and 10% are anaplastic oligodendrogliomas or oligoastrocystomas ( Fig. 3 ).
Irrespective of disease, the surgical goals for all malignant gliomas are the same: establishing a tissue diagnosis and cytoreduction and preserving or improving neurologic status. Although no prospective, randomized trials exist, dozens of studies have retrospectively examined the effect of extent of resection on survival for patients with malignant gliomas. Most of these studies have reported that greater extent of resection independently confers a survival benefit.
Many malignant gliomas are located in eloquent regions of the brain, which are not easily amenable to surgical resection. However, advances in fMRI and DTI have improved the surgeon’s ability to operate in these areas. By combining these imaging modalities with traditional intraoperative stimulation for cortical and subcortical mapping, extent of resection can be safely maximized.
Recent improvements in technology have also aided the surgeon to achieve maximal surgical resection. Specifically, the use of 5-aminolevulinic acid (an agent that accumulates as fluorescent porphyrins in portions of the tumor that contain an impaired blood-brain barrier) has been shown to increase extent of resection. In addition, the presence of residual tumor has decreased in cases that use iMRI.
Thus, all patients with surgically accessible malignant gliomas should undergo maximal, safe surgical resection, and evolving technologies should be used as needed to achieve this goal. Adjuvant therapy consists of chemotherapy and radiation; the specifics of these treatments are based on tumor pathology.
Low-grade gliomas
Low-grade gliomas (LGGs) encompass a heterogeneous group of World Health Organization (WHO) grade I and II gliomas and account for approximately 15% of all primary brain tumors. The management of WHO grade I and II LGGs is different ( Fig. 4 ).
Most WHO grade I lesions (pilocytic astrocytoma, subependymal giant cell astrocytoma, pleomorphic xanthoastrocytoma, subependymoma) are well demarcated from surrounding brain tissue and complete surgical resection is curative. Surgical resection is the gold standard; after resection, patients should be monitored with surveillance imaging.
WHO grade II lesions (diffuse astrocytomas, oligodendrogliomas, oligoastrocytomas) are molecularly distinct from grade I lesions and have a more invasive phenotype. However, they do not have the anaplastic features of a grade III lesion and in turn have a more favorable prognosis. Surgical resection is needed to ensure proper diagnosis and subsequent treatment. In addition, multiple studies have reported that extent of resection is associated with survival in patients with grade II LGGs. Hence, the surgical goal for all patients with grade II LGGs is gross total resection; for those lesions located in eloquent areas of the brain, the technologies discussed earlier are often used to achieve this goal. Adjuvant therapy is determined based on histopathology and molecular markers.
Ependymomas
Ependymomas are glial-based tumors that arise from the ependymal lining of the ventricular system ( Fig. 5 ). They are rare intracranial tumors and occur more commonly in children than adults. Most often, intracranial ependymomas are intimately associated with the ventricles, although ependymomas may occasionally occur at sites distant from the ventricular system. Surgical resection is critical to treatment; several studies have reported that extent of resection is associated with progression-free and overall survival. Thus, if complete resection is possible, this should be achieved. In addition, surgical resection can often restore normal cerebrospinal fluid (CSF) outflow in the case of an obstructing mass lesion, and this should be attempted. Given the propensity of this tumor to disseminate via CSF, staging of the entire neuroaxis is necessary to determine a comprehensive treatment plan. Need for adjuvant therapy is determined by tumor grade (WHO II vs III) and the presence of disseminated disease.
Pineal tumors
Pineal region tumors represent a heterogeneous group of lesions, ranging from benign to malignant. Overall, these are rare tumors, which occur more commonly in children than adults. Given their location deep within the brain, surgical resection of pineal region tumors is challenging ( Fig. 6 ). Before the advent of microsurgical techniques, surgical mortality was upwards of 50%. In current practice, with the use of the operative microscope and improvements in neuroanesthesia, the operative mortality has been reduced to less than 5%.