Abstract
Male breast cancer (MBC) is a rare disease, accounting for 1% of all breast cancers. Because of its rarity, it is treated similar to female breast cancer, although important differences exist. This chapter will address risk factors associated with MBC, clinical and pathologic features specific to MBC, and treatment and prognosis. Survivorship issues and surveillance recommendations unique to MBC will also be discussed.
Keywords
male breast cancer, risk factors, diagnosis, treatment, survivorship
Epidemiology
Male breast cancer (MBC) is a rare disease worldwide. As a result of its rarity, it is treated similarly to female breast cancer, but important differences exist. In the United States, it was estimated that 2600 men were diagnosed with breast cancer and 440 died from this disease in 2016. MBC accounts for less than 1% of all breast cancers and less than 0.5% of all male cancer deaths in the United States. Globally, the highest male incidence rate was observed in Israel at 1.24 per 100,000 man-years followed closely by the Philippines, Italy, and France. The lowest male incidence rate was recorded in Thailand at 0.16 per 100,000 man-years followed by Japan, Singapore, and Colombia.
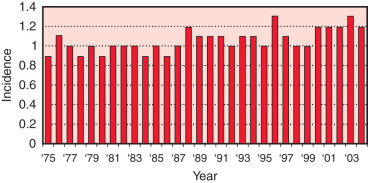
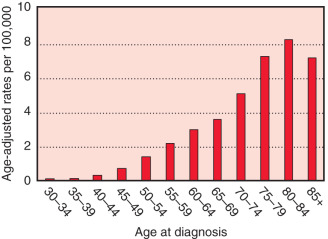
The worldwide female-to-male incidence rate ratio of breast cancer is 122 : 1. MBC compared with female breast cancers occur later in life with higher stage, higher grade, and more estrogen receptor (ER)-positive tumors. The median age of onset of MBC is 72 years of age, compared with 61 years in women. According to the US Surveillance, Epidemiology, and End Results (SEER) registry database, the incidence rates of MBC were slightly increasing from 1975 to 2004 (0.9–1.2 cases per 100,000 men at risk). A rapid increase in female breast cancer incidence was observed in the mid-1970s to mid-1990s in the United States and Europe largely due to the greater use of mammographic screening. Mortality rates in the late 1980s and 1990s tended to be lower than 3 decades earlier, likely owing to advances in diagnostics and therapeutics. The most recent SEER database analysis shows a decrease in breast cancer incidence and mortality in both men and women, but the trends were greater for women. Comparing patients diagnosed from 1996 to 2005 versus 1976 to 1985 and adjusting for age, stage, and grade, MBC death declined by 28% among men and 42% among women ( Figs. 76.1 and 76.2 ).
Racial/ethnic differences also exist. In the United States the ratio of female-to-male breast cancer is approximately 100 : 1 in whites and 70 : 1 in blacks. Age-adjusted incidence rates per 100,000 men are highest in blacks (1.65), intermediate in whites (1.31), and lowest in Hispanics (0.68) and Asian/Pacific Islanders (0.66). Blacks are also diagnosed at an earlier age and at a more advanced stage compared with other ethnicities. Similar to black women, black men have an increased breast cancer–specific mortality even after adjustment for clinical, demographic, and treatment factors.
The distribution of tumor subtypes is also different across racial/ethnic groups. In the largest population-based study evaluating breast tumor subtypes in 606 patients with MBC, 82.8% of white men (95% confidence interval [CI] 79.3%–86.4%) had hormone receptor–positive tumors, 14.6% had HER2-positive tumors (95% CI 11.3%–18%), and 2.6% had triple-negative breast cancer (95% CI 1.1%–4%). In contrast, among blacks, 73.3% had hormone receptor–positive tumors (95% CI 60.4%–86.3%), 17.8% had HER2-positive tumors (95% CI 6.6%–29%), and 8.9% had triple-negative tumors (95% CI 0.6%–17.2%); among Hispanics, 77.6% had hormone receptor–positive tumors (95% CI 67.6%–87.6%), 16.4% had HER2-positive tumors (95% CI 7.6%–27.5%), and 6% had triple-negative tumors (95% CI 0.3%–11.6%). Among the patients with hormone receptor–positive tumors, black and Hispanic men were more likely to have progesterone receptor (PR)-negative tumors than white men. No statistically significant differences in survival were observed according to tumor subtype ( p = .08). Among hormone receptor–positive patients, blacks experienced the worse survival.
Risk Factors
Several risk factors are associated with the development of MBC, including endocrine, nutritional, and genetic factors ( Box 76.1 ). In a large retrospective review of a US Veterans Affairs database assessing 642 cases of MBC, conditions associated with increased risk of MBC included diabetes, orchitis/epididymitis, Klinefelter syndrome, and gynecomastia. Among blacks, cholelithiasis emerged as a significant risk predictor. A large prospective study found family history, history of bone fracture, obesity, and low physical activity to be positively associated with MBC. Some of these identified risk factors are common to female breast cancer and suggest an importance of hormonal mechanisms.
Endocrine
Gynecomastia
Testicular conditions
Liver disease
Diabetes mellitus
Nutritional/lifestyle
Obesity
Low physical activity
Alcohol use
Genetic
Family history
Klinefelter syndrome
BRCA carrier ( BRCA2 > BRCA1 )
Cowden syndrome
Li-Fraumeni syndrome
Hereditary nonpolyposis colorectal cancer (Lynch) syndrome
The strongest risk factor for MBC is Klinefelter syndrome. This rare condition results from the inheritance of an additional X chromosome (XXY). Men with this condition have atrophic testes, gynecomastia, high serum levels of gonadotropins (follicle-stimulating hormone, luteinizing hormone), and low plasma levels of testosterone. It is hypothesized that the increased estrogen-to-testosterone ratio could, in turn, lead to abnormal hormonal stimulation of cell proliferation in mammary ductal epithelium. Of the few epidemiologic studies conducted in this area, the largest cohort study of 3518 men with cytogenetically diagnosed Klinefelter syndrome found 19- and 58-fold increases in incidence of and mortality from MBC, respectively, compared with the general population. Alteration of hormone levels, particularly the elevated ratio of estrogen-to-testosterone, administration of exogenous androgens, gynecomastia, and genetic factors are possible explanations for the high risk. Additional studies are needed to delineate which patients with Klinefelter syndrome are at increased risk for MBC, and the importance of patient education, self-examination, and regular examinations should be enforced.
Chronic liver diseases such as cirrhosis, chronic alcohol injury, and schistosomiasis have been associated with an increased risk of MBC. Cirrhosis limits the ability of the liver to metabolize endogenously produced estrogen, leading to a relative hyperestrogenic state with an imbalance in the estrogen-to-testosterone ratio. Similarly, ethanol, which has been associated with an increased risk of breast cancer in females, is a metabolic modifier for mammary epithelium and may promote the most carcinogenic pathway of estradiol metabolism to catechol estrogen. Very few cases of MBC have been documented in patients with chronic liver diseases, possibly due to the shortened life span associated with these disorders. Results from some studies have not found an association between liver cirrhosis and MBC.
Gynecomastia, when related to states of estrogen excess, has been associated with MBC. Gynecomastia is most often drug related, and several medications that cause gynecomastia have been associated with an increased risk of MBC. Breast cancer has been described in three men who were prescribed finasteride, a drug approved for the treatment of benign prostatic hyperplasia. Cases of MBC have also been reported with digoxin, thioridazine, and spironolactone, and in male-to-female transsexuals who were castrated and given high doses of estrogen. Testicular conditions have also been associated with an increased risk of MBC. These include orchitis, undescended testis (cryptorchidism), and testicular injury. Other conditions associated with an increased estrogen-to-testosterone ratio such as thyroid disease and marijuana use have not firmly established a link to MBC.
Experimental evidence suggests that prolactin may promote tumorigenesis in animal models; however, physiologic states of prolactin excess in humans (e.g., multiple pregnancies) do not confer an increased risk of breast cancer and may be protective. Several case reports have described the development of MBC in association with a prolactinoma, a setting in which low plasma testosterone levels are often observed. The association between prolactin excess and MBC remains unclear.
Androgens may convey a protective effect by inhibiting cell proliferation in breast tissue. In some reports, mutations in the DNA-binding domain of the androgen receptor (AR) gene have been implicated in the development of MBC. Conversely, a pathologic case study that analyzed tumor material from two series of patients with MBC without clinical evidence of androgen insensitivity reported no AR gene mutations. In a study of 43 MBC patients, AR expression by immunohistochemistry inversely correlated with survival.
Approximately 5% to 10% of female breast cancer cases are thought to be hereditary, with the majority of these cases associated with mutations in two genes: breast cancer type 1 and 2 susceptibility genes ( BRCA1 and BRCA2 ). These genes are inherited in an autosomal dominant pattern and confer a lifetime risk of female breast cancer ranging from 50% to 85%. Approximately 15% to 20% of MBC is associated with a positive family history for the disease compared with only 7% of the general male population.
BRCA2 mutations are more frequent than BRCA1 mutations. In an Italian series of 50 BRCA carriers, 92% harbored the BRCA2 mutation compared with 8% with the BRCA1 mutation. Inherited mutations in BRCA do not increase the risk of breast cancer to the same degree in males as in females. The breast cancer risk also appears to be higher with BRCA2 mutations as opposed to BRCA1. Men who carry a BRCA2 mutation have an approximate 6.5% cumulative risk for breast cancer by age 70, which is 100-fold higher than the general male population. A paucity of data exists correlating the risk for MBC in BRCA1 carriers. One Dutch and one American family have been described that carried the BRCA1 mutation; each had one case of MBC as well as multiple associated female breast cancer cases. A report from the National Cancer Institute Cancer Genetics Network suggests that the cumulative risk of breast cancer by age 70 in men harboring a BRCA1 mutation is 1.2% (95% CI 0.22%–2.8%) ( Fig. 76.3 ).
The classification of molecular subtypes based on immunohistochemical profiles as proposed in female breast cancer is still controversial in MBC. In one report of 382 MBC cases including 50 BRCA carriers, the immunophenotypic profiles differed between 4 BRCA1 – and 19 BRCA2 -asssociated patients, in whom complete ER, PR, and HER2 status were available. Of the 4 BRCA1 -related MBC cases, 3 showed a luminal A subtype, and 1 a triple-negative tumor. Of the 19 BRCA2 -related MBCs, 7 were luminal A, 9 luminal B, and 3 HER2-positive. Notably, all 7 triple-negative tumor cases were BRCA2 -mutation negative. In a multivariate logistic model, BRCA2 -associated MBCs showed positive association with high tumor grade (odds ratio [OR] 4.9, 95% CI 1.0–23.9) and inverse association with PR expression (OR 0.19, 95% CI 0.04–0.92), suggesting that the BRCA2 subgroup is characterized by a more aggressive phenotype.
Genetic mutations other than BRCA may predispose males to developing breast cancer. Cowden syndrome, an autosomal dominant cancer susceptibility syndrome, is associated with germline mutations in the tumor suppressor gene PTEN located on chromosome 10. This syndrome is characterized by multiple hamartomas and an increased risk for both male and female breast cancer and thyroid malignancies. Two cases of MBC have been reported with germline PTEN mutations and the Cowden syndrome phenotype. Other hereditary syndromes associated with MBC include Li-Fraumeni syndrome, caused by the TP53 mutation, and hereditary nonpolyposis colorectal cancer (Lynch) syndrome, caused by mutations in the mismatch repair genes.
Recent genome-wide association studies (GWAS) identified common single nucleotide polymorphisms (SNPs) that influence female breast cancer risk. A GWAS study of MBC compromising 823 MBC cases and 2795 controls were genotyped and validated in an independent sample set, and the SNP RAD51B was found to be significantly associated with MBC risk. Other studies have found genetic variants that influence susceptibility to breast cancer, which differ between male and female breast cancer.
Guidelines from the National Comprehensive Cancer Network (NCCN) recommend that genetic testing be offered to men who develop breast cancer as well as to families with a known BRCA mutation, a case of MBC, or the presence of female relatives with a history of breast or ovarian cancer that suggests the presence of an inherited breast or ovarian cancer syndrome. Furthermore, adherence to recommended screening guidelines for prostate cancer is advised because males with BRCA2 mutations have an elevated risk of prostate cancer.
Clinical Features
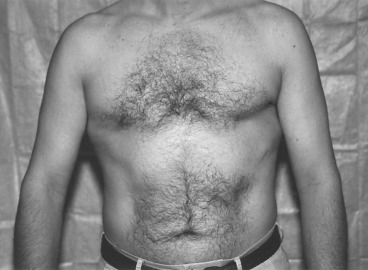
Similar to women, MBC typically presents as a painless lump. The mass is usually subareolar and less often in the upper outer quadrant. A slight predilection exists for the left breast. Nipple involvement is a fairly early event, occurring in 40% to 50%, with retraction in 9%, discharge in 6%, and ulceration in 6%. Bilateral MBC is rare with a reported incidence of 1.5% to 2% of all MBCs. Infrequently, MBC presents as an axillary nodal metastasis without a palpable breast lump. Other findings on examination for malignancy include fixation to skin or muscle and breast tenderness.
The majority of breast lesions in males are benign, with gynecomastia as the most common etiology. Other pathologic lesions in the male breast are related to the cutaneous and subcutaneous tissue and can include lipoma, breast abscess, metastatic lesion to the breast, and other primary malignancies such as sarcoma. Gynecomastia has been found in up to 55% of male breasts in a series of autopsy specimens. As opposed to MBC, gynecomastia usually presents as bilateral, symmetric breast enlargement with irregular borders in the absence of axillary lymphadenopathy or fixation to the chest wall. On mammography, MBC is usually subareolar and eccentric to the nipple. In contrast, gynecomastia appears as a round or triangular area of increased density positioned symmetrically in the retroareolar region. Calcifications are rarer and coarser than those occurring in female breast cancer. Because of the low incidence of MBC in the general population, there is no role for screening mammograms in men. One report described a male BRCA2 carrier who was diagnosed with breast cancer by screening mammography; clear guidelines have not been established for this population.
Diagnosis
The first step in the evaluation of a suspicious breast mass in a male is mammography. A mammogram can usually distinguish between malignancy and gynecomastia and is abnormal in 80% to 90% of MBCs. Mammographic features of malignancy include a dense mass generally without calcifications and often with spiculated, indistinct, or microlobulated margins. Sonography usually reveals an irregularly shaped hypoechoic mass, as seen in female breast cancers. Any cysts that are discovered on imaging should be sampled because simple cysts are rare in men and are associated with neoplastic papillary lesions. Likewise, radiologic features such as a well-defined lesion that would suggest a benign finding in a female are unreliable in men and require biopsy. In one series, MBC was manifested as a well-defined mass in 15% of cases using mammography and in 23% using sonography.
Several studies have suggested that mammography added no diagnostic information to the combination of physical examination and pathologic evaluation. In one retrospective analysis of 134 male patients with a history of a breast lump between 2001 and 2003 and undergoing mammographic imaging, only four cases of breast cancer were diagnosed. All four patients presented with a painless lump, for a mean duration of 7 months, in whom breast cancer was suspected by clinical examination and confirmed by biopsy. The use of breast MRI has not been widely studied in MBC, and no prospective data exist for its use in screening or diagnosis of MBC.
Once a suspicious breast mass is identified, biopsy is required to confirm the diagnosis and to assay for ER, PR, and HER2 status. Fine-needle aspiration (FNA) is a reliable procedure, which has been shown to avoid surgical biopsy in 59% of cases. However, in one report of 153 FNAs of the male breast, 13% did not provide sufficient tissue for diagnosis. Compared with FNA, core needle biopsy offers a more definitive histologic diagnosis, avoids inadequate samples, and usually distinguishes between invasive and in situ cancer.
Pathology
The most common histopathologic type of MBC is invasive ductal carcinoma, similar to female breast cancer, and accounts for 85% of all MBC cases. Conversely, invasive lobular carcinoma is much less frequent in males compared with females, constituting only 1.7% of MBCs. The rarity of lobular carcinoma in males may be due to the lack of acini and lobules in normal male breast tissue. The very rare cases of lobular carcinoma have occurred in men with Klinefelter syndrome, transsexuals taking estrogens, and men with prostate cancer. Ductal carcinoma in situ (DCIS) accounts for 20% to 25% of all cases of female breast cancer. In contrast, the frequency of DCIS in men ranges from 0% to 17%, with an average of 7%. Few case reports of lobular carcinoma in situ (LCIS) exist in the published literature. Paget disease of the breast has a higher incidence in males (5%) compared with females (1%–4%) and appears to have a worse 5-year survival in men. Likewise, invasive papillary carcinoma is more common in males (2%–4%) than in females (1%). All other subtypes of breast cancer, including inflammatory breast cancer, have been reported in men.
Immunohistochemical and molecular characteristics of MBC have shown a greater incidence of hormone receptor positivity and significantly less frequent overexpression of HER2/neu. In females with breast cancer, tumors are ER-positive in 77% of patients compared with 92% of ER-positive tumors in males. The incidence of HER2 overexpression is only 2% to 15% in MBC; approximately 18% to 20% of all female breast cancers overexpress HER2.
More information has become available regarding molecular markers in MBC. In a series of 134 cases of MBC, tumor samples were analyzed for ER, PR, HER2, AR, the proto-oncogene p53, the cell cycle regulatory protein cyclin D1, a marker of apoptosis bcl-2, among others. According to immunohistochemically defined molecular subtypes, the vast majority of cases were classified as luminal A (75%), whereas 21% of tumors were luminal B. No HER2-driven cases were identified; all HER2-positive cases (3%) showed ER positivity and were classified as luminal B. The remaining 4% of cases were basal-like. Expression of AR (81%), bcl-2 (75%), and cyclin D1 (77%) were very common, in line with previous studies. Approximately half of the tumors were positive for p21 (48%) and BRST2 (56%). p21, the most important downstream effector of p53, is a universal cyclin/cyclin-dependent kinase inhibitor which inhibits proliferation and has been associated with worse disease-free survival. Overexpression of p21 has been seen more frequently in MBC than in female breast cancer. In contrast, p53 accumulation (15%) was rare, somewhat lower than other reports in which up to 54% of samples were p53-positive. Expression of the basal markers CK5/6 (9%), CK14 (1%), and EGFR (12%) were encountered infrequently. This study also elucidated the clinical relevance of several biomarkers in MBC. PR-positive and bcl-2-positive tumors showed favorable histologic features, whereas HER2-positive, ki67-positive, and p21-positive tumors correlated with higher grade and mitotic count. p53 and BRST2 significantly predicted the presence of lymph node metastases, and PR negativity and p53 accumulation emerged as independent predictors of decreased survival.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree