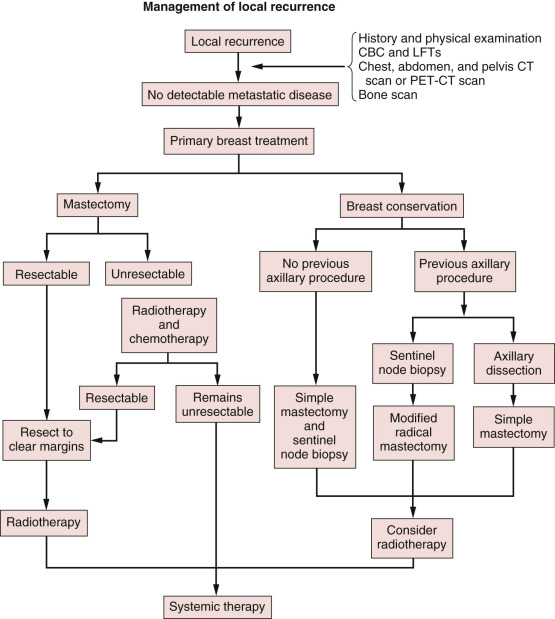
Abstract
As the number of breast cancer survivors increases, more women are at risk for locoregional recurrence of their initial tumor or the growth of a new primary breast cancer. Clinicians managing these patients should follow published breast cancer–specific and general cancer survivorship guidelines. Recent reports suggest that reducing the rate of locoregional failure improves overall survival. Gene expression studies have indentified several breast cancer subtypes that correlate with prognosis, response to therapy, and recurrence. Patients experiencing a locoregional recurrence require comprehensive multidisciplinary planning and treatment. Prior staging procedures, local treatments, and systemic treatments significantly influence the multimodal managment of these patients. Complete excision with negative margins and radiation therapy, if possible, is the preferred local therapy. Rarely, radical surgery and reconstruction may be required to achieve tumor-free margins. The type, dose, duration, and response to any initial systemic therapy must be carefully reviewed in the treatment planning of these patients. Ideally, all these patients should be presented at a multidisciplinary breast cancer conference for comprehensive multimodal treatment planning.
Keywords
locoregional recurrence, mastectomy, breast conservation, augmented breast
As the number of women undergoing treatment for breast cancer increases, more patients will experience local recurrence or develop a second primary breast cancer. The exponential increase in breast augmentation has led to more women potentially developing cancer in a previously augmented breast requiring a unique management approach. After mastectomy or breast conserving surgery, recurrence of disease can be local, regional or distant.
Local recurrence refers to reappearance of the original cancer in the ipsilateral treated breast or chest wall, whereas regional recurrence implies tumor recurring in the regional lymph nodes such as the ipsilateral axillary, supra/infraclavicular, or internal mammary lymph nodes.
This chapter addresses complex conditions requiring comprehensive multidisciplinary planning and treatment, such as locoregional recurrence (LRR) after mastectomy with or without reconstruction, LRR after breast conserving therapy (BCT), breast cancer in the augmented breast and breast cancer risk in the contralateral breast.
Locoregional Recurrence After Mastectomy
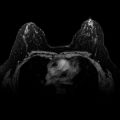
Although most women with breast cancer can be treated with BCT, some women still require or desire mastectomy. Locoregional recurrence after mastectomy refers to the reappearance of breast cancer in the skin flaps, in the mastectomy scar on the chest wall, or in the ipsilateral regional lymphatics (axillary, internal mammary, and supraclavicular lymph nodes). Locoregional postmastectomy recurrences are usually detected by physical examination and they represent a heterogenous group of lesions ranging from a small, solitary tumor nodule in the surgical scar to diffuse carcinoma en cuirass involving the entire chest wall and regional lymphatics ( Fig. 77.1 ).
In the last several decades, the number of breast cancer survivors has increased due to earlier diagnosis and more effective treatments. It is estimated that there are over 3.5 million women living in the United States with a history of breast cancer and approximately one-quarter million women will be newly diagnosed this year. There remains, however, considerable controversy regarding the appropriate follow-up after the initial breast cancer treatment. Logically, one would expect that intensive posttreatment surveillance would lead to earlier detection of recurrences and improved survival. However, expensive diagnostic tests to detect occult metastatic disease, such as serum markers, computed tomography (CT), magnetic resonance imaging (MRI), and positive emission tomography scans have not been shown to improve survival. Recent versions of the American Cancer Society, American Society of Clinical Oncology, and National Comprehensive Cancer Network (NCCN) guidelines for follow-up of breast cancer recommend that asymptomatic patients be followed with a detailed history, physical examination, and mammography. Furthermore, a recent Cochrane review examined all randomized controlled trials evaluating the effectiveness of follow-up strategies after the primary treatment of breast cancer. The findings of this review confirmed the conclusions of an earlier review that demonstrated that follow-up programs based on regular physical examinations and yearly mammography are as effective as more intensive approaches with respect to detection of recurrence, overall survival, and quality of life. The frequency of breast cancer follow-up is also not evidence-based. The American Society for Clinical Oncology recommends breast cancer patients be seen by a healthcare provider every 3 to 6 months for the first 3 years after initial treatment, every 6 to 12 months for years 4 and 5, and then annually thereafter. In addition to breast cancer–specific follow-up recommendations, clinicians should be aware of the NCCN guidelines for cancer survivorship that provide screening, evaluation, and management recommendations for all cancer patients after their initial treatment.
The coordination of care among providers managing breast cancer survivors is also important. One study from the United Kingdom demonstrated improved patient satisfaction when breast cancer follow-up was performed by a family practitioner compared with a specialist, but this satisfaction difference may have been due to patient familiarity with their family physician. In addition, the recent Cochrane review also concluded that follow-up care performed by general practitioners working in an ambulatory practice setting had comparable effectiveness to that delivered by hospital-based specialists. In summary, breast cancer follow-up necessitates an organized, team-based approach emphasizing health promotion, surveillance, screening, physical and psychosocial concerns, and care coordination.
The incidence of local recurrence after mastectomy varies from 7% to 32% depending on several factors, including tumor biology, the initial extent of disease (e.g., tumor size, lymph node, and margin status), the type of primary therapy (e.g., hormonal, chemotherapy, and/or radiotherapy), the length of follow-up, and the method of detection. The majority of chest wall recurrences appear within the first 2 years after mastectomy, and 90% occur within 5 years. Some solitary local recurrences result from incompletely excised tumor or tumor cells deposited at the time of surgery. Rarely, a new primary cancer develops in residual breast tissue remaining in the skin flaps at the time of mastectomy ( Fig. 77.2 ). In this circumstance, long-term disease-free survival may be possible with appropriate local therapy.
Recently gene expression studies have identified four major biological breast cancer subtypes: (1) luminal A (estrogen receptor [ER]/progesterone receptor [PR]–positive, HER2-negative, low/intermediate-grade), (2) luminal B (ER/PR-positive, HER2-negative, high-grade), (3) HER2-positive, and (4) triple-negative (ER-, PR-, HER2-negative) breast cancer. These breast cancer subtypes are associated with prognosis, response to therapy, and recurrence. Some evidence suggests that molecular profiling can stratify patients with respect to LRR more precisely than traditional determinants that focus on initial tumor burden. In a recent meta-analysis of 5418 patients undergoing mastectomy, patients with triple-negative cancers had a much higher relative risk of LRR compared with patients with non–triple-negative cancers. Patients with HER2-positive tumors treated with the anti-HER2 monoclonal antibody trastuzumab (Herceptin) have a lower risk of LRR. Interestingly, this fundamental difference in the risk of LRR based on hormone receptor and HER2 status remains present even in tumors less than 1 cm. Time to recurrence is also correlated with breast cancer molecular subtype with LRR in triple-negative and HER2-overexpressing cancers occurring within the first 5 years of diagnosis, and cancers with ER expression having a more prolonged time to LRR.
Gene expression profiling has also led to several commercially available predictive assays that may identify subgroups of patients who benefit from adjuvant endocrine treatment alone, avoiding the toxicity and risks associated with chemotherapy. For example, the 21-gene expression assay (Onco type DX-Genomic Health) or a 70-gene expression assay (MammaPrint-Agendia) can be used to predict the risk of recurrence in node-negative, ER/PR-positive tumors. However, the most recent updates of the NCCN breast cancer guidelines (Version 1. 2017) state that multigene assays may be used to help predict the risk of recurrence but have not been validated to predict the response to chemotherapy. Therefore, future clinical trials should incorporate molecular profiling when examining local and systemic treatment strategies.
Patients with advanced stage at initial diagnosis develop LRR more rapidly than do patients who present with earlier stage disease. Since most chest wall recurrences after mastectomy are detected by physical examination, patients must understand the importance of careful evaluation of the chest wall and axilla and bring any self-detected changes to a provider’s attention. Any suspicious lesion mandates a thorough evaluation and biopsy. If recurrence is confirmed histologically, the tissue must also be examined for hormone receptor status and HER2 overexpression because these factors may influence the type of systemic therapy. The majority of locoregional recurrences are isolated to the chest wall, whereas some chest wall recurrences also include a regional component in the lymphatic basin (i.e., axillary, internal mammary, and supraclavicular). Some patients present with concomitant distant disease at the time of locoregional recurrence, and therefore complete restaging is necessary at the time of locoregional recurrence.
The treatment of an isolated chest wall recurrence should include the combined modalities of surgery, radiation, and systemic therapy. Ideally, these patients should be presented at a multidisciplinary breast cancer conference for comprehensive multimodal treatment planning. Complete excision with negative margins and chest wall radiation, if possible, is the preferred local therapy. In some cases, skin grafting or flap coverage may be required to close the defect resulting from resection. Rarely, a formal chest wall resection and reconstruction may be required to achieve tumor-free margins. The functional consequences to the patient must be carefully considered before entertaining such radical resection. When surgical therapy is not possible or is too major, systemic therapy may be given first to downstage the recurrent disease before resection.
Regional recurrence refers to the return of breast cancer in the ipsilateral regional lymphatics that drain the breast (axillary, internal mammary, or supraclavicular nodes). The axilla is the primary site of drainage for most breast cancers and therefore the most common site for regional recurrence. Most axillary lymph node recurrences are discovered as a palpable mass on routine follow-up examination. Rarely, axillary recurrence presents with brachial plexopathy symptoms or lymphedema without palpable lymphadenopathy. Patients with these symptoms should undergo CT scan or MRI to determine whether the cause of these symptoms is radiation fibrosis or axillary recurrence.
The risk of axillary recurrence is also dependent on many factors including tumor size, the number and extent of nodal involvement, the type of axillary surgery and whether radiation was given. Historical data suggest the axillary recurrence rate is approximately 17% for patients with a clinically negative axilla who receive no axillary treatment, whereas the axillary recurrence rate after an axillary lymph node dissection (ALND) is 1% to 3%. Axillary recurrences are divided into three main categories: isolated axillary recurrence, axillary recurrence concurrent with a breast recurrence, and axillary recurrence simultaneous with distant disease. Isolated axillary recurrences are rare, and they are more commonly a component of chest wall recurrence. The potential morbidity of an untreated axillary recurrence is significant, and symptoms resulting from brachial plexus involvement include intractable pain, motor and sensory impairment, decreased arm mobility, and lymphedema. Unfortunately, the treatment of axillary recurrence after axillary dissection is often palliative. In one study involving 145 patients with LRR treated with combined modality therapy, the 5-year survival rate for those patients with an isolated axillary recurrence was 50%. In another study, de Boer and associates identified 59 patients with an axillary recurrence treated with a combination of chemotherapy, hormonal therapy, radiotherapy, and surgery. Complete surgical eradication was achieved in only 34 patients (58%), and the overall 5-year actuarial survival rate was 39%.
Sentinel lymph node (SLN) biopsy has essentially replaced axillary dissection in the management of most patients with breast cancer. Several studies suggest axillary recurrence rates are low in women who undergo SLN biopsy with no axillary dissection. The American College of Surgeons Oncology Group (ACOSOG) Z0011 trial prospectively examined the LRR and overall survival of patients with SLN metastases undergoing BCT randomized to undergo ALND after SLN biopsy or no further axillary specific treatment. At a median follow-up of 9.25 years, there was no statistically significant difference between the two groups in local recurrence-free survival. The 10-year cumulative locoregional recurrence was 6.2% with ALND and 5.3% with SLND alone. Therefore SLND without ALND provides excellent local-regional control for selected patients with early-stage breast cancer treated with BCT and adjuvant systemic therapy.
The incidence of internal mammary (IM) node metastasis is primarily dependent on tumor size and location in the breast (i.e., inner vs. outer quadrants) and the histology of the axillary lymph nodes. Routine removal of the IM nodes does not have an impact on LLR or overall survival for patients with breast cancer. However, the emergence of SLN biopsy led to a renewed interest in the clinical significance of the IM lymph nodes. Lymphoscintigraphy studies demonstrate that between 18% and 35% of patients have breast lymph drainage to the IM nodes, but isolated IM nodal drainage occurs in only 5% to 8% of patients. The incidence of IM nodal drainage is also dependent on the site of radionuclide injection with peritumoral injections have a much higher likelihood of IM nodal drainage than subareolar or subdermal injections. The recent revision of the eighth edition of the American Joint Commission on Cancer (AJCC) tumor, lymph node, and metastasis (TNM) staging manual for breast cancer does not have any major changes to N classification. A positive IM node found by SLN biopsy and not by imaging is given an N1 designation, whereas a positive IM node found by preoperative imaging (excluding lymphoscintigraphy) is given an N2 designation. If a positive IM node is found in conjunction with a positive axillary node, an N3 designation is given. The clinical outcome for patients with isolated positive IM nodes is similar to those patients with isolated positive axillary lymph nodes. In a multiinstitutional study by Krag and associates, the IM node was the only positive sentinel node in 3% of patients. Most patients with tumor-containing IM nodes also have concurrent involved axillary nodes. Patients with metastasis to the axillary and IM nodes have a worse overall survival than patients with metastasis to a single lymphatic basin. In a study by Sugg and coworkers, 65 of 72 (90.2%) patients with involved IM nodes also had accompanying metastases to axillary nodes.
The status of the IM nodes does not alter the treatment recommendations for the vast majority of breast cancer patients. However, a subset of patients with small breast tumors with tumor-free axillary nodes may have treatment recommendations altered by a positive IM node biopsy. In the study by Sugg and coworkers, less than 2% of patients fell into this specific subgroup. The concealed location of the IM lymph nodes in the intercostal space behind the sternal border makes detection of recurrence by physical examination difficult. Internal mammary nodal recurrence presents as a parasternal mass, swelling, or pain. Isolated IM node recurrences are uncommon, and CT scan or MRI may detect them. In a study by van Rijk and colleagues, only 1 of 803 patients with a sentinel node biopsy recurred solely in the IM lymph nodes. In a similar study by Cranenbroek and associates, nearly 6000 breast cancer patients were followed for recurrence, and only 6 patients developed isolated IM nodal metastases.
Supraclavicular lymph node metastasis as a first site of recurrence of breast cancer is also relatively uncommon, occurring in less than 2% of patients after mastectomy. The supraclavicular space is defined by the internal jugular vein, the omohyoid muscle and tendon, the clavicle, and the subclavian vein. In the eighth edition of the AJCC TNM staging system for breast cancer, supraclavicular nodal involvement was classified as N 3 disease. This classification was changed based on data suggesting that patients with supraclavicular metastases who undergo aggressive multimodal treatment have similar outcomes to patients with stage III locally advanced disease. The majority of patients with metastasis to the supraclavicular lymph nodes also have positive axillary lymph nodes. Patients with more than four positive axillary lymph nodes have radiation fields expanded to include the supraclavicular region. Isolated supraclavicular recurrence is unusual, and it is usually simultaneous with local or distant recurrence. A study by van der Ploeg and coworkers followed 748 patients with breast cancer who had a tumor-free sentinel node and found that only 2 patients (0.25%) developed isolated supraclavicular nodal metastases. In a study by Chen and colleagues, the 5-year overall survival in patients treated for supraclavicular lymph node metastases was 33.6%. Similar to other LRR, the treatment of supraclavicular nodal metastases requires a combination of surgery, chemotherapy, and radiation.
The heterogeneity of patients with LRR after mastectomy complicates the design of prospective randomized trials examining this group. Therefore, most studies examining locoregional recurrence after mastectomy have been small, single-institution, retrospective series. In these retrospective studies, patients have received diverse adjuvant therapies, making any comparison between studies difficult. Historically, local recurrences after mastectomy have carried a poor prognosis, with an overall 5-year survival rate of approximately 35% from the time of recurrence after mastectomy. Because locoregional recurrence is an independent predictor of simultaneous or subsequent distant metastases, patients with locoregional recurrence after mastectomy require a metastatic workup. Multiple local recurrences that arise after a short disease-free interval may represent hematogenous spread of tumor cells to the mastectomy site. Therapy in this group of patients tends to be palliative, but efforts toward locoregional control even in patients with metastatic disease should be maximized.
In selected patients with isolated chest wall recurrences (i.e., ribs, intercostal musculature), radical chest wall resection may be indicated to provide palliation and long-term disease-free survival. Recently, Shen and colleagues from the University of Texas MD Anderson Cancer Center reported the largest contemporary experience for patients with isolated chest wall recurrences. They identified 76 patients, of whom 44 were treated surgically and 32 nonsurgically. The 5-year overall survival was approximately 40%, and complications related to radical surgical resection occurred in 25% of patients. Surgically treated patients were more likely to have triple-negative breast cancer at recurrence, and 95% received preoperative systemic therapy. For hormone receptor–positive recurrence, 5-year progression-free survival was significantly higher among surgical patients. These data are similar to other historical and contemporary series of radical resection for isolated chest wall recurrences. Both Shah and Urban and Toi and associates reported a 5-year survival rate of 43% and 47%, respectively, using full-thickness chest wall resection for isolated chest wall recurrences. Some investigators have demonstrated a significant difference in survival rates after chest wall resection between patients with greater than a 2-year disease-free interval versus patients with less than a 2-year disease-free interval. Large anterior chest wall resections involving multiple ribs and/or the sternum require stabilization with prosthetic material to prevent paradoxical chest wall motion. Soft tissue coverage may be achieved after chest wall resection through the use of pectoralis, latissimus, or rectus muscle flaps. In summary, patients with isolated chest wall recurrences requiring radical resection are best treated at centers with multidisciplinary teams experienced in performing complicated resections and sophisticated reconstructions.
Recent data support the hypothesis that reducing the rate of locoregional failure for breast cancer improves overall survival. In addition, because locoregional recurrence after mastectomy is associated with such a poor outcome, prevention of locoregional recurrence at the time of initial treatment is an important goal. Postmastectomy radiation therapy has been shown to significantly decrease the rate of locoregional recurrence in high-risk patients. Historically, indications for postmastectomy radiation have included four or more tumor-containing lymph nodes, tumor size 5 cm or greater, inadequate margins, or skin invasion. However, data from randomized prospective trials conducted in Canada and Europe demonstrate that postmastectomy radiation in women with one to three positive lymph nodes improves not only local control but also survival. The magnitude of the survival benefit from postmastectomy radiation in these studies was similar to the survival benefit seen from adjuvant chemotherapy in previous studies. Questions remain, however, regarding the generalizability of these data because of differing surgical and radiotherapy techniques. In addition, the survival benefit specific to postmastectomy nodal irradiation needs to be further defined by ongoing studies. CT-assisted treatment planning may limit the cardiopulmonary toxicity associated with postmastectomy radiation. Finally, the optimal integration of postmastectomy radiation with systemic therapy and reconstructive surgery requires additional investigation.
If locoregional recurrence is often a manifestation of systemic disease and chemotherapy affects systemic relapse, then chemotherapy should also affect locoregional relapse. Despite this line of reasoning, the effect of chemotherapy on locoregional recurrence has been difficult to prove because of selection and other confounding factors. Patients with locally advanced disease who have a good response to preoperative chemotherapy have a significantly reduced risk of local recurrence. Because patients with locally advanced disease are also treated with mastectomy and chest wall radiation therapy, it is difficult to sort out the impact of chemotherapy alone on local recurrence. The optimal sequence and timing of adjuvant radiation and chemotherapy in the overall management of breast cancer remain to be defined. Although radiation oncologists emphasize the importance of adjuvant radiotherapy in achieving optimal local disease control, medical oncologists argue that potential gains in terms of local control may be at the expense of increased distant relapse. The integration of these modalities requires individual patient assessment to maximize locoregional control and minimize distant relapse. Delaying radiation therapy until after the completion of chemotherapy in patients at greatest risk for systemic relapse appears to have little negative effect on local control.
The recent publication of the results of the CALOR trial (Chemotherapy as Adjuvant for LOcally Recurrent Breast Cancer) validated the use of adjuvant chemotherapy for patients with completely resected isolated local or regional recurrence of breast cancer. In this multicenter, randomized trial, 85 patients with histologically proven and completely excised LRR were randomized to investigator-determined, multidrug chemotherapy or no chemotherapy. Patients with ER-positive LRR received adjuvant endocrine therapy, radiation therapy was required for patients with microscopically involved surgical margins, and anti-HER2 therapy was optional. At a median follow-up of 4.9 years, adjuvant chemotherapy increased the risk of disease-free and overall survival by 41% and 59%, respectively. In this study, adjuvant chemotherapy was most effective in patients with ER-negative recurrences, whereas longer-term follow-up was required for patients with ER-positive recurrences.
In summary, local relapse after mastectomy should be treated aggressively for palliation and improved survival ( Fig. 77.3 ). Prior staging procedures, local and systemic treatments significantly influence the multimodal management of these patients. For example, the use of SLN biopsy during initial therapy requires axillary restaging at the time of recurrence. In addition, with the increasing use of postmastectomy chest wall irradiation, many postmastectomy recurrences occur in previously irradiated patients. Patients with recurrence after mastectomy who have not previously received radiation should receive radiation therapy in addition to surgery to decrease the risk of another local relapse. The high risk of simultaneous or subsequent distant metastases in the setting of isolated locoregional recurrence after mastectomy justifies the administration of systemic chemotherapy. The type, dose, duration, and response to any initial systemic therapy must be carefully reviewed in the treatment planning of patients with LRR. Finally, the sequencing of treatment modalities should be determined through careful multidisciplinary evaluation of each patient.
Recurrence in the Reconstructed Breast
Over the past several decades, advancements in plastic surgery have revolutionized breast reconstruction for patients undergoing mastectomy. Overall, approximately 40% of patients having a mastectomy in the United States undergo breast reconstruction and the rate of immediate reconstruction appears to be increasing. Breast reconstruction can be performed with prosthetic implants, autologous tissue or a combination of both techniques. Approximately 60% of breast reconstructions involve the combination of tissue expanders and implants, whereas 16% are implants only, and 25% employ autologous tissue transfer. In the past decade, there has been a trend toward higher proportions of breast conserving surgery–eligible patients undergoing mastectomy, breast reconstruction, and bilateral mastectomy. Kummerow and colleagues recently studied more than 1.2 million adult women treated at centers accredited by the American Cancer Society and the American College of Surgeons Commission on Cancer to determine trends in mastectomy for early-stage breast cancer. They found that from 2003 to 2011, the use of mastectomy increased 34%, and from 1998 to 2011, rates of breast reconstruction increased from 11.6% to 36.4% and rates of bilateral mastectomy for unilateral disease increased from 1.9% to 11.2%.
Despite these recent data, controversy continues regarding the optimal timing of reconstruction. Historically, an empirical 2-year interval between mastectomy and reconstruction was recommended based on the assumption that most local recurrences occurred during this critical time interval. That assumption has been challenged by studies demonstrating that chest wall recurrence rarely occurs in the first 2 years postoperatively in patients with stage I disease. Other skeptics of immediate breast reconstruction were fearful that delaying postmastectomy radiation therapy after reconstruction might increase local recurrence rates and that the reconstructed breast might mask the signs and symptoms of a recurrence. Many studies have since shown no increase in the rate of locoregional recurrence or distant metastasis in patients who undergo immediate breast reconstruction and that the postradiation effects on the reconstructed breast are well tolerated and easily managed.
Advocates of immediate breast reconstruction cite potential benefits such as improved self-image, superior cosmetic results, elimination of subsequent surgeries and anesthetic risks, and an overall decrease in cost as reasons not to delay reconstruction. Along with these benefits, potential problems and conflicting reports must be considered regarding the timing of breast reconstruction. For instance, the complication rate after immediate breast reconstruction has been reported in one study to be as low as 15%, with only 9% of patients undergoing autologous tissue transfer requiring additional operative procedures. On the other hand, one retrospective review of 50 patients reported a much higher complication rate of 50%, prolonged hospitalization, and a more frequent need for blood transfusions associated with immediate breast reconstruction. Potentially, wound-healing complications after immediate reconstruction may also significantly delay the initiation of adjuvant therapies, but several studies have found this delay to be of no statistical significance with regard to recurrence or mortality rates. In a recent comprehensive meta-analysis of 31 studies involving 139,894 patients, Zhang and associates found no difference in disease-free/overall survival or LRR between women undergoing immediate breast reconstruction after mastectomy and those undergoing mastectomy alone.
The surgical management of breast cancer has evolved over the past century from radical resections to more conservative procedures with equivalent oncologic outcomes and improved cosmesis. In 1991 Toth and Lappert first reported the technique of a skin-sparing mastectomy (SSM) that included resection of the nipple-areola complex, the biopsy scar, and removal of the breast parenchyma with preservation of the breast skin envelope to improve cosmetic outcome. Since that initial report, SSMs with or without nipple preservation have gained acceptance as an option for immediate breast reconstruction because this technique preserves the skin envelope and thereby provides the best cosmetic outcome. In patients without locally advanced or large tumor burdens, an SSM with immediate reconstruction has proven to be an oncologically safe operation with no increased rate of locoregional recurrence.
Investigators at the University of Alabama examined the factors associated with local recurrence in 173 patients with invasive breast cancer who underwent SSM and immediate breast reconstruction over an 11-year period. The locoregional relapse rate after SSM and immediate breast reconstruction was 4.5%, and the median follow-up was 73 months. Factors associated with locoregional recurrence were tumor stage and differentiation. The authors concluded that locoregional relapse was an independent predictor of survival after SSM, but it is a function of tumor biology rather than surgical modality, supporting the practice of less aggressive oncologic resections. A more recent study from the University of Texas MD Anderson Cancer Center examined the rates of local, regional, and systemic recurrence, and survival in 1810 breast cancer patients who underwent SSM or conventional mastectomy (CM) The local, regional, and systemic recurrence rates did not differ significantly between the SSM and CM groups. After adjusting for clinical TNM stage and age, disease-free survival rates between the SSM and CM groups did not differ significantly.
There are conflicting data regarding the effect of radiation therapy on reconstructed breasts. Some studies have demonstrated high rates of flap necrosis requiring additional surgery and impaired cosmetic outcomes related to capsular contractures in women that undergo prosthetic reconstruction, whereas other investigations have shown no difference in outcome.
Liang and colleagues reviewed 191 patients receiving postmastectomy radiation therapy from 1997 to 2001 and found that 82 patients had a transverse rectus abdominis muscle (TRAM) flap reconstruction and 109 patients received no reconstruction. In general, the indications for postmastectomy radiation therapy included tumor size of more than 5-cm, involved lymph nodes, and positive or close surgical margins. The patients were followed for a median time of 40 months, and complications, recurrence, and distant metastasis were observed. The researchers found no statistical differences between patients who had received postmastectomy radiation, whether they underwent a TRAM reconstruction or had no reconstruction. They concluded that immediate TRAM flap reconstruction could be considered a feasible treatment for breast cancer patients requiring postmastectomy radiotherapy.
Immediate breast reconstruction may also interfere with the design of postmastectomy radiation fields. The contour of the reconstructed breast may lead to an inability to cover all treatment targets within the radiation fields and increase the volume of normal tissue irradiated. Therefore radiation given to patients who have had immediate breast reconstruction may not be as effective and may increase the risk of radiation-related complications. As an intermediate compromise, some reconstructive surgeons have suggested a staged approach, with initial placement of a tissue expander followed by conversion to autogenous flap reconstruction after completion of all oncologic therapy.
While the use of autologous fat transfer or lipofilling to correct volume/contour defects and asymmetry after breast cancer surgery has increased dramatically, oncological concerns remain. While most clinical studies fail to point out a significant increase in LRR in patients who receive fat transfer after breast cancer surgery, more prospective studies are needed with a sufficient follow-up time and analysis of critical factors involved.
In summary, immediate breast reconstruction is best offered to patients who are anticipated to be at lower risk for postoperative adjuvant therapies. However, evidence is growing that such reconstruction is an acceptable component of the multimodality treatment for breast cancer with similar outcomes and complication risks. The coordination of the multidisciplinary breast team, including the medical, radiation, and surgical oncologist as well as the reconstructive surgeon, is essential to provide optimal care to the patient with breast cancer.
Recurrence After Breast Conserving Therapy
BCT consists of resection of the primary cancer with a margin of normal-appearing tissue followed by adjuvant radiation therapy. For many women with early invasive breast cancer (stage I, IIA, and IIb) BCT is preferable to total mastectomy. BCT produces equivalent survival rates while preserving the breast, thereby enhancing cosmesis and patient satisfaction. Several randomized prospective studies have identified that BCT results in disease-free and overall survival rates equal to mastectomy. Although breast conservation rates vary geographically, approximately 60% of women with newly diagnosed breast cancer in the United States will be treated with BCT. This translates to approximately 110,000 partial mastectomies yearly that have the corresponding potential to develop locoregional recurrence. Improvements in systemic therapy together with the increasing use of neoadjuvant chemotherapy, partial breast irradiation, and SLN biopsy may impact the incidence of ipsilateral breast tumor recurrence (IBTR) after BCT and also influence the subsequent management of these patients.
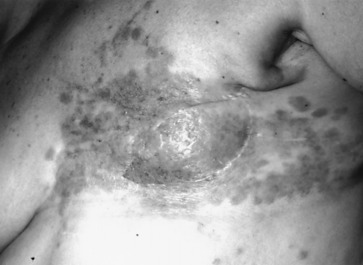
Early detection of local recurrence after BCT is important. IBTR after BCT is detected as a palpable mass on physical examination or as an abnormality on follow-up mammography. Because the lumpectomy scar and radiation fibrosis may produce radiographic distortion, a baseline mammogram is required approximately 6 months posttreatment. Patient migration may complicate the continuity of follow-up care; therefore an experienced examiner is required to follow the irradiated breast. Any change detected on breast examination should be thoroughly evaluated, and, similar to the situation with a mass in a previously untreated breast, the presence of a mass within a normal mammogram should still be investigated for the possibility of a local recurrence or second primary cancer. Rarely, local recurrences after breast conserving surgery can present with inflammatory breast changes such as warmth, erythema, and peau d’orange and are often misdiagnosed as postoperative infection ( Figs. 77.4 and 77.5 ).
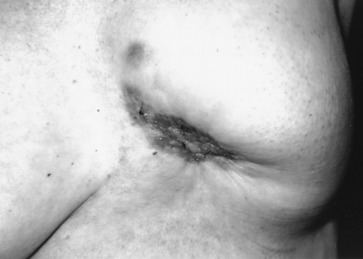
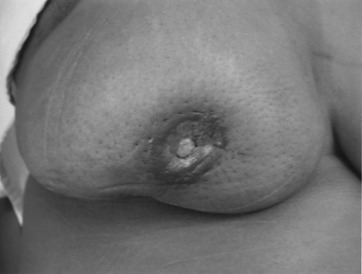
There are differences in the time to recurrence and clinical characteristics of locoregional failures after BCT versus mastectomy. Local recurrences tend to occur later after breast conserving surgery, particularly if patients initially received chemotherapy or hormonal therapy. Although most local recurrences after mastectomy are detected by physical examination, local recurrences after lumpectomy are detected solely by mammography approximately 40% to 75% of the time, by physical examination alone in 10% to 30%, a combination of mammography and physical examination in 10% to 25%, and by other imaging modalities such as MRI in 5% of cases. In general, locoregional recurrence after mastectomy has a worse prognosis than recurrence after breast conserving surgery.
In the 20-year follow-up data from the National Surgical Adjuvant Breast and Bowel Project Protocol B-06, the local recurrence rate was 39% for lumpectomy alone but decreased to 14% when lumpectomy was followed by radiation therapy. Women who received radiation had their recurrences later in their postoperative course, with 31% developing after 10 years. These data are in contrast to the lumpectomy-only group, with 73% of local recurrences occurring within the first 5 years after surgery. The Early Breast Cancer Trialists’ Collaborative Group meta-analysis of 36 breast cancer trials, which involved more than 17,000 women, showed an isolated breast tumor recurrence rate of 6.7% after BCT. It has been difficult in the past to determine a subgroup of women who might not require radiation after lumpectomy. Prospective data suggest that women 70 years of age or older with small ER-positive tumors treated with lumpectomy with tumor-free margins plus an aromatase inhibitor without breast irradiation experience a locoregional recurrence rate of less than 5%.
The majority of local recurrences in the ipsilateral breast after breast conserving surgery occur in the proximity of the primary excision. It can be difficult, however, to distinguish a late local recurrence from a metachronous second primary breast cancer after breast conserving surgery. Local recurrences can develop in or near the lumpectomy site, whereas second primary cancers develop in other breast quadrants. If the initial tumor was an invasive cancer then the majority of subsequent recurrences will be invasive, however, if the initial tumor was noninvasive or in situ cancer, then 50% of the recurrences will be invasive and the remainder will be noninvasive recurrences. Genomic profiling may provide a useful tool to distinguish a recurrence of the original lesion from a metachronous tumor. Local recurrence after BCT may be a marker for aggressive disease at the time of lumpectomy. Predetermined tumor biology should not, however, lessen the importance of careful patient selection and meticulous surgical technique to minimize the risk of local recurrence.
The rate of local recurrence has been shown to be dependent on a multitude of factors, including patient age, tumor size, stage, grade, method of detection, and family history. In patients receiving preoperative chemotherapy, poor clinical response and residual axillary disease after chemotherapy have been shown to be independent predictors of local recurrence. Other factors that may favor recurrence after lumpectomy include positive surgical margins at the time of lumpectomy, lymphatic invasion, anaplasia, associated in situ disease in both the primary cancer and surrounding parenchyma, tumor necrosis, invasive lobular carcinoma, inadequate radiation dose, and a delay of radiation therapy after lumpectomy. Because many of these variables are interrelated, it is difficult to establish independent prognostic factors for local recurrence. In addition, studies that attempt to identify risk factors for local recurrence after breast conservation suffer from patient heterogeneity and treatment selection biases.
Until recently, the lack of an accepted definition of a positive margin and a failure to standardize pathologic assessment of the surgical margin made it difficult to precisely define the impact of a positive margin on local recurrence after breast conserving surgery. However, a recently published report from a consensus panel convened by the Society of Surgical Oncology and the American Society for Radiation Oncology states that “no ink on tumor” should be the standard pathologic margin assessment for patients treated BCT. This recommendation was based on a meta-analysis of 33 studies, with a median follow-up of more than 6.5 years where a positive margin defined “as ink on tumor” was associated with a greater than twofold increase local recurrence. Interestingly, wider margins were not associated with a lower recurrence rate. Regardless of the definition of a positive margin, it is important to ensure that all suspicious microcalcifications have been removed on postexcision mammography before administering radiation therapy. If margins are positive or close, factors to consider when contemplating reexcision include patient age, the extent of the close margin (focal vs. diffuse disease), differences between radiographic and pathologic tumor size, and the morbidity involved with a reexcision lumpectomy. Again, these patients should be presented at a multidisciplinary tumor board to determine the need for additional surgery. At the University of Florida, the use of frozen section of shaved margins performed during lumpectomy resulted in lower reexcision rates and a local recurrence rate of 2% for invasive cancer with a median follow-up of 5.6 years.
The relationship between extensive intraductal component and local recurrence is also controversial. If one assumes that tumor cells spread locally via the breast ducts, then a greater intraductal component might increase the risk of local recurrence. Reports indicate that the presence of extensive intraductal component alone is not, however, a contraindication to breast conserving surgery, but its presence may require a more extensive resection to achieve uninvolved margins. In addition, the relationship between infiltrating lobular histologic subtype and risk of local recurrence after mastectomy is unsettled. Lobular cancers are diffusely infiltrative, with individual tumor cells being difficult to identify pathologically at the tumor margin. Mammography also tends to underestimate the pathologic extent of invasive lobular cancer. Therefore it may be more difficult to obtain histologic tumor-free surgical margins for patients with invasive lobular cancer. Several reports have documented, however, that select patients with invasive lobular cancer can be treated with lumpectomy, axillary dissection, and radiotherapy with an acceptable local recurrence rate. Therefore lobular histology by itself is not a contraindication to breast conserving surgery. Breast MRI may be particularly helpful in selecting patients with invasive lobular cancer for breast conserving surgery.
Preoperative or neoadjuvant chemotherapy may be used to downsize tumors that are too large to meet the criteria for BCT. Before chemotherapy, the placement of a radiopaque clip using mammographic or ultrasound guidance should be employed to identify the tumor site should the patient experience a complete clinical response. Studies demonstrate that breast conservation rates are higher after preoperative chemotherapy but that there is no survival advantage over postoperative adjuvant chemotherapy. At the University of Texas MD Anderson Cancer Center, a prognostic index was developed to predict local recurrence for patients undergoing breast conservation surgery after neoadjuvant chemotherapy. This index included four factors previously found on retrospective analysis to be significant: clinical N2 or N3 disease, residual pathologic tumor size less than 2 cm, multifocal residual tumor, and lymphovascular invasion. Unfortunately, three of these factors are discovered postoperatively in the pathology report and therefore are not useful in preoperative decision-making.
The treatment of local recurrence after BCT is dependent on the type of recurrence as well as the initial treatment of the primary tumor. Salvage mastectomy is considered the standard treatment for local recurrence after breast conserving surgery. Doyle and associates found a 10-year survival of 64% for 93 patients with local recurrences after breast conserving surgery treated with salvage mastectomy for recurrent invasive cancer. Of these patients with recurrence, 44% were free of systemic metastasis. These data compare favorably to series reported by other investigators in which local recurrence after breast conservation was treated with mastectomy. Several series have recently demonstrated that repeat lumpectomy with salvage radiotherapy to the bed of the recurrent tumor is well tolerated and provides reasonable local control. A study by Alpert and colleagues compared outcomes of salvage mastectomy to salvage breast conserving surgery in 166 women who experienced an IBTR. With a median length of follow-up of 13.8 years, patients who underwent repeat lumpectomy had outcomes comparable to those who underwent salvage mastectomy but remain at continued risk for IBTR. Other studies have provided further support for repeat lumpectomy for patients with IBTR after breast conserving surgery. A recent analysis by Jobsen and colleagues demonstrated a pattern in risk of IBTR over time, with 2 peaks, first at approximately 5 years and a second, much higher peak at approximately 12 years, especially for women ≤40 years old. They also noted that the absence of adjuvant systemic therapy and the presence of lymphovascular invasion were independent prognostic factors of IBTR for women ≤40 years old with tumor-free resection margins. For women >40 years old, the presence of lymphovascular invasion and the presence of lobular carcinoma in situ were independent risk factors. The introduction of newer local radiation techniques such as partial breast irradiation and combined hyperthermia and radiation may provide new opportunities in the local management of IBTR.
There are limited data addressing the role of chest wall or regional lymphatic radiation after mastectomy for postlumpectomy recurrence. Postmastectomy radiation may be considered for patient with recurrences greater than or equal to 5 cm in size, close or positive margins after mastectomy, or four or more positive nodes.
The management of the axilla in patients who experience local recurrence after breast conserving surgery depends on several factors, including whether the recurrence is noninvasive or invasive, previous axillary surgery, radiation therapy, and whether the status of the axillary nodes will change treatment decisions. If clinically suspicious axillary nodes are present, then ultrasound-guided fine-needle aspiration of the lymph nodes is appropriate. If the axilla is clinically negative, then repeat axillary staging with lymphatic mapping and sentinel lymph node biopsy is appropriate. Surgeons from the Memorial Sloan Kettering Cancer Center recently reported reoperative SLN biopsies performed in patients suffering a local recurrence after BCT. They concluded that reoperative SLN biopsy is feasible in the setting of local recurrence after previous lumpectomy and radiation. In addition, lymphoscintigraphy identified more sites of nonaxillary drainage; the number may be greater in the setting of reoperative SLN biopsy than in the initial sentinel lymph node procedure.
Immediate reconstruction should be used with caution and is best offered to patients who are anticipated to be at lower risk for postoperative adjuvant therapies. If immediate breast reconstruction is considered after salvage mastectomy, autogenous tissue transfer (i.e., TRAM flap, deep inferior epigastric perforator [DIEP] flap) is preferable compared with placement of an implant in an irradiated field.
The value of systemic adjuvant therapy in patients suffering an IBTR after BCT remains unproven. If IBTR is in fact a marker for increased risk of distant failure and death, then one would assume that chemotherapy would be indicated. Unfortunately, few well-designed studies have addressed this important topic.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree