Fig. 12.1
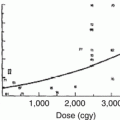
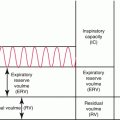
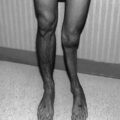
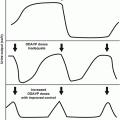
Top, delayed radiation injury in the wall of the ileum, approximately 10 years after exposure to undetermined (kilorad) dose of external radiation for adjacent intra-abdominal neoplasm (hematoxylin and eosin stain, ×21). Note chronic ulceration (left third) and extensive fibrosis of submucosa and subserosa. Villous atrophy is slight in this case (From Fajardo [18]). Bottom, histologic picture of the small bowel at the time of obstruction, demonstrating severe villous blunting, distended lymphatics, and an abnormally dense mucosal round cell infiltrate. The normal columnar epithelium is lost, with only low cuboidal cells present. Villi are shortened (hematoxylin and eosin stain, ×10) (From Donaldson et al. [1])
12.2.2 Clinical Manifestations
Clinical signs and symptoms of GI tract toxicity are related to the specific tissues involved and to the severity of injury. Mild injury to GI tract tissues may be asymptomatic and noted as an incidental finding at the time of a diagnostic imaging procedure, surgery, or autopsy. More severe injury produces chronic or persistent symptoms associated with inflammation and fibrosis of specific tissues. Upper GI symptoms are most common, with a cumulative incidence of 26 %, and lower GI complications are reported with an incidence of 15 % [7]. Common constitutional symptoms referable to the GI tract include dysphagia, odynophagia, indigestion/heartburn, vomiting, abdominal pain (which may be focal or generalized), colic, constipation/obstipation, diarrhea, and GI bleeding with or without anemia. The clinical sequelae of fibrosis include partial or complete bowel obstruction from strictures or adhesions. Chronic enteritis and bowel resection may result in malabsorption, bowel ulceration/perforation, or fistula formation.
Nonspecific symptoms of anorexia, fatigue, and wasting may also be observed. Individuals with chronic hyperchloremic metabolic acidosis resulting from excess GI bicarbonate loss may present with obtundation and confusion. This complication is rare, but can develop following ureterosigmoidostomy or ileal or jejunal loop procedures. A young child with upper GI tract obstruction and reflux may present with aspiration pneumonia. Older age at diagnosis, intensified therapy, abdominal radiation, and abdominal surgery increase the risk of certain GI complications [7].
12.2.2.1 Radiation
Radiation injury of the GI tract has been the most extensively studied cause of fibrosis and enteritis. According to data from cohorts of adults treated with radiation for abdominopelvic tumors, radiation complications typically develop within 5 years after treatment in individuals who experienced acute GI toxicity [24]. However, primary presentations of strictures or inflammatory lesions may occur as late as 20 years after radiation [17, 25]. The risk of radiation injury to the GI tract is related to the cumulative radiation dose, daily fraction dose, and extent of treatment volume [26, 27]. Fixed loops of the duodenum and terminal ileum are more prone to radiation injury than the esophagus or other intestinal sites [6, 25]. The incidence of small bowel fibrosis is about 5 % after 40–50 Gy and rises to 40 % when doses exceed 60 Gy [26, 28]. Chronic GI tract injury is uncommon after treatment doses below 42 Gy given over 4–4.5 weeks.
Limited studies are available describing the chronic effects of GI radiation therapy in children [17]. Many of the data on the risk of bowel adhesions and obstruction in this population are confounded by the fact that most of the cancer survivors had prior abdominal surgery as a risk factor for late gastrointestinal effects [17]. Donaldson et al. comprehensively evaluated intestinal symptoms in 44 children with Wilms tumor, teratoma, or lymphoma (including Hodgkin lymphoma) treated with whole abdominal (10–40 Gy) and involved field (25–40 Gy) radiation [1]. Additional interventions predisposing to GI tract toxicity in the group included abdominal laparotomy in 43(98 %) and chemotherapy in 25 (57 %). Late small bowel obstruction was observed in 36 % of patients surviving 19 months to 7 years, which was uniformly preceded by small bowel toxicity during therapy. Infants and children younger than 2 years appeared to experience more acute and chronic GI toxicity following intensive radiation or combined modality therapy compared to older children.
Several reports document GI toxicity in long-term survivors of genitourinary rhabdomyosarcoma [2, 3, 29]. Investigators from the Intergroup Rhabdomyosarcoma Study evaluated late effects in long-term survivors of paratesticular and bladder/prostate rhabdomyosarcoma [5]. Radiation-related complications occurred in approximately 10 % of patients and included intraperitoneal adhesions with bowel obstruction, chronic diarrhea, and stricture or enteric fistula formation. Similar findings were observed in another small cohort of survivors of paratesticular rhabdomyosarcoma treated at a single institution [29]. A report on long-term survivors of germ cell tumors reported up to 25 % had gastrointestinal dysfunction in patients who had received 25–30 Gy to the abdomen and pelvis. The late effects included chronic diarrhea or constipation, stool incontinence, bowel obstruction, rectal prolapse, strictures, or fistula formation [30]. The small number of patients and GI events reported in these studies precluded correlation of host and treatment factors predicting GI toxicity.
Radiation can enhance the risk of late postsurgical small bowel obstruction in long-term childhood survivors, but this complication has been rarely observed with contemporary techniques and doses. Donaldson reported only one case of late bowel obstruction in 79 surgically staged pediatric patients with Hodgkin lymphoma treated with radiation (35–44 Gy) [31]. Similarly, high-dose radiation used in earlier protocols for Hodgkin lymphoma in adolescents and young adults has been associated with a 1 % incidence of late onset nonspecific abdominal pain attributable to retroperitoneal fibrosis involving the genitourinary tract rather than the GI tract [32]. However, children irradiated at lower doses for Wilms tumor less commonly develop chronic small bowel obstruction [17].
Thoracic radiation, particularly when administered to young children at high cumulative doses (40 Gy or more), predisposes to the development of esophageal strictures [4, 33]. The frequency of stricture formation after mediastinal radiation has ranged from 17 % to 42 % in older series [34–36]. This complication is uncommonly observed following contemporary treatment for pediatric tumors which have reduced or eliminated radiation in many frontline therapies. Acute radiation esophagitis developing during radiation administration is likely the nidus for stricture formation or tracheoesophageal fistulas; fungal esophagitis may also enhance the risk of this complication [33, 37]. Subacute or chronic candidal esophagitis may predispose to stricture formation that usually involves the upper third of the esophagus [37, 38] (Figure 12.2). Strictures developing in individuals with chronic candidal esophagitis have been seen in association with intramucosal pseudodiverticulitis, an inflammatory disorder characterized by digitation of excretory ducts of the submucosal glands. Another characteristic histopathology includes the development of mucosal bridges that may result in webs. Similarly, the administration of radiation-enhancing chemotherapeutic agents like doxorubicin may augment the risk of stricture formation by inducing recurrent episodes of esophagitis through a “recall” phenomenon [39–41]. This premise concurs with the increased frequency of strictures observed in patients treated with combined modality therapy including radiation and radiomimetic chemotherapy.
12.2.2.2 Chemotherapy
In general, chemotherapy plays a less prominent role in the development of chronic enteritis. Multiple chemotherapeutic agents produce acute GI toxicity and typically manifest as mucositis; complete resolution of symptoms after completion of therapy is the usual clinical course. Anthracyclines and dactinomycin have potent radiomimetic effects that enhance acute GI toxicity and likely contribute to late onset radiation-related GI toxicity [40–42]. Rapid chemotherapy-induced tumor lysis has been associated with intestinal necrosis and fistula formation [43]. Both alkylating agents and anthracyclines were associated with dose-dependent risk of upper GI and liver complications in the CCSS cohort. Vincristine exposure is associated with late liver and lower GI complications [7].
12.2.2.3 Surgery
Abdominal surgery is associated with a lifelong risk of developing adhesive and obstructive complications involving the GI tract. Numerous pediatric studies describe adhesive or obstructive complications that most commonly develop within the acute or subacute postoperative period [44–46]. In contrast, information about very late onset adhesive and obstructive complications in childhood cancer survivors are more likely to be found in manuscripts describing global long-term outcome after pediatric sarcomas, in which the incidence of complications related to adhesions and obstructions is approximately 10 % [3, 5, 29]. Notably, in addition to laparotomy, risk factors for postoperative complications included intensive combination chemotherapy including radiomimetic agents and radiation therapy. Investigators from the National Wilms Tumor Study Group observed small bowel obstruction in 7 % of patients; however, the incidence of late onset obstruction (more than 1 year from laparotomy) occurred in fewer than 2 % of patients, with events rarely reported more than 5 years postoperatively [44]. Jockovich et al. evaluated long-term complications of laparotomy in a cohort of 133 pediatric and adult patients who had undergone staging laparotomy with splenectomy before treatment of Hodgkin lymphoma [45]. At a median follow-up of 15.7 years (range, 2.5–28 years) after laparotomy, the most frequent surgical complication was small bowel obstruction, which occurred in 9.8 % and required surgical intervention in 6.8 %. Patients younger than 15 years of age at the time of laparotomy had a higher risk of small bowel obstruction compared to older patients, perhaps related to the increased intensity of the pediatric surgical staging protocols. An increased frequency of obstructive complications is also anticipated in survivors who have undergone multiple laparotomies for staging and assessment of tumor response [31, 42].
Rarely, a temporary or permanent enterostomy is required for the management of cancer-related GI toxicity, for example, after resection of ischemic bowel, for a refractory GI tract stricture or fistula, or for chronic fecal incontinence. Affected survivors must cope with physical and psychological issues related to stoma maintenance. Finally, excess gastrointestinal bicarbonate loss associated with ureterosigmoidostomy or ileal or jejunal loop procedures predisposes to hyperchloremic metabolic acidosis [47]. These individuals are also at increased risk of developing adenocarcinoma of the large bowel [48].
12.2.2.4 Hematopoietic Stem Cell Transplant (HSCT)
The incidence and severity of delayed GI tract toxicity following allogeneic hematopoietic stem cell transplantation are related to the cumulative radiation dose used in the conditioning regimen, the presence of GVHD, or a combination of both. Whereas GI tract toxicity is frequent in the acute transplant setting, chronic late problems affecting GI tissues are relatively uncommon. Xerostomia may result from sclerodermatous changes of the mucous membranes and salivary glands and predispose to accelerated tooth decay and periodontal disease [49]. Esophageal stricture formation is the most common form of delayed GI toxicity and may require dilation procedures to maintain satisfactory oral intake [50, 51]. Affected tissues show characteristic weblike intraluminal membranes that form strictures; some patients also demonstrate perimuscular fibrosis similar to that seen in scleroderma [50, 51]. GVHD of the small bowel may result in chronic diarrhea from malabsorption (Fig. 12.3). Steatorrhea and diarrhea may also signal the presence of bacterial overgrowth with stasis syndrome. As a result, many individuals with chronic GVHD involving the GI tract are anorexic and undernourished. Endoscopic evaluation with biopsy is generally required to identify the specific GI pathology and provide appropriate therapeutic interventions.
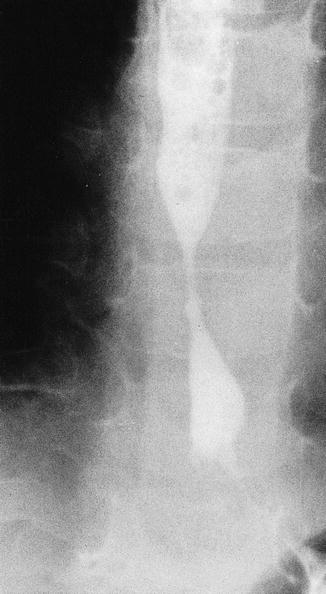

Fig. 12.2
Barium study showing distal esophageal stricture in a 5-year-old girl previously treated for ALL. The distal location of the stricture and its endoscopic appearance suggest that gastroesophageal reflux and chronic esophagitis may have played a role in the pathogenesis of the stricture (Courtesy of Dr. S. Kocoshis)
12.2.3 Detection and Screening
Assessment of cancer-related GI tract sequelae should begin with a thorough history with close attention to symptoms referable to GI tract pathology including dysphagia, odynophagia, vomiting, chronic abdominal pain, chronic constipation or diarrhea, hematochezia, or melena (Tables 12.1 and 12.2). Anorexia, intolerance to certain foods, fatigue, and impaired weight gain or growth may be secondary nonspecific complaints. Physical examination should assess nutritional status, which can be inferred by height and weight, and abdominal findings suggesting GI pathology like distension and pain. Although not routinely used in pediatric assessments, a digital rectal exam and fecal occult blood test should be performed on children presenting with signs and symptoms of GI tract pathology.
Table 12.1
Chemotherapy-associated gastrointestinal and hepatic late effects
Therapeutic agent | Potential late effects | Modifying factors | Health screening and management |
|---|---|---|---|
Dactinomycin | No known late effects Dactinomycin has been associated with acute venoocclusive disease, from which the majority of patients recover without sequelae | Treatment: abdominal radiation | History and physical exam annually Laboratory: ALT, AST, bilirubin, baseline at entry into long-term follow-up, then as clinically indicated Screen for viral hepatitis in patients with persistently abnormal liver function Hepatitis A and B immunizations for patients lacking immunity Gastroenterology/hepatology consultation as clinically indicated |
Mercaptopurine | Hepatic dysfunctiona | Medical conditions: viral hepatitis, VOD, thiopurine S-methyltransferase, deficiency, iron overload | |
Thioguanine | Sinusoidal obstruction syndrome/VODb | ||
Methotrexate | Hepatic dysfunctiona | Treatment: abdominal radiation Medical conditions: viral hepatitis |
Table 12.2
Radiation-associated gastrointestinal and hepatic late effects
Treatment fields | Potential late effects | Modifying factors | Health screening and management |
|---|---|---|---|
Spinal (cervical, thoracic, whole) Chest (mediastinal, mantle, whole lung) Abdomen (upper quadrant, flank, whole) Total body irradiation Total lymphoid irradiation | Esophageal stricture | Treatment: higher radiation dose, radiomimetic chemotherapy (e.g., doxorubicin, actinomycin) Medical conditions: gastroesophageal reflux disease, severe/chronic candidal esophagitis, chronic graft-versus-host disease | History and physical exam annually Gastroenterology consultation as clinically indicated |
All abdominal (upper quadrant, flank, whole) and pelvic Spinal (thoracic, lumbar, sacral, whole) Total body irradiation Total lymphoid irradiation | Bowel obstruction | Treatment: higher radiation dose to bowel, especially dose >45 Gy, abdominal surgery | History and physical exam annually KUB and surgical consultation as clinically indicated |
Chronic enterocolitis Fistula, strictures | Treatment: higher radiation dose to bowel, especially dose > 45 Gy, abdominal surgery | History and physical exam annually Laboratory: serum protein, albumin annually in patients with chronic diarrhea or fistula Gastroenterology and surgical consultation as clinically indicated | |
Cholelithiasis | Treatment: abdominal radiation, abdominal surgery, ileal conduit, total parenteral nutrition, hematopoietic stem cell transplant for marrow failure syndrome Medical conditions: obesity | History and physical exam annually Gallbladder ultrasound and surgical consultation as clinically indicated | |
Gastrointestinal and hepatobiliary malignancy | Treatment: higher radiation dose to bowel, especially dose >30 Gy, higher daily fraction dose, alkylating agent chemotherapy | History and physical exam annually Oncology and surgical consultation as clinically indicated See Table 12.3 for colorectal cancer screening guidelines | |
Whole abdomen Hepatic Total body irradiation | Hepatic fibrosis Cirrhosis | Treatment: radiation dose >40 Gy to at least 1/3 of liver volume or 20–30 Gy to entire liver Medical conditions: chronic hepatitis, iron overload Health behaviors: alcohol use | History and physical exam annually Laboratory: ALT, AST, bilirubin baseline at entry into long-term follow-up Screen for viral hepatitis in patients with persistently abnormal liver function Hepatitis A and B immunizations for patients lacking immunity Gastroenterology/hepatology consultation as clinically indicated |
Aside from screening levels of vitamin B12 and folate, laboratory studies are largely obtained to assess secondary effects of GI tract pathology. Anemia and microcytosis may reflect iron deficiency from GI tract blood loss or from malabsorption. Macrocytic anemia may result from vitamin B12 or folate deficiency associated with chronic malabsorption or resection of the terminal ileum. Albumin and serum protein are useful to assess protein stores in patients with chronic diarrhea or fistulas and should be monitored periodically in individuals with chronic malabsorption. A hemoglobin and hematocrit provides useful information in patients who present with hematochezia, but further radiographic and endoscopic evaluation is typically needed to identify the site and specific pathological lesion. Gastroenterology or surgical consultation may be required to establish the diagnosis or provide remedial interventions.
In the absence of abdominopelvic radiation fields, the preferred screening evaluations for GI tract malignancies are the same as for the asymptomatic general population: beginning at age 50, patients should initiate screening with tests that can detect cancer (e.g., guaiac-based fecal occult blood test, immunochemical fecal occult blood test, or stool for exfoliated DNA) and advanced lesions (e.g., flexible sigmoidoscopy, colonoscopy, double-contrast barium enema, computed tomography colonography) (Table 12.3) [52]. Several studies have described an excess risk of gastrointestinal malignancies in childhood cancer survivors treated with abdominal/pelvic radiation [20–22]. Since the median reported age of onset and time since primary cancer of this complication is 33 years age (range 9.7–59 years) and 23 years (range 2–49 years), respectively, it seems prudent to consider an earlier onset of screening colonoscopy in at-risk childhood cancer survivors or in survivors with risk who are symptomatic. The current Children’s Oncology Group Guideline recommends monitoring for occult colorectal malignancies to begin 10 years after radiation or at age 35 years (whichever occurs last) in asymptomatic survivors (Table 12.3) [53]. While the efficacy of this approach has not been validated in longitudinal trials, this represents a conservative strategy that would increase the likelihood of early detection of an occult GI malignancy of the lower GI tract.
Table 12.3
Colorectal cancer (CRC) screening in childhood cancer survivors
CRC risk groups | Risk group definition | Recommended surveillance |
|---|---|---|
Average risk related to cancer treatment | Survivor without high-risk cancer treatment exposures, familial or hereditary risk, or history of inflammatory bowel disease | Beginning at age 50 yearsa: Annual high-sensitivity guaiac-based fecal occult blood test or immunochemical fecal occult blood test combined with one of the following: Flexible sigmoidoscopy every 5 years or Colonoscopy every 10 years or Double-contrast barium enema every 5 years or Computed tomography colonography every 5 years |
High risk related to family history or genetic syndrome | Family history of CRC or adenomatous polyps in first-degree relative before age 60 or in 2 or more first-degree relatives at any age Known or suspected presence of familial adenomatous polyposis (FAP) or hereditary nonpolyposis colorectal cancer (HNPCC) | Early and heightened surveillance with colonoscopy based on specific conditionb: Beginning at age 40 or 10 years before the youngest case of first-degree relative with CRC or adenomatous polyps Beginning at age 10–12 years for FAP Beginning at age 20–25 years or 10 years before the youngest case in the family for HNPCC |
High risk related to inflammatory bowel disease | History of chronic ulcerative colitis or Crohn’s disease | Early and heightened surveillance with colonoscopy every 1–2 years: Beginning 8 years after the onset of pancolitis or 12–15 years after the onset of left-sided colitis |
High risk related to cancer treatment | Treatment with radiation fields involving the colon and rectum: all abdominal (e.g., upper quadrant, flank, whole) and pelvic fields, spinal (thoracic, lumbar, sacral, whole), total body irradiation,c total lymphoid irradiation | Beginning at age 35 years or 10 years postradiation therapy (whichever occurs last)c: Colonoscopy every 5 years |
12.2.4 Management of Established Problems
Optimal management of cancer-related GI complications requires cooperation between the survivor’s primary physician, gastroenterologist, and surgeon. Conditions requiring intervention most often result from primary or secondary consequences of chronic inflammation or fibrosis of GI tissues. Esophagitis and gastritis are usually managed by dietary modification and a variety of pharmacologic agents, including H2 antagonists and proton pump inhibitors, which eliminate or reduce acid production by the parietal cells. Prokinetic agents are also helpful if acid reflux is contributing to chronic esophagitis. Adjunctive agents like Carafate, which binds to the proteins of denuded mucosa, may also be used as a physical barrier to mucosal irritation.
Upper GI tract strictures are best evaluated by barium swallow (Fig. 12.2) followed by endoscopy. Repeated endoscopy and dilation procedures may be required for long-term management of esophageal strictures. Concurrent evaluation and treatment of peptic esophagitis is important to reduce the risk of further mucosal injury. Surgical procedures that reduce or prevent reflux may also be undertaken to minimize the risk of recurrent stricture formation. Patients who have unrevealing barium swallow study or upper endoscopy may have dysphagia resulting from abnormal motility associated with neuronal injury; this can be identified through manometric studies. Chronic constipation and obstipation may be predisposed by more severe motility abnormalities. Aggressive treatment with laxatives and enemas is required for individuals who develop stool impaction. Regular treatment with stool softeners and peristaltic stimulants reduces the risk of this complication.


Fig. 12.3
Colonic mucosa in graft-versus-host disease. Glands show architectural disruption with infiltration of lymphocytes accompanied by epithelial cell necrosis and dropout. Hematoxylin and eosin stain, 20× original magnification (Courtesy of Dr. J. Jenkins)
Chronic enteritis may be associated with malabsorption. Affected patients present with chronic diarrhea, abdominal distension, failure to thrive, and other signs of protein deficiency. In cases with malabsorption, contrast studies are used to determine the site of pathology and small bowel biopsies to define the histologic features. Individual tests of absorption, including the d-xylose absorption test, lactose breath hydrogen test, 72-h fecal fat determinations, serum B12 and folate levels, measurement of stool pH and reducing substances, and the Schilling test, are used to define the defect. Bacterial contamination of the small bowel may also predispose to malabsorption; strictures and blind loops may also contribute to this complication. Intubation and quantitative bacterial cultures of the small bowel may be required to confirm the diagnosis, especially in the absence of the ileocecal valve or in the presence of small bowel stasis. Bacterial overgrowth responds to appropriate antimicrobial therapy like tetracycline or metronidazole. Chronic diarrhea associated with bile salt malabsorption after ileal resection may improve with cholestyramine. Refractory malabsorption associated with villous atrophy may require enteral or parenteral nutritional support.
While the management of acute GI bleeding is generally easily accomplished in the primary care setting, evaluation and treatment of chronic GI blood loss associated with chronic enterocolitis or proctitis usually requires more extensive evaluation by subspecialists to localize the site of bleeding. Following contrast diagnostic imaging studies, endoscopic evaluation is used to confirm inflammation, ulceration, or less commonly malignancy. Biopsy will determine the etiology of mucosal lesions. Only rarely are more aggressive interventions, for example, enterolysis, tagged red blood cell study, or exploratory laparotomy, needed to localize the site of GI tract bleeding.
Patients presenting with signs and symptoms of bowel obstruction should be promptly evaluated with abdominal radiographs, decompression (if clinically indicated), and the appropriate contrast imaging. Chronic or refractory intestinal obstruction associated with strictures or adhesions may eventually require surgical intervention for definitive correction.
12.3 Hepatobiliary Tree
12.3.1 Pathophysiology
12.3.1.1 Normal Anatomy and Physiology
The liver is the largest organ in the body and consists of right and left lobes joined posteroinferiorly at the porta hepatis. The gallbladder lies under the visceral surface of the liver. The hepatic lobule, which contains a central vein that is a tributary of the hepatic vein, is the basic ultrastructural unit of the liver. The central vein of each hepatic lobule drains into the inferior vena cava. Columns of hepatocytes radiate from the center of each lobule and are separated by sinusoids. Hepatic sinusoids are lined with reticuloendothelial or Kupffer cells. The hepatic lobule is divided into three functional zones that each receives blood of varying nutrient and oxygen content. Zone 3, which receives the least oxygen and nutrients, is the most vulnerable to injury. Hepatic arterioles, portal vein radicles, and branches of the left and right hepatic ducts are located in portal triads between the hepatic lobules. The left and right hepatic ducts fuse at the porta hepatis to form the common hepatic duct. The cystic duct, which drains the gallbladder, joins the hepatic duct to form the common bile duct that drains into the duodenum.
Liver function is diverse and includes synthesis of enzymes, albumin, coagulation proteins, urea, and steroids such as cholesterol and primary bile acids, conjugation of bilirubin, detoxification of drugs, and storage of fat-soluble vitamins. Hepatocytes are also responsible for gluconeogenesis and glycolysis. The Kupffer cells, which engage in phagocytosis and secrete cytokines, play a role in immune regulation.
12.3.1.2 Changes Induced by Cytotoxic Therapy
Fibrosis, which is generally periportal and concentric (Fig. 12.4), is the most frequently described long-term complication of antineoplastic therapy observed in the liver [56]. Hepatic fibrosis may be associated with fatty infiltration, focal necrosis, nodular regeneration or cirrhosis, and portal hypertension. Chronic hepatitis, regardless of its etiology, is characterized by portal-periportal lymphoplasmacytic infiltration with varying degrees of fibrosis and piecemeal necrosis. The histopathology of sinusoidal obstruction syndrome (SOS) [formerly termed venoocclusive disease, VOD] demonstrates endothelial injury, fibrin deposition, and microthrombi in hepatic sinusoids; if progressive, fibrous obliteration of central veins, centrilobular necrosis, and hepatic outflow obstruction ensue. GVHD is associated with hepatocellular necroinflammatory changes suggestive of chronic active hepatitis, a paucity of interlobular bile ducts, and intrahepatic cholestasis (Fig. 12.5) [14, 19]. Cell-mediated immune dysregulation is implicated in the pathophysiology of chronic GVHD in most studies. However, findings of deposits of immunoglobulin and complement along the dermal-epidermal junction in 85 % of individuals with chronic GVHD suggest that humoral mechanism may play a role as well [57]. Hepatocellular carcinoma has been rarely reported in patients treated with chemotherapy and radiation therapy for Wilms tumor and in long-term survivors of childhood leukemia with chronic hepatitis C infection [58–60].
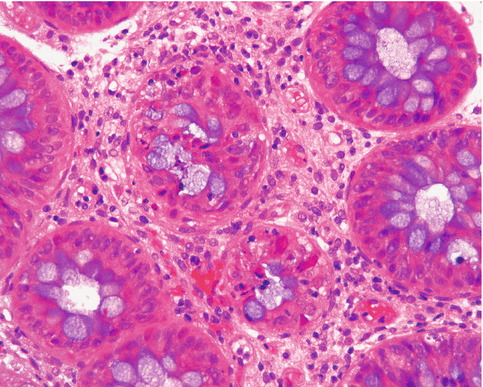
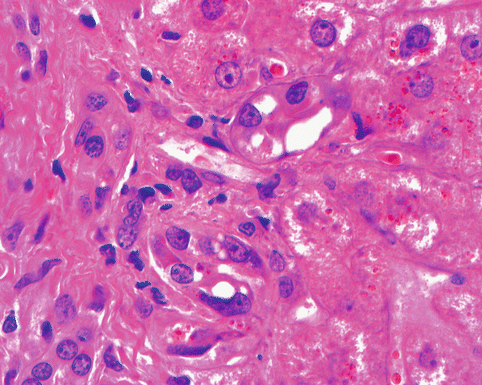

Fig. 12.4
Chronic methotrexate liver damage. There is fatty change together with chronic portal inflammation and fibrosis. Hematoxylin and eosin stain, ×180. Reprinted with the kind permission of Dr. R.S. Patrick and Chapman Hall, Ltd.)

Fig. 12.5
Hepatic portal triad in graft-versus-host disease. Bile ducts show epithelial damage in the form of irregular cellular and nuclear size and spacing around the duct lumen with some epithelial cells completely missing. Epithelial cell cytoplasm is vacuolated and shows variable eosinophilia. In this example a paucity of lymphocytes is noted. Hematoxylin and eosin stain, 60× original magnification (Courtesy of Dr. J. Jenkins)
12.3.2 Clinical Manifestations
Liver injury related to treatment for childhood cancer is most often subclinical and may develop without a history of prior acute toxicity. Asymptomatic abnormalities of serum transaminases (alanine aminotransferase and aspartate aminotransferase) are most commonly observed. In the setting of chronic GVHD, alkaline phosphatase and bilirubin may also be elevated. However, bilirubin elevation is variable and does not correlate with clinical outcome. Patients with chronic liver dysfunction frequently report constitutional complaints like pruritus, fatigue, and weight loss. In contrast, hepatic sinusoidal obstruction syndrome (or venoocclusive disease) is characterized by the acute onset of jaundice, right upper quadrant pain, hepatomegaly, ascites, liver dysfunction, and thrombocytopenia. The clinical course of sinusoidal obstruction syndrome varies from mild and reversible to life threatening with progressive hepatic failure. The impact of acute sinusoidal obstruction syndrome on long-term hepatic function has not been established. Chronic progressive hepatic fibrosis associated with cirrhosis may cause portal hypertension and resulting sequelae like hypersplenism and variceal bleeding. If cirrhosis leads to liver decompensation, clinical signs and symptoms of hepatic synthetic dysfunction appear including ascites and coagulopathy. As hepatic failure ensues, affected individuals manifest progressive metabolic derangements and encephalopathy.
12.3.2.1 Radiation
The majority of reports describing acute radiation hepatopathy in pediatric patients involve obsolete radiation technology and treatment approaches. Radiation-induced liver disease (RILD) typically presents in the first 12 weeks after completion of radiation and histologically demonstrates endothelial cell injury [61]. Persistent or late onset radiation hepatopathy after contemporary treatment is uncommon in the absence of other predisposing conditions, for example, viral hepatitis. The low frequency of hepatopathy after contemporary radiation suggests that the liver has the potential for complete resolution of acute hepatic injury, but this has not been confirmed in prospective studies in pediatrics.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree