Stage
Weeks
Major developments
Embryonic
4–9
Formation of major airways
Pseudoglandular
5–17
Formation of bronchial tree and portions of respiratory parenchyma
Birth of the acinus
Canalicular
16–27
Last generations of the lung periphery formed
Epithelial differentiation
Air–blood barrier formed
Saccular
24–38
Expansion of air spaces
Surfactant detectable in amniotic fluid
Alveolar
36–3 years
Secondary septation
The alveoli phase of development extends from 36 weeks gestation through about 3 years of age. At birth, there are few alveoli present, but potential airspaces are identifiable as smooth-walled ducts and saccules with thickened septa. Inflation of the saccules occurs at birth. The septa become thin and grow into air spaces, forming partitions within the pouches. Within a few months the infant’s alveoli resemble those of the adult, with greatly increased surface area available for gas exchange. Completion of the vascularization process during this time results in single capillary networks associated with each area of gas exchange [118, 150]. After birth, minor structural changes continue to occur. The alveolar surfaces become more complex, and the alveoli become more numerous with the increase in body size.
The most rapid phase of pulmonary growth occurs within the first few years of life, followed at 4–6 years of age by a slow growth phase. According to autopsy studies [99], the maximum numbers of alveoli are achieved by approximately 8 years of age. After this point it is believed that alveolar surface volume increases without an increase in alveolar number [149]. However, recent studies have suggested that formation of new alveoli may continue into adolescence [99]. In regard to musculoskeletal development, radiographic measurement of lung diameters demonstrates linear growth during childhood and a spurt at puberty [142].
11.1.2 Pathophysiologic Changes Induced by Cytotoxic Therapy
Primary lung malignancies are very rare in children; however, the lung is a common site for metastases, sometimes even years after the completion of definitive therapy. Therapy-related pulmonary toxicity is due to either local therapies (radiation or surgery) directed at the lung parenchyma and chest wall or chemotherapy that is administered systemically but likewise negatively affects these organs and structures. Of course, many patients receive multimodality treatment that potentially compounds acute and late toxicity.
The pathogenesis of pulmonary toxicity secondary to cytotoxic therapy is largely based on animal experimentation. However, it is believed to occur through one of four mechanisms of injury: (1) DNA damage from the drug or radiation itself, (2) damage inflicted by free radical generation, (3) allergic response to the cytotoxic agent, and (4) subsequent injury induced by the inflammatory response to the primary damage itself. Pulmonary fibrosis, which mediates many of the long-term effects, can arise from collagen deposits that occur after cellular damage. In addition, the breakdown of actual lung tissue can trigger an inflammatory reaction that activates increased production of elastin by actin-expressing smooth muscle cells. This also results in pulmonary fibrosis. While there are several postulated means of injury to the pulmonary tissue, the common finding in all is diffuse alveolar damage. The cytotoxic changes start as endothelial blebs in the alveolar capillaries and lead to capillary leak syndrome. These are then associated with interstitial edema. There is destruction and a resulting decrease in number of Type I pneumocytes, as well as reactive changes and proliferation of Type II pneumocytes. More recently, progress has been made in understanding the molecular and cellular events after radiation lung injury, leading to clinically and histologically recognizable changes. The process appears to be dynamic and to involve proinflammatory cytokines, profibrotic cytokines, chemokines, and adhesion molecules in modulating and recruiting immune cells to the sites of radiation lung injury [24]. Long-term effects on the lungs are the result of this complex process.
11.1.2.1 Pathophysiology of Chemotherapy-Induced Disease
Drug-related pulmonary disease may be the result of toxicity, allergy, or idiosyncrasy [39]. Toxicity, with a dose–response, has been shown for bleomycin, chlorambucil, and the nitrosoureas. Pulmonary damage, likely mediated through allergic mechanisms, is caused by cyclophosphamide, methotrexate, procarbazine, and bleomycin. Pulmonary disease has also been associated with mitomycin, cytosine arabinoside, the vinca alkaloids, and alkylating agents.
Bleomycin may be the most commonly recognized cause of pulmonary toxicity; the pathogenesis of bleomycin injury has been studied extensively [29, 58, 71]. Lung injury following low-dose bleomycin may be idiosyncratic, possibly attributable to genetically impaired drug metabolism. Having inherently low levels of bleomycin hydrolase [76], an enzyme that inactivates bleomycin, the lung is particularly vulnerable to bleomycin injury. Mouse data demonstrate that strain sensitivity to bleomycin is related to different levels of bleomycin hydrolase activity [62]. Hence, individual variations in bleomycin sensitivity may be explained at least in part by genetically determined levels of bleomycin hydrolase activity. Free radical formation and oxidative damage also play a role in bleomycin-induced lung injury. Fibrosis after bleomycin therapy develops under the influence of immune processes that include activation of effector cells, including alveolar macrophages, and release of cytokines. Tumor necrosis factor may play a pivotal role [82, 106]. Pathology demonstrates endothelial and Type I cell necrosis with Type II hyperplasia and hyaline membranes. Bleomycin-induced pulmonary effects usually occur during or within a year of treatment.
Alkylating agents, such as the nitrosoureas, are known to cause late-onset pulmonary fibrosis. The fibrosis noted after nitrosourea therapy demonstrates less inflammation than bleomycin-induced fibrosis, but consistency in Type I depletion and Type II hyperplasia with excess collagen deposition. The formation of free radicals and lipid peroxidation of phospholipid membranes may also be the mechanism by which cyclophosphamide and mitomycin damage the capillary endothelium [28]. Permeability increases, resulting in interstitial edema. Hyaline membranes form as plasma proteins, and fluid enters the alveoli through the denuded epithelium. Type I pneumocytes swell, become necrotic, and are replaced by cuboidal cells. Proliferation of fibroblasts then occurs. This process may evolve slowly, with fibrosis increasing over a period of years. Interstitial pneumonitis (either the desquamative type that appears to be an earlier stage or the usual type with fibrinous exudation, hyaline membranes, and interstitial fibrosis) is also seen with alkylating agents. This pneumonitis may lead to the development of chronic pulmonary fibrosis that is characterized by the enhanced production and deposition of collagen and other matrix components. Pulmonary veno-occlusive disease, with vasculitis and intimal fibrosis resulting in pulmonary hypertension, has been reported after either bleomycin or mitomycin [34].
11.1.2.2 Pathophysiology of Radiation-Induced Disease
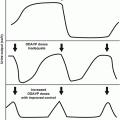
Similar histopathologic changes and resultant physiologic abnormalities are found in the lung following radiotherapy and chemotherapy. Subclinical injuries resulting from radiation to the lung are most likely present in all patients, even after very small doses of radiation. Studies of the immunological regulation of inflammation after radiation in animals have revealed a complex interaction between local tissues, resident cells, and circulating immune cells, mediated through chemokines, adhesion molecules, inflammatory cytokines, and fibrotic cytokines. Chemokine monocyte chemotactic protein-1 (MCP1) [66], adhesion molecules (intercellular adhesion molecule-1 [ICAM-1]) [56, 69], and interferon-inducible protein-10 (IP-10) [66] appear to be involved in initiating radiation lung injury [24, 54, 56, 66, 124, 128, 148]. Afterward, there appears to be a cascade of proinflammatory cytokines and fibrotic cytokines (Fig. 11.1) [129].
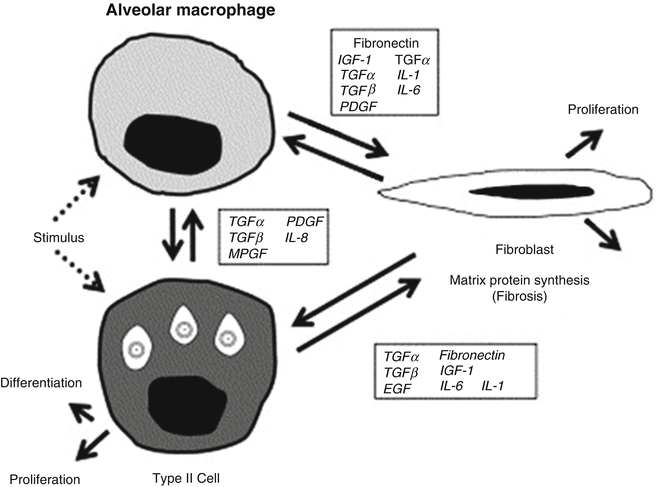

Fig. 11.1
Cell–cell interaction and control of gene expression by growth factors in lung injury (With permission from Rubin et al. [129])
In the first few days to weeks after irradiation, ultrastructural alterations in the capillary endothelial lining become evident. The cells become pleomorphic and vacuolated and may slough, thereby producing areas of denuded basement membrane and occlusion of the capillary lumen by debris and thrombi [51, 79, 86, 116]. There is exudation of proteinaceous material into the alveoli, leading to impairment of gas exchange. Studies have shown that radiation-induced lung injury is characterized by alveolar infiltrates of mononuclear cells, primarily CD4+ T cells and macrophages/monocytes (mononuclear alveolitis), and that there is a relative scarcity of neutrophils [40, 43], a common marker for infectious processes. Lavage fluids obtained from bronchoscopy have confirmed this finding in patients with active pneumonitis [84, 98, 124]. In a few weeks the interstitial edema organizes into collagen fibrils, which eventually leads to thickening of the alveolar septa. These exudative changes may resolve in a few weeks to a few months. However, depending on the volume of lung parenchyma irradiated, the total dose, and the dose per fraction, the changes can result in an acute radiation pneumonitis.
Although no specific lesion is entirely characteristic of pneumonitis, current evidence suggests that damage to the Type II pneumocyte and to the endothelial cell is closely linked to the pneumonitic process [21, 113, 151, 152]. The type II pneumocyte, which produces surfactant and maintains patent alveoli, has been well studied. After radiation exposure a rapid decrease in the content of cytoplasmic surfactant-containing lamellar bodies occurs, followed by the ultimate sloughing of some of the cells into the alveolar lumen [111, 112]. Changes in the surfactant system that lead to alterations in alveolar surface tension and low compliance are most likely a direct result of the radiation [113, 130, 131], although it has been postulated that the changes indirectly result from exudation of plasma proteins [52]. Endothelial cell damage results in changes in perfusion and permeability of the vessel wall. Endothelial leakage and increased permeability allow immune cells to undergo transendothelial migration and extravasation from the vascular compartment into the alveolar space.
Late lung injury is characterized by progressive fibrosis of the alveolar septa, which become thickened by bundles of elastic fibers. The alveoli collapse and are obliterated by connective tissue. The mechanisms of chronic injury may be related to the effects of radiation on the pulmonary vasculature (endothelial cells) or somatic cells. The nature of the triggering event in the pathogenesis of radiation-related lung fibrosis is complex. The classic hypothesis that fibrosis is a connective tissue replacement process following parenchymal cell death has been challenged, and the exact mechanisms of early injuries leading to the late effects are not entirely understood. Cytokine-mediated multicellular interactions that initiate and sustain the fibrogenic process take place within hours to days after radiation in animal research models. Experimental data suggest that the progression of the initial lung injury to the pneumonitic phase may be the result of a cytokine and cellular interaction, which subsequently regulates the fibrotic phase of the presentation [24, 127–129]. In addition, it has been recently hypothesized that chronic hypoxia, and the perpetual injury to normal tissue through reactive oxygen species, may also be a contributing mechanism to chronic and progressive fibrosis [158]. Studies in animals have confirmed the protective effect of fractionated radiation therapy, which is several small doses of radiation as compared to a comparable dose delivered in a single large treatment, indicating a significant degree of recovery of lung tissue between fractions [141, 160].
11.1.2.3 Categorization of Pulmonary Disease
Lung disease may be categorized as follows: interstitial, obstructive, restrictive, or a combination. Most long-term toxicity is the result of interstitial disease, which involves inflammation and fibrosis of the alveolar walls and changes in the capillary endothelium and alveolar epithelial lining cells, as described below. The histologic hallmarks of interstitial lung disease are proliferation of fibroblasts and excessive deposition of collagen [135]. Interstitial lung disease also may impact the small airways. As alveolar–capillary membrane destruction is an integral part of interstitial disease, pulmonary function tests demonstrate a decrease in the measured diffusing capacity [135].
Pulmonary disease after chemotherapy or radiation therapy may also have an obstructive component. Obstructive diseases result from airway narrowing. This may be due to bronchospasm, mucus, or luminal narrowing as a result of edema and inflammation or scarring [97]. Airway narrowing due to disease can be detected as a decrease in expiratory airflow. Pulmonary function tests demonstrate a decrease in the ratio of the volume exhaled in 1 s (FEV1) to the total exhaled forced vital capacity (FVC).
Restrictive lung diseases occur as a result of alterations in the elasticity of the pulmonary system [42], which may be due to parenchymal disease originating in the lung or from structural anomalies of the chest wall. In the healthy lung, collagen and elastin fibers contribute to the formation of an organized web, which has a significant ability to stretch and recoil [42]. Disruption of this organization from inflammation and fibrosis occurs as a result of injury and response to cytotoxic therapy, thereby decreasing the elasticity of the lung and resulting in restrictive disease. Additionally, in the child, cytotoxic therapy may impair the proliferation and maturation of alveoli, leading to inadequate alveoli number and lung growth and resulting in chronic respiratory insufficiency.
Restrictive lung disease may also occur as a result of growth impairment of the lung or musculoskeletal structures, which is predominantly a consequence of radiation therapy. Inhibition of growth of the thoracic cage (i.e., muscle, cartilage, and bone) can limit chest wall compliance, with resultant restrictive problems. Naturally, younger children are more vulnerable to chronic respiratory damage from impairment of the normal growth and development of the lungs and the thoracic cage.
In restrictive respiratory disease, pulmonary function testing demonstrates an increased FEV1/FVC ratio with an increased maximal expiratory airflow. With more advanced restrictive disease, total lung capacity, vital capacity, and lung volumes are decreased, with evidence of uneven distribution of ventilation [97]. Please refer to Sect. 11.3.1 for a detailed discussion of pulmonary function tests.
11.2 Clinical Manifestations
11.2.1 Long-Term Effect in Pediatric Population
Potential pulmonary complications of therapy leading to a range of respiratory manifestations have been reported by the Childhood Cancer Survivor Study. Study participants were asked whether they had ever been told by a physician, or other healthcare professional, that they have or had a particular diagnosis, such as pulmonary fibrosis. This self-report study demonstrated that long-term survivors described a statistically significant increased relative risk of lung fibrosis, recurrent pneumonia, chronic cough, pleurisy, use of supplemental oxygen, abnormal chest wall, exercise-induced SOB, bronchitis, recurrent sinus infection, and tonsillitis for all time periods, including during therapy, from the completion of therapy to 5 years off therapy and >5 years after therapy. Significant associations existed between the development of fibrosis and treatment with radiation therapy and between the use of supplemental oxygen, recurrent pneumonia, chronic cough, and pleurisy and treatment with radiation therapy and/or multiple chemotherapy agents [92]. In regard to mortality, a large retrospective study of greater than 20,000 5-year survivors of childhood cancer reported a significant excess rate of deaths largely due to treatment-related causes rather than progression or recurrence of the primary disease; furthermore, pulmonary causes accounted for excess mortality risk (standard mortality ratio, 8.8) second only to death from secondary malignancy (standard mortality ratio, 15.2) [7].
Pulmonary toxicity is frequently reported in survivors of Hodgkin lymphoma, but it also complicates the cure of survivors of germ cell tumors, rhabdomyosarcoma, neuroblastoma, bone tumors, and acute lymphoblastic leukemia (ALL) [16, 55, 61, 67, 72, 94, 101, 102]. Although many of the patients that have been studied received radiation therapy, the intensified use of chemotherapy accounts for some or all of the toxicity in subsets of survivors. In fact, pulmonary complications are a major cause of morbidity and mortality following hematopoietic stem cell transplant [145], which is now an established treatment approach for many pediatric malignancies.
Studies with long-term follow-up suggest that the cumulative incidence of pulmonary complications increases with increasing time since treatment [63]. This appears to be far in excess of decreases in lung function with normal aging, suggesting that the effects of early lung injury from cancer therapy compound expected decreases and that survivors should be monitored indefinitely for new-onset pulmonary morbidity as they age.
11.2.2 Radiotherapy: Clinical Presentations
Pneumonitis and pulmonary fibrosis are the two most important consequences of irradiation of the lung. Pulmonary fibrosis occurs in almost 100 % of patients receiving high doses of radiation, but it may not be of clinical significance if the volume is small. The clinically significant presentation of pulmonary toxicity is usually pneumonitis, due to its prevalence and potential morbid outcome. The presentation varies with the type of lung injury present. Often there are complaints of a nonproductive cough, fever, and dyspnea. However, the presentation can also be quite acute with respiratory insufficiency and acute respiratory distress syndrome (ARDS). Other presentations include bronchospasm, pleural effusion, bronchiolitis obliterans, pulmonary veno-occlusive disease, sarcoidosis, pulmonary alveolar proteinosis, pneumothorax, and pulmonary hemorrhage.
When radiation therapy is the only modality used, radiation pneumonitis follows the completion of the definitive course of treatment. Cough, pink sputum, dyspnea, and pleuritis are common complaints during the subacute pneumonitic phase, which generally occurs 1–3 months after completion of radiation. When chemotherapy is administered in conjunction with radiation, as in total body irradiation (TBI) and BMT-conditioning regimens, reactions can occur during treatment. The fibrotic phase of radiation injury starts 3–6 months after completion of radiation and is progressive. The clinical manifestations of fibrosis are worsening dyspnea, increasing probability of oxygen dependence, and declining pulmonary function test results.
11.2.2.1 Subacute Radiation Pneumonitis
Subacute pneumonitis is a pneumonopathy that usually occurs 1–3 months after the completion of radiation. It is well described in the adult literature after the treatment of lung cancer [5, 6, 122], but little of the data from such studies are relevant to modern pediatric cancer therapies. Pneumonitis can occur unexpectedly, with little or no warning. Because of this, many attempts have been made to identify clinical risk factors. The factors that influence risk include total lung radiation dose, irradiated lung volume exceeding 20 Gy vs 25 Gy vs 30 Gy, mean lung dose, fractionation of radiotherapy, daily fraction size, performance status, pre-treatment pulmonary function, gender, low pre-treatment blood oxygen, and high C-reactive protein [49, 59, 64, 74, 123, 125, 136].
Symptoms of subacute pneumonitis syndrome include low-grade fever and nonspecific respiratory symptoms such as congestion, cough, and fullness in the chest. In more severe cases, dyspnea, pleuritic chest pain, and nonproductive cough may be present. Later, small amounts of sputum, sometimes bloodstained, may be produced. Physical signs in the chest are usually absent, although a pleural friction rub or pleural fluid may be detected. Evidence of alveolar infiltrates or consolidation is sometimes found in the region corresponding to pneumonitis. This results from an acute exudative edema that is initially faint but may progress to homogenous or patchy air space consolidation. Frequently there is an associated volume loss in the affected portion of the lung.
CT studies of the lung have been used to evaluate lung density in this situation. Because of its sensitivity to increased lung density, CT has been favored for radiographic detection of pulmonary damage in humans [81, 85, 155]. CT findings demonstrate a well-defined, dose–response relationship [85]. Four patterns of radiation-induced changes have been defined in lung on CT: homogenous (slight increase in radiodensity), patchy consolidation, discrete consolidation, and solid consolidation [81]. These patterns, corresponding to both pneumonitic and fibrotic phases, have varying timetables and may appear weeks to years after radiotherapy.
11.2.2.2 Radiation Fibrosis
In contrast to the acute reaction, clinically apparent chronic effects of cytotoxic therapy may be observed from a few months to years following treatment, even though histologic and biochemical changes are evident sooner. Pulmonary fibrosis develops insidiously in the previously irradiated field and stabilizes after 1–2 years.
The clinical symptomatology related to the radiographic changes is proportional to the extent of the lung parenchyma involved and preexisting pulmonary reserve. After thoracic radiation, restrictive changes gradually develop and progress with time [51]. Gas exchange abnormalities occur approximately at the same time as the changes in lung volumes. These abnormalities consist of a fall in diffusion capacity, mild arterial hypoxemia that may manifest only with exercise, and a low or normal PaCO2 level. The changes appear to be consistent with a parenchymal lung defect and ventilation–perfusion inequality that results in a component of effective shunt [52]. Radionuclide evaluations have demonstrated that underperfusion, rather than underventilation, is the cause of these inequalities, reflecting radiation injury to the microvasculature [119, 120, 157]. Larger doses of irradiation cause reductions in lung compliance that start at the time of pneumonitis and persist thereafter [117]. The compliance of the chest wall is usually much less affected in adults and adolescents than in young children, in whom interference with growth of both lung and chest wall leads to marked reductions in mean total lung volumes and diffusion capacity (DLCO) [165]. Whole-lung irradiation in doses of 11–14 Gy has resulted in restrictive changes in the lungs of children treated for various malignancies [10, 83, 93]. Consequently, RT in younger children, particularly those younger than 3 years old, results in increased chronic toxicity [93]. One to 2 years after radiation, clinical symptoms stabilize and are often minimal if fibrosis is limited to less than 50 % of one lung [134]. A mild deterioration in pulmonary function may be demonstrated as fibrosis develops. There is a reduction in maximum breathing capacity that is particularly evident in patients with bilateral radiation fibrosis. Tidal volume usually decreases, and breathing frequency tends to increase, resulting in an overall moderate increase in minute ventilation [139]. Most studies have found these changes to persist indefinitely, with little recovery unless there are concurrent improvements in pulmonary function with lung tumor response [41, 45, 50]. Functional compensation by adjacent lung regions [100] limits the effect of radiation on pulmonary function tests when small volumes of lung are irradiated. Dyspnea and progressive chronic cor pulmonale leading to right heart failure may occur when >50 % of a lung is irradiated.
Radiologic changes consistent with fibrosis are seen in most patients who have received lung irradiation, even if they do not develop acute pneumonitis. Chest radiographs have linear streaking, radiating from the area of previous pneumonitis and sometimes extending outside the irradiated region, with concomitant regional contraction, pleural thickening, and tenting of the diaphragm. The hilum or mediastinum may be retracted with a densely contracted lung segment, resulting in compensatory hyperinflation of the adjacent or contralateral lung tissue. This is usually seen 12 months to 2 years after radiation. When chronic fibrosis occurs in the absence of an earlier clinically evident pneumonitic phase [113, 126, 140], chest radiography generally reveals scarring that corresponds to the shape of the radiation portal. Eventually, dense fibrosis can develop, especially in the area of a previous tumor [127]. CT is currently favored to image regions subjected to RT [81, 85, 155]. Magnetic resonance imaging (MRI) is being explored and may have promise in accurately distinguishing radiation fibrosis from recurrent tumor. Although radiation tolerance doses may be exceeded, not all patients will develop complications, given that the sensitivity to radiation varies from patient to patient.
11.2.2.3 Radiation Tolerance Doses and Tolerance Volumes
Radiation-associated sequelae are dependent on radiation dose and fractionation, as well as volume of lung exposed. With high doses exceeding clinical thresholds (8.0–12.0 Gy, single dose), pulmonary reactions clinically express themselves as a pneumonitic process 1–3 months after the completion of thoracic irradiation. Lethality can occur if both lungs are irradiated to high doses (approximately 8–10 Gy, single dose, or greater than 20–25 Gy in a fractionated schedule) or if threshold doses of drugs are exceeded. Recovery from pneumonitis usually occurs, however, and is followed almost immediately by the second phase, which is progressive fibrosis. The clinical pathologic course is biphasic and again dependent upon the dose and volume of lung exposed. Lower doses of lung irradiation (approximately 7 Gy, single dose, or 15–18 Gy in a fractionated schedule) produce subclinical pathologic effects that can be expressed by added insult, such as infection or drugs.
Single Dose, Whole Lung Volume
Total body irradiation (TBI) in the setting of bone marrow transplantation (BMT) and half-body irradiation (HBI) initially used single doses of 8.0–10.0 Gy, without lung correction factors for lung density [44, 45, 47, 50–53, 57, 58, 68, 69, 71]. Death resulting from interstitial pneumonitis was often attributed to secondary opportunistic infection after BMT for leukemia. Pulmonary failure 1–3 months later was mistaken for lymphangitic carcinomatosis after HBI. At autopsy, radiation pneumonopathy became evident. Studies of fatal pneumonitis following TBI and HBI conducted by Keane et al. [71] and Fryer et al. [44] provided precise dose–response curves for injury, both with and without lung inhomogeneity correction. The threshold dose for fatal pneumonitis was 7.0 Gy with the TD5 (tolerance dose for 5 % probability of death) at 8.2 Gy, the TD50 at 9.3 Gy, and the TD90 at 11.0 Gy, corrected for pulmonary transmission. The dose–response is so sharp that a difference of 2.0 Gy could change zero mortality to 50 % lethality.
Fractionated Dose, Whole Lung Volume
The tolerance of the whole lung to fractionated doses of radiation is well described, particularly in Wilms’ tumor patients [10, 15, 83, 93, 115, 165]. In the absence of chemotherapy and with daily doses of 1.3–1.5 Gy, the TD5 is 26.5 Gy and the TD50 is 30.5 Gy. Young children experience more chronic toxicity at lower doses than older children and adults because of interference with lung and chest wall development, in addition to fibrosis and volume loss [96]. After 20 Gy, mean total lung volumes and DLCO are reduced to 60 % of predicted values [165]. Even within the dose range currently used for whole lung irradiation (11–14 Gy), restrictive changes occur [10, 83, 93].
Whole Lung Volume, Dose Rate
Fractionated Dose, Partial Lung Volume
Clinical tolerance of partial lung volumes to fractionated radiation is not well quantified. There are, however, some relevant data from Mah and colleagues [85]. They showed that, using an increase in lung density within the irradiated volume on CT in the posttreatment period as an endpoint, each 5 % increase in effective dose was associated with a 12 % increase in the incidence of pneumonitis. Doses above 30 Gy over a period of 10–15 days, and 45–50 Gy over a period of 25–30 days, caused radiographic changes in 30–90 % of patients. The need for a clinical guideline in estimating radiation injury prompted the collaborative work by a task force to address the normal tissue tolerance in the standard, fractionated radiation setting. Information was obtained from a diverse group of patients afflicted with various diseases of the thoracic region, but mostly from patients with Hodgkin’s disease, lung cancer, or a disease requiring large-volume irradiation (hemibody or total body radiation) [37]. The doses agreed on by the physicians in the taskforce are shown in Table 11.2. It is important to note that these values were defined for adult, not pediatric, patients.
Table 11.2
Lung tolerance dose (TD) in fractionated radiotherapy
Lung volume | TD 5/5a | TD 50/5b |
|---|---|---|
1/3 | 4,500 cGy | 6,500 cGy |
2/3 | 3,000 cGy | 4,000 cGy |
3/3 (whole lung) | 1,750 cGy | 2,450 cGy |
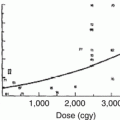
More recently, as part of the Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) effort, a logistic regression fitted to radiation pneumonitis vs mean lung dose was created from data from all published studies, again, predominantly involving adult patients, of a significant size that had extractable complication rates binned by mean dose (Fig. 11.2). The authors note that some of the variation around the fitted curve is possibly explained by differences in patient selection, as well as differences in the grade of RP reported in the various studies; however, there is a relatively small 68 % confidence interval (stippled lines). Of importance is the gradual increase in dose–response, which suggests that there is no absolute “safe” mean lung dose below which pneumonitis is certain not to develop.
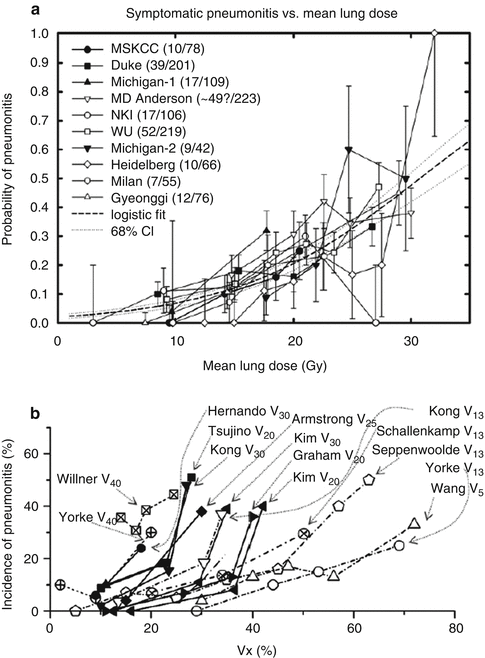

Fig. 11.2
Rate of radiation pneumonitis after fractionated partial lung radiotherapy (RT) related to (a) mean lung dose and (b) different values of Vx. Confidence intervals shown are _1 standard deviation (With permission from Marks et al. [87])
An international effort similar to the above QUANTEC analysis is currently underway specific to the pediatric population.
11.2.3 Chemotherapy: Clinical Manifestations
As increasing numbers of patients are cured with chemotherapy, reports of agents responsible for acute, and possibly chronic, pulmonary toxicity are expanding. Drug-related lung injury is most commonly an acute phenomenon, occurring during or shortly after the chemotherapeutic agent(s) is administered [28].
11.2.3.1 Patterns of Toxicity
Three typical patterns of pulmonary toxicity have been described: acute hypersensitivity (or inflammatory interstitial pneumonitis), noncardiogenic pulmonary edema, and pneumonitis or fibrosis.
Hypersensitivity reactions are rare but can be induced by such agents as methotrexate, procarbazine, bleomycin, BCNU, and paclitaxel. Cough, dyspnea, low-grade fever, eosinophilia, “crackles” on exam, and interstitial or alveolar infiltrates are noted. These reactions occur during therapy and usually resolve with discontinuation of the offending drug and, potentially, corticosteroid use.
Noncardiogenic pulmonary edema, characterized by endothelial inflammation and vascular leak, may arise upon initiation of treatment with methotrexate, cytosine arabinoside, ifosfamide, cyclophosphamide, and interleukin-2 [28, 77, 146]. All-trans retinoic acid (ATRA) syndrome, a potentially fatal cytokine release syndrome, occurs in 23–28 % of patients receiving ATRA. Pulmonary edema has also been described in patients treated with bleomycin who are exposed to supplemental oxygen. These acute reactions generally have a good prognosis. Hypersensitivity reactions and noncardiogenic pulmonary edema are unlikely to result in late-onset pulmonary toxicity.
Drug-induced pneumonitis or fibrosis has a similar clinical presentation to that described after RT. Bleomycin, the nitrosoureas, and cyclophosphamide are most commonly the etiologic agents, although methotrexate and vinca alkaloids have also been implicated [28]. This syndrome is particularly worrisome because symptoms may not be detectable until months after a critical cumulative dose has already been reached or exceeded. In addition, persistent subclinical findings may indicate a potential for late decompensation.
11.2.3.2 Specific Agents
Bleomycin
The incidence of bleomycin pulmonary toxicity is 6–10 %, with a mortality of 1–2 %. One study in children with rhabdomyosarcoma exposed to bleomycin demonstrated an incidence of toxicity of 70 % based on decreased DLCO [67]. A risk factor for bleomycin-induced pulmonary toxicity is the cumulative dose with a 10 % risk at doses of 400–500 IU/m2 [14, 132] although injury may occur at doses as low as 20 IU/m2. The elderly [14] and children or adolescents [44] may be more sensitive, especially when bleomycin is administered in conjunction with RT. Of children treated for Hodgkin’s disease with 70–120 IU/m2 of bleomycin [44], 9 % had grade 3 or 4 pulmonary toxicity, according to DLCO. Three patients (5 %) had clinical symptomatology, and one patient died. Only one patient had received RT. Although pediatric trials now use a significantly lower maximal dose than many adult studies, 80 % of the drug is excreted by the kidney, which can result in an increased risk of toxicity due to renal insufficiency [114, 143]. Other chemotherapeutic agents such as cisplatin, cyclophosphamide, doxorubicin, methotrexate, and vincristine [8, 121] may also increase risk. Exposure to high levels of oxygen or to pulmonary infection, especially within a year of treatment, is associated with a risk for immediate progressive respiratory failure [47]. Risks associated with surgery after treatment with bleomycin may be due to fluid overload [35]. These risks may persist for longer periods of time. There may be a potential increase in pulmonary toxicity with the use of granulocyte colony stimulating factor (G-CSF), which is mediated via the increased numbers of neutrophils [30].
Patients with acute bleomycin toxicity most commonly present with dyspnea and a dry cough. Fine bibasilar rates may progress to coarse rales involving the entire lung. Radiographs reveal an interstitial pneumonitis with a bibasilar reticular pattern or fine nodular infiltrates. In advanced cases, widespread infiltrates are seen, occasionally with lobar consolidation [132]; however, the consolidation may involve only the upper lobes. Large nodules may mimic metastatic cancer [89]. Loss of lung volume may occur. Pulmonary function testing reveals a restrictive ventilatory defect with hypoxia, hypocapnia, and chronic respiratory alkalosis due to impaired diffusion and hyperventilation [154]. The DLCO is thought by some to be the most sensitive screening tool for bleomycin toxicity [154]. In patients who develop mild toxicity, discontinuation of bleomycin may lead to a reversal of the abnormalities [32], but some patients will have persistent radiographic or pulmonary function abnormalities [9, 107, 164].
Nitrosoureas
The risk of nitrosourea pulmonary toxicity is age and dose dependent with patients who have received higher doses of nitrosoureas (e.g., greater than 1,500 mg/m2 in adults and 750 mg/m2 in children) more likely to present with an interstitial pneumonitis identical to that seen after bleomycin therapy [1]. Fibrosis may be early onset or late onset. Radiation therapy also increases risk, as does underlying pulmonary abnormality, such as chronic obstructive pulmonary disease, although this is rarely a factor in children. Bone marrow transplant patients may develop pulmonary fibrosis with BCNU as one of the contributing etiologies [103]. As part of a preparative regimen including etoposide and melphalan, BCNU at 600 mg/m2 was associated with unacceptable pulmonary toxicity, but doses of 450 mg/m2 were tolerated in the acute period [2]. Chemotherapy prior to bone marrow transplant may induce inflammatory changes that render the lung more susceptible to further, potentially irreversible, injury with high-dose therapy [12]. Although pulmonary fibrosis has been most commonly associated with BCNU, it has been described after other nitrosoureas as well [13, 33]. Bibasilar rales with a bibasilar reticular pattern may be seen on chest radiograph, and restrictive ventilatory defects are seen as well. Abnormalities may be restricted to the upper lobes. A decreased diffusion capacity may precede all other signs [137]. Discontinuation of therapy may alter the course of BCNU-induced pulmonary disease. However, once pulmonary infiltrates are noted, the disease may be irreversible [162]. In a documented study, 47 % of survivors of childhood brain tumors treated with BCNU and radiation died of lung fibrosis, 12 % within 3 years of treatment, and the remainder 6–17 years posttreatment. Additional patients were known to have pulmonary fibrosis and remained at risk for late decompensation. In this study, age was a risk factor. The median age of the patients who died was 2.5 years, while the median age of survivors was 10 years. In fact, all patients treated under the age of 5 years had died [104].
Cyclophosphamide
Fibrosis after treatment with cyclophosphamide is rare, with a reported incidence less than 1 %. However, one study [72] found that 4 of 15 children treated with high-dose cyclophosphamide without mediastinal RT had significantly decreased forced vital capacities; 2 of these children also had a decreased FEV1. In addition, one of the children had pulmonary fibrosis and a chest wall deformity. Two children who received more than 50 g/m2 of cyclophosphamide had delayed (greater than 7 years) fatal pulmonary fibrosis, with severe restrictive lung disease. Severely decreased anteroposterior chest dimensions in these patients were attributed to inability of the lung to grow in accordance with body growth. Fibrosis may also develop late after prolonged treatment with relatively low doses of cyclophosphamide. Although there may be recovery if symptoms occur during therapy and the drug is discontinued with administration of corticosteroids, the course may be one of progressive fibrosis nonetheless.
Hematopoietic Stem Cell Transplant (HSCT)
Patients who are treated with HSCT are at risk of pulmonary toxicity because of multiple potential factors, such as preexisting pulmonary dysfunction; the preparative conditioning regimen, which may include cyclophosphamide, busulfan, carmustine, and total body irradiation (TBI); and the presence of graft-vs-host disease [21, 78, 134, 162]. Although most transplant survivors are not clinically compromised, restrictive lung disease may occur. Obstructive disease is less common, as is the recently described late-onset pulmonary syndrome, which includes the spectrum of restrictive and obstructive disease. Bronchiolitis obliterans, with or without organizing pneumonia, diffuse alveolar damage, and interstitial pneumonia, may occur as a component of this syndrome, generally 6–12 months after transplant. Cough, dyspnea, or wheezing may occur with either normal chest radiograph or diffuse or patchy infiltrates; however, most patients are symptom-free [78, 162]. Cerveri et al. [21] evaluated pulmonary function tests in survivors of pediatric HSCT at baseline and at 3–6, 12, and 24 months after transplant. Before transplant, at 3–6 months after transplant, and at 24 months after transplant, 44 %, 85 %, and 62 % of children, respectively, had abnormal pulmonary function tests. A restrictive abnormality was most common at 3–6 months after transplant.
Other Agents
Acute pulmonary effects have occurred with cytosine arabinoside (noncardiogenic pulmonary edema) [3, 57] and vinca alkaloids in association with mitomycin (bronchospasm or interstitial pneumonitis) [29, 53], but delayed pulmonary toxicity has not been described. Hypersensitivity reactions to the antimetabolites (methotrexate, mercaptopurine, and azathioprine) may cause either a desquamative interstitial pneumonitis or an eosinophilic pneumonitis [77, 155, 159]. Recovery usually occurs within 10–45 days after methotrexate-induced pulmonary toxicity [144].
However, long-term follow-up of 26 childhood leukemia survivors revealed that 17 (65 %) patients had one or more abnormalities of vital capacity, total lung capacity, reserve volume, or diffusion capacity [138]. All children with these deficiencies were diagnosed and treated before age 8. The findings have also been attributed to an impairment of lung growth, which normally proceeds exponentially by cell division during the first 8 years of life. Other studies have also demonstrated long-term changes in pulmonary function in survivors of ALL treated without spinal radiation or bone marrow transplant [102].
Busulfan can result in late pulmonary fibrosis, with no consistently identified risk factors. Unlike many other agents, the risk does not appear to be dose related. The clinical and pathologic picture is like that of bleomycin-induced fibrosis. The mortality from busulfan fibrosis is high [1]. Although reports of pulmonary toxicity with other agents are rare, pneumonitis and fibrosis should be considered in the differential of patients presenting with respiratory symptoms. New agents may also present a risk for late pulmonary toxicity. See Table 11.2 [1, 90].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree