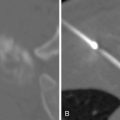
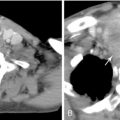
Key points
- •
Intraarterial procedures are useful for pain palliation, local disease control and as an adjunct to a planned surgical intervention.
- •
Meticulous angiographic technique and careful monitoring of the patient’s neurologic status is essential for preventing complications during embolization of tumors in the spine.
- •
Specific strategies for embolization of tumors in the spine are discussed.
Background
The arterial system provides an extensive route of access to tumors of the musculoskeletal system, allowing for the precise delivery of embolic agents or antineoplastic chemotherapy. This chapter reviews how intraarterial procedures can be used as a tool for pain palliation, local disease control, and as an adjunct to support surgical interventions.
Patient selection
Patients with primary or secondary musculoskeletal neoplasms often receive care from a multidisciplinary team of physicians, consisting of medical oncologists, orthopedic surgeons, radiation oncologists, and interventional radiologists, who make the collective decision to incorporate the use of intraarterial (IA) therapy. Evaluation of the patient in a clinic setting is essential for providing the patient with education regarding the potential risks and benefits of the procedure. A complete history and physical examination is necessary to document symptoms and their severity and to assess baseline physical function. The use of subjective pain assessment scales (Brief Pain Inventory or Memorial Pain Assessment Card) and the quantification of narcotic medications to a morphine-equivalent dose can be useful tools for determining treatment efficacy in follow-up. Patients with mild pain, <4 on a 10-point scale, usually do not experience significant pain relief following intervention and may not be appropriate candidates for arterial embolization. Patients who undergo a procedure for pain palliation should be counseled on the expected onset, degree, and duration of symptom relief. These patients require close clinical follow-up after the procedure to determine clinical effectiveness. Repeat embolizations may be indicated if pain symptoms recur or develop in a new location.
The diagnostic cross-sectional imaging must be reviewed for procedural planning. Routine laboratory values including coagulation studies and serum creatinine must be evaluated. Normal cardiac and renal function is required for the administration of chemotherapeutic agents. Patients with uncorrectable coagulopathy, renal insufficiency, severe allergic reaction contrast media, or severe atherosclerotic disease may not be candidates for angiography. ,
Technique
Intraarterial procedures are typically performed under monitored, moderate sedation using a combination of fentanyl and midazolam. Adequate hydration is recommended prior to angiography as the use of significant amounts of contrast media may be required. The placement of a urinary catheter may be helpful for monitoring urine output during the procedure and in the postoperative recovery period. Antibiotic prophylaxis is not routinely given.
Intraarterial infusion catheter placement for limb infusion
After arterial access is achieved using Seldinger’s technique, a guiding 4-F or 5-F catheter is directed into the main arterial trunk supplying the tumor. This is usually the common femoral artery for lower extremity lesions and the axillary artery for upper extremity lesions. Image acquisition during angiography should be performed in at least two different projections (anterior and lateral or oblique projections) to allow for the adequate evaluation of the arterial supply to the entire lesion. Angiography should be performed using a power injector to deliver contrast at 3 to 5 mL per second for a total volume of 12–20 mL. Image projections and contrast injection rates should be documented so that subsequent infusion sessions can use the same parameters, allowing for accurate assessment of tumor response during treatment.
Ideally, the catheter should be positioned to deliver the chemotherapy to the entire tumor while minimizing the exposure of uninvolved tissues. Once the catheter position has been optimized, an infusion wire (0.035 in. diameter, 145 cm length) with either a 6- or 12-cm working tip is inserted coaxially through the guide catheter. A longer working tip length is helpful in cases where the catheter must be placed to cross an arterial bifurcation point. For example, if a lesion derives supply from both the deep femoral and superficial femoral arteries, the working tip of the infusion wire should be placed in the deep femoral artery and extend back into the common femoral artery to ensure that chemotherapy is delivered through all arteries supplying the lesion. The infusion wire may be advanced through the guide catheter to the appropriate location in the artery; however, when feasible, a pull-back technique, where the guide catheter is retracted to uncover the infusion wire, should be used to minimize the risk of intimal injury. Following infusion wire placement, contrast injection is performed to verify the appropriate placement.
Bland arterial embolization
For embolization procedures, the arterial access can be secured with the placement of a 5-F sheath, which facilitates subsequent catheter exchanges and manipulations. A diagnostic angiogram is performed from the parent artery supplying the lesion using a 4-F or 5-F guide catheter to create a vascular map, defining the pathologic vessels that need to be targeted for embolization. Subselective catheterization of the vessels feeding the tumor is performed using a 3-F microcatheter, inserted coaxially through the guide catheter. Embolization of the tumor from a subselective location allows for tumor devascularization while minimizing the risk for nontarget embolization. Embolization is carried out until stasis of the vessel has been achieved. Postembolization angiography provides an assessment of the percentage reduction in tumor vascularity.
Spinal angiography
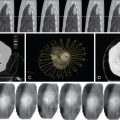
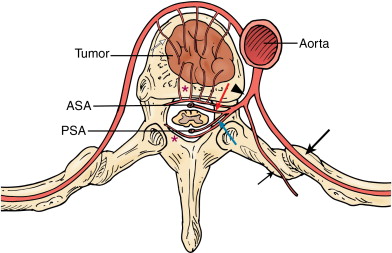
The spine is a common location for bone metastases, and preoperative embolization of spinal tumors is routinely performed to reduce intraoperative blood loss. A comprehensive knowledge of the vascular anatomy of the spine and spinal cord is essential ( Figure 30-1 ). There are three types of radicular arteries, classified according to their contribution to the spinal arteries. Simple radicular arteries supply the nerve roots and dura but do not supply the spinal cord; radiculopial arteries supply the posterior spinal arteries; and the radiculomedullary arteries supply the anterior spinal arteries, which provide the major blood supply to the spinal cord. Six to eight radiculomedullary arteries are present, each of which bifurcates near the midline into ascending and descending branches that form the anterior spinal artery. The blood supply to the spine and spinal cord via the radiculomedullary arteries varies depending on the region of the spine. In the cervicothoracic (C1-T2) and thoracic regions, an average of three to four radiculomedullary arteries supply the spinal cord. In the lower thoracic and lumbar region, a single large radiculomedullary artery, called the artery of Adamkiewicz, supplies the spinal cord and its origin can be located anywhere between T8 and L3. ,
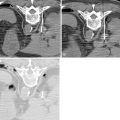
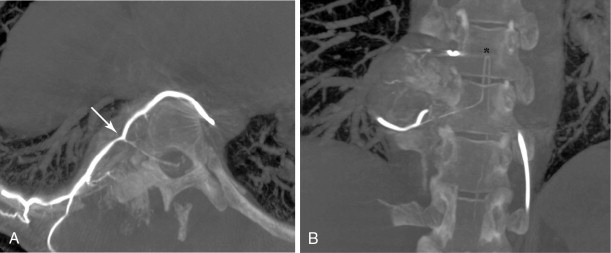
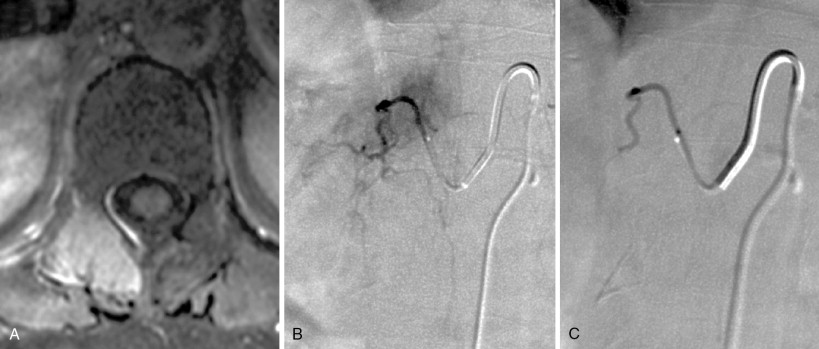
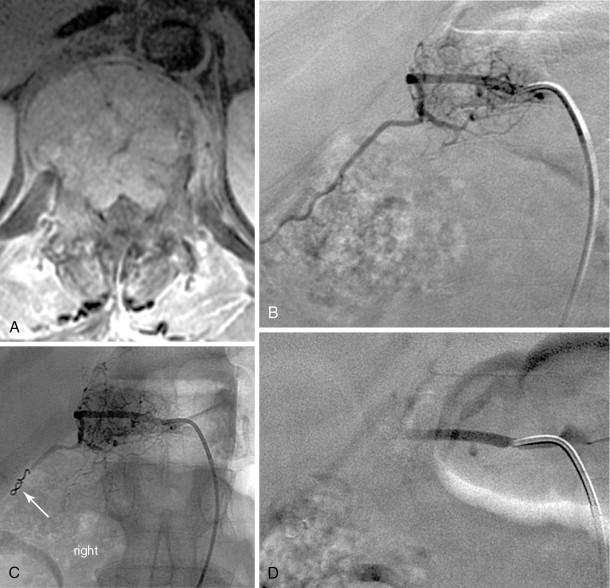
A 5-F catheter is typically used to select the segmental artery. Digital subtraction angiography is performed using hand injections of 3–5 mL of noniodinated contrast. The segmental arteries on both sides, two levels above and below the tumor site, should be interrogated to define the arterial supply to the tumor and the proximity of the origin of the anterior spinal artery, if present. Table 30-1 lists the arteries supplying the vertebral column that need to be investigated for tumor embolization in each spinal region. It is recommended that images be acquired with a large field of view at a high frame rate (6/second) until the draining veins are visualized. These techniques are designed to maximize visualization of the feeding arteries of the spinal tumor and the radiculomedullary arteries. The radiculomedullary arteries have a classic hairpin turn as they join the anterior spinal artery, which appears as a midline vessel. In some cases, the use of cone-beam computed tomography (CBCT) with 3D soft tissue reconstructions has been helpful as a problem-solving tool for difficult cases and can provide information about feeding arteries, tumor vascularity, and radiculomedullary arteries ( Figure 30-2 ).
| Cervicothoracic Spine | Thoracolumbar Spine | Sacrum |
|---|---|---|
| Vertebral arteries Ascending cervical trunk Deep cervical trunk Descending branches of thyrocervical trunk Supreme intercostal artery Intercostobrachial trunk | Segmental arteries (intercostal or lumbar) Iliolumbar arteries Median sacral artery | Iliolumbar arteries Internal iliac arteries Median sacral artery Lateral sacral arteries |
In spinal embolization, selective delivery of embolic agents to the tumor and protection of the radiculomedullary arteries are essential to reduce the risk of nontarget embolization, which may have catastrophic consequences. A coaxial microcatheter system permits safe, selective catheter position and minimizes nontarget embolization. Although small particles (100–300-μm) can be routinely used in embolization of peripheral bone tumors, medium-sized particles (250–500-μm) are recommended for embolization in the spine. Medium-sized particles will occlude tumor vessels proximal to or at the capillary bed without blocking collateral arterial supply to the spinal cord, thus reducing the risk of cord ischemia and paralysis. Table 30-2 lists some of the common embolic agents and their characteristics.
| Embolic agents | Characteristics |
|---|---|
| Particles (PVA/Embosphere) | Inert, water-insoluble, nonabsorbable, defined sizes |
| Gel foam/sponge | No defined sizes, provides temporal occlusion, degraded by enzyme |
| Alcohol/ N -butyl-cyanoacrylate | Liquid agents, high risk of nontarget embolization, increased rates of neurologic complications and skin/muscle necrosis; use should be limited to experienced operators |
| Metallic coils | Single use for proximal embolization is ineffective, frequently used in conjunction with particle embolization |
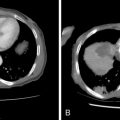
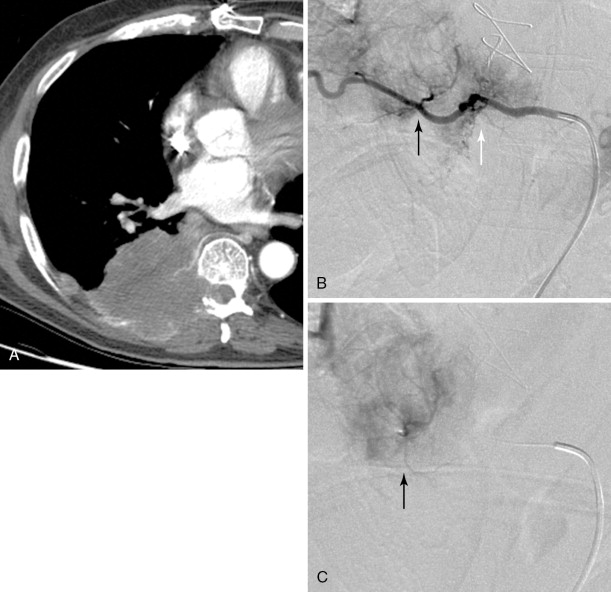
Strategies and techniques of spinal embolization depend on the angiographic findings: the size and number of feeding vessels to the tumor and the presence or absence of visualization of the spinal artery. Table 30-3 summarizes five common patterns of angiographic findings seen in hypervascular spinal tumors and proposes specific embolization strategies for each pattern ( Figures 30-3 to 30-7 ). In addition to the five common angiographic patterns identified, other angiographic features warrant technical consideration. Arteriovenous shunting may be present on the segmental angiogram, and occlusion of the shunt can be attempted with large embolic agents or coils; however, if a large shunt persists, embolization is contraindicated because of the risk of pulmonary and systemic embolism. Occasionally, the radiculomedullary artery may only be visible following subselective catheterization or may be visible via an intersegmental anastomosis one level above or below the selected segmental artery. Embolization in both these cases is contraindicated if the feeding arteries are too close to the origin of the radiculomedullary artery. Lastly, the flow dynamics of the spinal tumor may change during the embolization procedure. The radiculomedullary artery may not be seen during preembolization subselective angiography because of diversion of contrast into the high-flow tumor but will become angiographically apparent once embolization has been initiated and flow to the tumor has been reduced. Therefore, frequent angiographic investigation and careful monitoring of the patient’s clinical status during the procedure are necessary to prevent nontarget embolization. If the patient reports altered sensation, onset of paresthesia, or muscle spasm during the embolization procedure, this may be a sign of nontarget embolization of a radiculomedullary artery and the procedure should be terminated. In patients undergoing the procedure for preoperative purposes, if embolization of the spinal tumor was not feasible or if only a portion of the tumor could be embolized, this should be communicated to the surgeon so that additional precautions, if needed, can be taken during surgery.
| Angiographic Pattern | Spinal Artery | Embolization Strategies/Techniques | Category |
|---|---|---|---|
| Single or few feeding vessels that can be catheterized | Not visualized | Superselective catheterization of the feeding vessels followed by particulate embolization | A-1 |
| Small feeding vessels that cannot be catheterized | Not visualized | Distal coil embolization of the segmental artery followed by particulate embolization | A-2 |
| Origin of feeding vessels distal to that of the radiculomedullary artery | Visualized | Superselective catheterization distal to radiculomedullary artery followed by particulate embolization +/- distal coil embolization | B-1 |
| Origin of feeding vessels close to that of radiculomedullary artery | Visualized | No embolization | B-2 |
| Origin of feeding vessels proximal to that of radiculomedullary artery | Visualized | Unless superselective catheterization of the feeding arteries is possible, do not embolize | B-3 |

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree