Key points
- •
Resection and chemotherapy are the mainstays of treatment for most advanced gynecologic cancers, but interventional strategies play an important role in the diagnosis and treatment of patients who are not surgical candidates.
- •
Image-guided biopsy is suggested in cases of suspected ovarian cancer with peritoneal carcinomatosis to help guide neoadjuvant chemotherapy and avoid unnecessary surgery in cases of nongynecologic primary cancers presenting with omental metastases.
- •
Percutaneous thermoablative techniques, including radiofrequency and cryoablation, can be safely used to treat liver, pelvic, and peritoneal metastases in women with unresectable or suboptimally resected disease.
- •
Image-guided placement of semipermanent indwelling peritoneal catheters is an effective alternative to serial paracentesis for patients with refractory ascites.
- •
Transarterial catheter embolization can be used for control of massive pelvic hemorrhage from advanced gynecologic malignancies.
- •
Thermoablative techniques, such as high-intensity focused ultrasound (HIFU) and radiofrequency ablation (RFA), may be employed in the treatment of nonmalignant gynecologic conditions such as cervical ectopy and abdominal wall endometriosis.
Introduction
Interventional strategies are limited in the primary treatment of gynecology cancers, but they are increasingly employed as secondary therapies for patients with nonresectable disease, and image-guided techniques are used for the biopsy and management of symptoms related to these malignancies. This chapter presents the interventional techniques used in the diagnosis, management, and treatment of gynecologic neoplasms. Ovarian cancer is highlighted in this chapter because it is the leading cause of death in women with gynecologic cancers, often presenting in advanced stages and with frequent recurrence that limit surgical options. However, many of these techniques can be applied in other gynecologic cancers, though their use is not as frequent. Various interventional therapies for benign gynecologic conditions are also described, with uterine leiomyomas covered in a separate, dedicated chapter.
Interventions in gynecologic oncology
Biopsy of peritoneal carcinomatosis in suspected ovarian cancer
Surgical cytoreduction followed by chemotherapy is the mainstay of therapy for patients with ovarian cancer. Often patients will have debulking surgery prior to tissue diagnosis, ovarian cancer may be presumed based on elevated CA-125 and imaging findings. In patients with unresectable disease at the time of presentation or in those with medical comorbidities that preclude surgery, induction or neoadjuvant chemotherapy is typically administered first. Unfortunately, the diagnosis of ovarian cancer is not always clear and a tissue diagnosis is warranted prior to these treatments. Additionally, some medical therapies rely on pathologic or molecular analysis.
Advanced ovarian cancer can sometimes be indistinguishable from peritoneal and adnexal metastases from other primary tumors, and up to 20% of women undergoing surgical debulking for suspected ovarian cancer are found to have metastatic disease from a nongynecologic source. , Moreover, in women with a history of a primary cancer known to demonstrate peritoneal spread, such as breast or gastrointestinal tract malignancies, new ovarian or peritoneal lesions do not always represent spread of disease. At least two series have demonstrated a diagnosis of a different primary Müllerian cancer (ovarian or primary peritoneal) on resection. , There also can be considerable overlap with the imaging appearance of benign complex ovarian masses. In these situations, a site-specific diagnosis is imperative to avoid unnecessary surgery or inappropriate chemotherapy.
Image-guided biopsy (IGB) of omental, peritoneal and pelvic masses is a safe and effective alternative to surgical biopsy via exploratory laparotomy or laparoscopy. , In the largest published study to date of IGB in 149 women with peritoneal carcinomatosis, a site-specific diagnosis was made in 93% of patients. Smaller series in women with peritoneal carcinomatosis and suspected ovarian cancer also had favorable results, with reported accuracy rates of 92% and 87%.
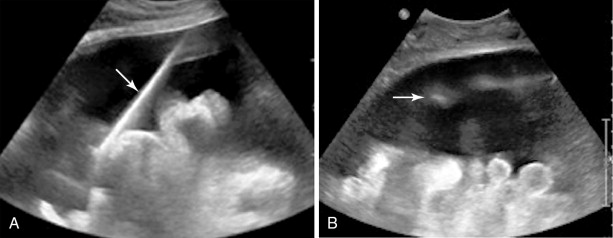
Both ultrasound and CT-guided techniques have been described, modality selection depends largely on the patient and his or her disease burden as well as operator comfort. , For patients with bulky disease likely to be visualized sonographically, ultrasound may be the appropriate choice as it is typically faster, allows for real-time visualization of vasculature, and spares the patient and physician from ionizing radiation. However, isolating peritoneal or omental masses from the adjacent bowel under ultrasound guidance can be challenging for a less experienced operator. CT may be a better choice in these cases or with smaller, more inaccessible lesions. Often CT and ultrasound are used in conjunction, as the portability of ultrasound allows operators to image the target lesions using both modalities in the same room and choose the most appropriate modality at the time of the procedure ( Figures 18-1 and 18-2 ).
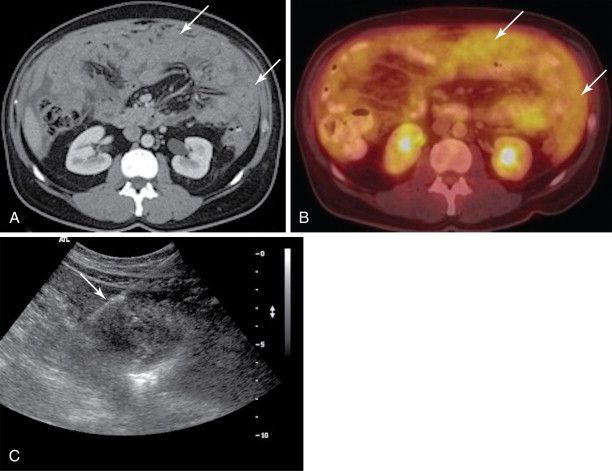

All patients should undergo a diagnostic CT of the abdomen and pelvis with intravenous and oral contrast shortly prior to the procedure to identify disease burden and distinguish peritoneal disease from adjacent bowel or liver. Ultrasound-guided biopsies require no patient preparation, though one may request a full bladder for a better acoustic window. Multiple authors have described CT-guided techniques that employ oral contrast prior to the procedure to opacify and help identify bowel from adjacent disease. , , With both techniques, the procedure can be combined with a diagnostic or therapeutic drainage of ascites. Although trace fluid may improve sonographic visualization, removal of large-volume ascitic fluid prior to biopsy can help minimize the risk of hemorrhage.
The safety of larger-gauge (18 gauge and below) core biopsy needles for use in peritoneal or omental disease is well documented, these are preferred to the finer gauge (20-gauge and higher) self-aspirating needles because the increased specimen size allows for assessment of the tissue architecture and further analysis with immunohistochemistry if necessary. , Coaxial systems, commonly available as a 17/18-gauge or 19/20-gauge introducer/biopsy needle combinations, are preferred as they allow the operator to take several specimens through a single needle track. This is also advantageous for small, difficult-to-reach lesions as the introducer needle can help anchor the target lesion, facilitating multiple passes.
Both CT- and ultrasound-guided biopsies are quick, taking roughly 15–20 minutes and can safely be performed in an outpatient setting. Other than local pain and bruising at the puncture site, there have been few reported complications, the most serious being a rectus sheath hematoma that was managed conservatively and focal abdominal wall cellulitis. Though there is a theoretical risk of tracking tumor into the abdominal wall, as this has been reported in a laparoscopy scar, there have been no reported cases following IGB or during percutaneous interventional treatments. IGB is fast, safe, accurate and an important tool in the diagnosis and management of women with suspected ovarian cancer.
Interventional techniques for local control of metastatic disease
For women with advanced-stage ovarian cancer and peritoneal, nodal, or hepatic metastases, there is a survival benefit to aggressive cytoreduction despite widespread disease. In a study of patients with Stage IV ovarian cancer, Bristow et al. reported a median survival of 50.1 months for women with optimal debulking of both their intra- and extrahepatic disease, defined as a maximal diameter of residual tumor ≤1 cm. However, anatomic limitations meant that optimal debulking was achieved in only 16% of patients, and in women with successful resection of their extrahepatic disease but residual tumor in the liver, the median survival fell to 27.0 months. Their experience reflects a challenge for many oncologists and surgeons treating women with ovarian cancer, as many of them present with advanced disease and roughly 50% will have hepatic involvement at the time of death.
Percutaneous thermoablative techniques, including radiofrequency ablation (RFA), microwave ablation, and cryotherapy, are well-established tools for treating hepatic tumors, and the techniques are described elsewhere in this book. Although more commonly used in the treatment of hepatocellular carcinoma or colorectal metastases, both have been applied in the treatment of hepatic metastases from ovarian cancer as well as nodal and peritoneal metastases from gynecologic cancers. ,
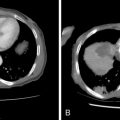
Although RFA of hepatic tumors is typically reserved for patients with disease confined to the liver, this is not the case for ovarian cancer, as it can be used as part of the overall strategy of cytoreduction. Unfortunately, the literature on the use of RFA in ovarian cancer is limited to a few case studies, small series, or cases in a larger series of hepatic metastatic lesions. In the largest reported series of six patients, Gervais et al. achieved a mean local progression-free interval of 2.5 years after percutaneous RFA: RFA can also be used as an adjunct to surgical resection when there is bilobar involvement or when intraoperative ultrasound demonstrates small, deep parenchymal lesions that may have been missed on preoperative CT or MR: In a three-person case series of patients undergoing resection and intraoperative RFA, Mateo et al. reported progression-free survivals of 9, 13, and 39 months. The minimally invasive nature of RFA also offers the option of following patients with the test of time approach in which lesions are ablated with close imaging surveillance in an effort to minimize the number of hepatic resections that would not benefit the patient either because of rapid progression of extrahepatic disease or lack of disease elsewhere , ( Figure 18-3 ).
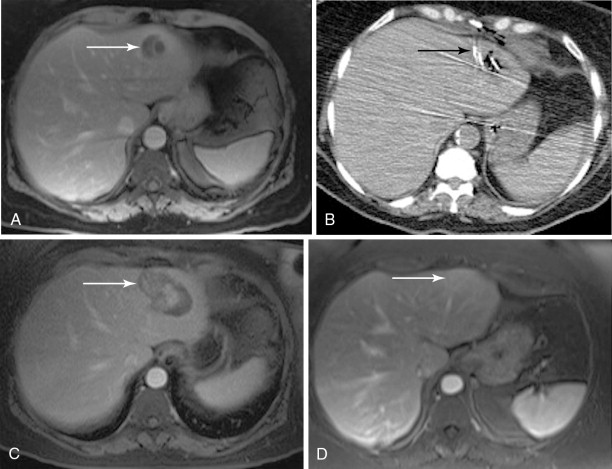
Data on the application of cryoablation for gynecologic malignancies is more sparse and limited to a handful of patients in larger series. To our knowledge, the use of cryoablation for hepatic lesions from metastatic gynecologic disease has been reported in eight cases: one percutaneous and seven intraoperative. Unfortunately, half of these patients succumbed to their disease shortly after treatment without follow-up of their hepatic lesions. Each of the four remaining patients demonstrated recurrence of their hepatic disease within a year of treatment.
Our experience includes nine women with 27 metastatic lesions from advanced ovarian or endometrial cancer treated with RFA (70%), cryoablation (26%), and microwave ablation (4%). Complete ablation was achieved with 14 lesions (52%) with an average local progression-free survival of 18 months. In the other 13 cases (44%), partial ablations were performed for debulking and palliative purposes. All patients survived the procedure without complication. Of our 8 patients with adequate follow-up, 2 (25%) remained free of disease, 1 (12.5%) developed distant metastases, and 1 (12.5%) had both local and distant progression. The other 4 women died from extensive metastatic disease at 1, 12, 14, and 21 months following their ablation sessions. Though limited, our own experience with percutaneous thermoablative techniques has also demonstrated that these techniques are safe and can be effective for local control or palliation.
Transarterial chemoembolization (TACE) has been successfully employed in the treatment of hepatocellular carcinoma (HCC) as well as colorectal and neuroendocrine metastases, and the first case series of its use for ovarian metastases was recently published. Sixty-five patients with no response, disease progression on, or toxicity to platinum and taxane-based chemotherapy underwent a minimum of three (mean 5.03) TACE procedures. The chemotherapeutic protocol evolved over the course of their experience, with the initial protocol of only mitomycin eventually supplemented with gemcitabine, and then later a therapeutic regimen of mitomycin, cisplatin, and gemcitabine was used. The embolization material was standardized to include a maximum of 10 mL lipiodol followed by an injection of 200–450 mg of degradable starch microspheres (200 μm) for vessel occlusion. A 14-month median survival was achieved, with survival rates of 58% at 1 year, 19% at 2 years, and 13% at 3 years. Survival times from the start of therapy showed no significant difference among the groups treated with the different chemotherapeutic regimens. From a treatment perspective, they selected perhaps the most challenging subset of ovarian cancer patients, as the presence of capsular or shallow lesions from peritoneal seeding was an exclusion criterion. Patients with deeper parenchymal lesions from hematogenous spread have poor survival compared to patients with resectable disease from peritoneal spread. These results compare favorably to survival times of 9 and 10 months reported for other cases of nonresectable hematogenous ovarian metastases. Although more data are needed regarding the use of TACE in these patients, it appears to be a reasonable strategy in the management of patients with unresectable hepatic involvement.
Finally, the safety and feasibility of high-intensity focused ultrasound (HIFU), a newer transcorporeal thermoablative technique, for treating liver tumors has been validated, but to date, no report exists of its use in patients with hepatic metastasis from gynecologic cancers. Nevertheless, this is likely to be a promising noninvasive treatment option for these patients in the near future.
Intraperitoneal catheters for palliative treatment of refractory malignant ascites
Recurrent ascites can be a significant source of distress for patients with advanced gynecologic malignancies and negatively affect the quality of their last few months of life. The large-volume ascites that develop in these patients can cause orthopnea, dyspnea, abdominal discomfort, vomiting, lower-extremity edema, and even difficulty with ambulation. There is no standardized approach for the management of rapidly accumulating ascites beyond treatment of the primary disease. Diet modification and diuretic use are not very effective. Internal peritoneovenous or transjugular intrahepatic portosystemic shunts (TIPS) are not commonly used in the setting of cancer. Often these patients will undergo repeat paracentesis for palliative relief. However, each procedure introduces a risk of infection, peritonitis, bleeding, bowel perforation, and hypotension. Moreover, ascites reaccumulates quickly, necessitating frequent trips to the hospital for drainage, sometimes multiple trips per week. Because of the pain and inconvenience of repeat procedures, many patients delay interventions for as long as possible and present with tense ascites, requiring large-volume taps, which increase the risk for hypotension and renal insufficiency related to large fluid shifts and protein loss. ,
Indwelling, semipermanent intraperitoneal drains are an effective alternative to serial paracentesis, allowing patients or their caregivers to safely manage their ascites at home by removing small aliquots of liquid with vacuum bottles at regular intervals. A variety of catheters have been used for this purpose, including tunneled Tenckhoff® catheters, tunneled and untunneled Pleurex® catheters, a Cope-type loop drainage catheter, a tunneled silastic catheter with Dacron cuff, tunneled and untunneled peritoneal catheters, and tunneled peritoneal Port-A-Caths®. Tunneled catheter systems are generally preferred, as they are associated with a lower risk of peritonitis than the untunneled systems.
These intraperitoneal drains can be placed under CT or ultrasound guidance ( Figure 18-4 ). An 18-gauge needle fitted with a syringe is first used to access the peritoneal cavity. Once the cavity has been entered and ascitic fluid aspirated, a 0.035-in. guide wire is inserted through the needle and then the needle is removed. For tunneled systems, the fenestrated end of the catheter is attached to a tunneler, then passed subcutaneously from an incision approximately 5 cm away to the puncture site, pulling the catheter subcutaneously until the plastic cuff comes to rest under the skin approximately 1 cm from the second incision. Serial dilations are performed so that a 16-Fr peel-away introducer could be placed over the guide wire and into the peritoneal cavity. The catheter is then inserted through the peel-away introducer until all of the fenestrations are within the peritoneal cavity. The catheter is clamped closed, the incisions sutured, and the catheter is sutured to the skin. , Contraindications to placement include coagulopathy, active abdominal infection, and the presence of loculated ascites.