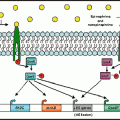
Fig. 5.1
Functional classes of enteric neurons and their major projections to the mucosa, submucosal neurons, and smooth muscle coats in the intestinal wall of a large mammal, such as a pig. Note that the mucosa receives both afferent and efferent innervation. CM circular muscle; IPAN intrinsic primary afferent neuron; ISMP and OSMP inner and outer submucosal plexuses; LM longitudinal muscle; MP myenteric plexus; MUC mucosa (from Linden and Farrugia 2008)
5.2.4 Diffuse and Organized Gut-Associated Lymphoid Tissue
The gut-associated lymphoid tissue (GALT) represents the largest component of the common mucosal immune system and functions to control intestinal infections. It consists of a diffuse lymphoid compartment containing large populations of lymphocytes and antigen-presenting cells (e.g., macrophages, dendritic cells) in the intestinal lamina propria and Peyer’s patches, which are organized lymphoid follicles covered by a single layer of specialized epithelial cells (i.e., M cells). Studies of germ-free and gnotobiotic rodents have shown that microflora play a role in the development of the GALT (Bauer et al. 2006). As the inductive site for mucosal immunity, Peyer’s patches play a critical role in sampling of luminal contents and initiating adaptive immune responses towards potentially harmful microorganisms and antigenic materials (Mowat 2003). This includes the generation of immunoglobulin A (IgA)-producing lymphoblasts. These cells mature in the system circulation and then traffic as plasma cells to mucosal effector sites in the gut lamina propria. Neurons and nerve fibers exist in close proximity to lamina propria leukocytes, including mast cells (Wood 2007) and lymphocytes (Downing and Miyan 2000). Peyer’s patches are highly innervated (Defaweux et al. 2005; Vulchanova et al. 2007; Chiocchetti et al. 2008). Antigen-specific secretory IgA synthesized in these plasma cells is the major immunoglobulin secreted onto mucosal surfaces and plays an important role in mucosal protection; furthermore, constitutively produced secretory IgA is thought to regulate the gut microfloral population (Suzuki et al. 2007; Macpherson and Slack 2007). In addition to their important immunological role, Peyer’s patches are exploited as portals of entry into the body for several species of enteropathogenic bacteria (Clark and Jepson 2003).
5.3 The Enteric Nervous System, Mucosally Directed Nerves, and Gut Bacteria
5.3.1 Organization of the Enteric Nervous System
The mammalian ENS originates mainly from the vagal neural crest during gestation and constitutes two or more distinct ganglionated plexuses (Burns and Thapar 2006). These include the submucosal (Meissner) and myenteric (Auerbach) plexuses, which are organized into an interconnected neural network of reflex arcs containing intrinsic primary afferent neurons, interneurons, and excitatory or inhibitory, motor and secretomotor neurons, each with distinct plurichemical coding (Furness 2006). Thus, the intestine is the only visceral organ capable of executing complex preprogrammed behaviors that can occur independently of the central nervous system.
The myenteric plexus, which is located between the longitudinal and circular smooth muscle layers, coordinates intestinal propulsion and segmentation. It is well known that neurally mediated disruptions in myoelectrical activity and mechanical functions of the intestine can alter the enteric content of microorganisms (Scott and Cahall 1982).
Neurons in the submucosal plexus(es) regulate active ion transport and paracellular permeability of the intestinal epithelium, as well as relay sensory information from the mucosa to the myenteric plexus and central nervous system (Furness 2006). Species differences have been observed in the structure and chemical coding of submucosal neurons. Rodents possess a single submucosal ganglionated plexus whereas larger mammals such as cattle and pigs have both inner (Meissner’s) and outer submucosal (Schabadasch) plexuses (Fig. 5.1). Humans possess a third submucosal plexus, which appears to be similar in neurochemical coding to the outer submucosal plexus (Timmermans et al. 2001).
5.3.2 The ENS and Gut Bacteria
Submucosal and myenteric nerves play an important defensive role in the initial stages of bacterial infection, by coordinating the intestinal secretory and propulsive functions necessary to dilute and purge enteropathogens (Spiller 2002). Ingested pathogenic bacteria can alter enteric neural activity and plasticity, either through (1) direct interactions with nerve cell bodies and fibers, or (2) induction of inflammatory responses in neurons and neighboring cells, such as enteric glia, or (3) the release of neuroactive exotoxins (Lundgren 2002). Enteric neuropeptides mediate or mitigate these bacterial effects on the ENS. Some gut neuropeptides, including SP, neuropeptide Y, and neurotensin, even possess inherent antimicrobial activity (Brogden et al. 2005).
LPS at high concentrations (100 ng/ml) produces death in cultured myenteric neurons, an effect that can be prevented by the enteric neurotransmitter vasoactive intestinal peptide (VIP) (Arciszewski et al. 2005, 2008). Bacteroides fragilis infections alter the relative proportions of substance P- and somatostatin-expressing colonic neurons and fibers (Gonkowski et al. 2003). Intestinal infection with the causative agent in porcine proliferative enteropathy, Lawsonia intercellularis, is associated with an increase in the number of submucosal neurons immunoreactive for the gut peptides VIP, galanin, somatostatin, and calcitonin gene-related peptide (Pidsudko et al. 2008). The probiotic bacterium Lactobacillus reuteri appears to modulate enteric neurotransmission linked to colonic motility by affecting the gating properties of neuronal cation channels (Wang et al. 2010). Several disease-causing bacteria, including enteropathogenic Escherichia coli, Salmonella Typhimurium, and Shigella dysenteriae, induce the expression of receptors for the enteric neuropeptide galanin on colonic epithelial cells; this effect is not produced by normal colonic microflora. Galanin released by colonic submucosal nerves acts upon these epithelial cell receptors to stimulate active transepithelial anion secretion, which in turn contributes to secretory diarrhea (Hecht et al. 1999; Matkowskyj et al. 2000). The gut neuropeptide substance P (SP), which activates intestinal defenses against bacteria, has been shown to reduce host susceptibility to enteric Salmonella infections (Pascual 2004). This phenomenon appears to be due to the involvement of this neuropeptide and its cognate receptors in Salmonella-induced gut inflammation (Walters et al. 2005).
Toxins from Clostridium difficile, Shigella spp., Campylobacter spp., and other enteropathogens can evoke the release of inflammatory mediators, which can acutely alter neuronal activity and chronically produce structural and chemical changes in the ENS (Vasina et al. 2006; Lomax et al. 2006). The copious diarrhea associated with Vibrio cholerae infections has been attributed in part to cholera toxin-evoked increases in the activity of enteric neurons expressing VIP, a potent secretogogue (Mourad and Nassar 2000). Clostridium difficile toxin B appears to activate VIPergic submucosal neurons via an interleukin 1-dependent mechanism (Neunlist et al. 2003). Fluid secretion in diarrhea associated with Salmonella enterica and enterotoxigenic E. coli infections may involve enteric neural circuits as well (Lundgren 2002).
5.3.3 Catecholamines in the ENS
Both DA and NE are synthesized in and released from enteric nerves innervating the intestinal mucosa (Wu and Gaginella 1981; Llewellyn-Smith et al. 1981, 1984; Eisenhofer et al. 1997; Vieira-Coelho and Soares-da-Silva 1993; Wang et al. 1997; Li et al. 2004; Lomax et al. 2010). Enteric dopaminergic neurons appear to reside within the intestinal wall, but the noradrenergic innervation of the intestine originates in neurons lying outside the gut wall are located in prevertebral ganglia (Anlauf et al. 2003; Li et al. 2004; Furness 2006). These “extrinsic” sympathetic nerve fibers may co-contain peptide neurotransmitters such as neuropeptide Y or somatostatin (Timmermans et al. 1997; Straub et al. 2006, 2008). Butyrate, which is produced by colonic bacteria, has been found to transcriptionally activate the gene encoding tyrosine hydroxylase, the rate-limiting enzyme in catecholamine synthesis (Patel et al. 2005). Epinephrine is not synthesized in enteric nerves because phenylethanolamine N-methyltransferase, the enzyme catalyzing epinephrine synthesis from norepinephrine, does not appear to be expressed in the digestive tract (Black et al. 1981; Bäck et al. 1995; Kennedy and Ziegler 2000; Costa et al. 2000). Interestingly, the growth of enteropathogens such as enterohemorrhagic Escherichia coli O157:H7 (EHEC) and Salmonella enterica is preferentially enhanced by catecholamines that are normally present in the GI tract (NE and DA) in comparison to epinephrine, and growth of the more exclusive enteric pathogen Yersinia enterocolitica is stimulated by NE and DA but not epinephrine (Freestone et al. 2007a). The direct action of NE on bacterial growth is unlikely to be mediated by conventional adrenergic receptors, which recognize both NE and epinephrine (cf. Sect. 5.3.4 below).
In addition to neuronal cells, there is accumulating evidence that immune cells are capable of synthesizing, releasing, and degrading catecholamines. As there is an abundance of immunocytes within the GALT, these cells may represent an alternate, non-neuronal source of NE and DA in the intestinal wall (Flierl et al. 2008).
5.3.4 Catecholamine Receptor Pharmacology
Norepinephrine and DA activate their cognate G protein-coupled receptors expressed on closely apposed neurons, IECs, and other target cells, which influence overall mucosal function and alter intestinal susceptibility to infection. Receptors for NE and DA have been defined over approximately four decades through the development and use of highly selective receptor agonists and antagonists in functional pharmacological investigations and, more recently, through molecular cloning and structure-function studies in isolated cells and transgenic animals.
The receptor concept was based at the turn of the last century on the powerful and physiologically relevant approach of defining binding interactions of endogenous substances or their synthetic homologs with specific receptors that are linked to a biological response. Biochemical analyses of selective ligand-binding site interactions that were developed some 60 years later provide valuable information on the affinities (K d, dissociation constant) and competitive interactions of ligands at specific binding sites as well as the relative density (B max) of the binding sites. Because they do not measure the biological activity of the ligands examined, however, they do not truly define a “receptor,” which is an entity that is functionally coupled to intracellular signal transduction pathways and mediates a biological function. Binding studies, if carefully executed, provide information on a specific binding site for an endogenous or synthetic ligand, which can serve as supporting evidence for the presence of the receptor in a biological system. Through GTPγ35S binding assays (Harrison and Traynor 2003), it is now possible to assess the effectiveness of ligands to activate G proteins coupled to a particular receptor and this approach affords a better approximation of drug activity (e.g., it is possible to distinguish an agonist from an antagonist). There is generally a good concordance in the results of studies determining the affinities and competitive interactions among ligands for adrenergic receptors (ARs) and dopaminergic receptors (DRs) by functional means with affinities and interactions of the same ligands determined biochemically at specific binding sites.
Presently, nine ARs are classified into alpha- and beta-AR types; there are two subtypes of alpha-ARs (alpha1 and alpha2) having three isoforms each, and three subtypes of beta-ARs. Compared to epinephrine, NE has relatively higher binding affinity for alpha-ARs and beta1– and beta3-ARs, but lower affinity for beta2-ARs. Two main DR types exist through gene duplication events in the vertebrate lineage (D1R and D2R); from these, five receptor subtypes have been defined. Our understanding of the nature of these receptors and their relationships to G proteins and downstream intracellular signaling cascades is evolving (Strange 2008). For example, there is accumulating evidence that these receptors may form homo- or heterodimeric complexes on cell membranes that possess a pharmacological profile that differs from that of the monomeric receptor(s) or may be involved in the process of receptor down-regulation. With respect to ARs, this dimerization phenomenon appears to occur between different ARs as well between ARs and other classes of G protein-coupled receptors, including chemokine receptors (Milligan et al. 2005; Hague et al. 2006); dopamine receptors are known to form heterodimeric complexes with adenosine receptors (Fuxe et al. 2005).
5.3.5 Catecholamine Receptors and Mucosal Function
Enteric dopaminergic and alpha1-, alpha2-, or beta-adrenergic binding sites have been detected on submucosal nerves or IECs in some species, notably the guinea pig and rat (Chang et al. 1983; Cotterell et al. 1984; Senard et al. 1990; Valet et al. 1993; Vieira-Coelho and Soares-da-Silva 2001; Baglole et al. 2005). Catecholamine receptors are probably expressed by immunocytes and enteroendocrine cells as well. NE alters immunocyte function (Elenkov et al. 2000; Meredith et al. 2005; Kin and Sanders 2006) and modulates serotonin release from enterochromaffin cells in the intestinal mucosa (Pettersson 1979; Simon and Ternaux 1990; Schäfermeyer et al. 2004). In summary, enteric catecholamines, NE in particular, can potentially modulate crosstalk between several different types of cells in the intestinal mucosa and submucosa.
Norepinephrine alters active, transepithelial ion transport in the intestinal mucosa through interactions with functionally defined alpha-ARs and to a lesser extent the beta-ARs. Depending upon the animal species and intestinal segment examined, this action is mediated indirectly through enteric nerves or by direct effects on IECs (Brown and O’Grady, 1997; Horger et al. 1998). In addition, NE modulates epithelial growth and turnover (Tutton and Helme 1974; Tutton and Barkla 1977; Olsen et al. 1985), paracellular permeability (Lange and Delbro 1995), and the vectorial secretion of secretory IgA towards the gut lumen (Schmidt et al. 1999, 2007). Dopamine affects active ion transport through direct and indirect actions on enteric adrenergic and dopaminergic receptors (Donowitz et al. 1982, 1983; Vieira-Coelho and Soares-da-Silva 1998; Al-Jahmany et al. 2004).
5.3.6 Enteric Nerves, Catecholamines, and IEC:Bacteria Interactions
Both NE and DA have been shown to alter the mucosal attachment or invasiveness of bacterial pathogens such as EHEC or serovars of Salmonella enterica not always through direct contact with these bacteria, but rather by acting on cells of the intestinal mucosa (Table 5.1). The actions of these catecholamines on bacteria–mucosa interactions have been examined in mucosal explants mounted in Ussing chambers (Brown and O’Grady 2008). This apparatus has been used for decades in studies of transepithelial ion transport, and more recently in investigations of bacteria–host interactions (Ding et al. 2001; Crane et al. 2006). This system extends the viability of mucosal explants under quasi-physiological conditions, allows for continuous, tangential flow of bacteria across a fixed mucosal surface area, and permits the selective contact of drugs and bacteria with the luminal or contraluminal surfaces of intestinal tissues (Fig. 5.2).
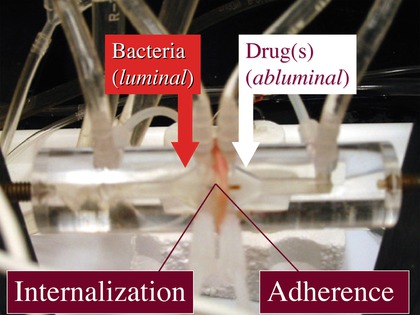
Table 5.1
Functional evidence for mucosal alpha-adrenergic receptors influencing EHEC adherence to explants of porcine cecal and colonic mucosae
Pharmacological characteristic | Supporting evidence | References |
|---|---|---|
Selective agonism | At equimolar concentrations, UK14,304 (alpha2-adrenoceptor agonist), but neither phenylephrine (alpha1) nor isoproterenol (beta) increase EHEC adherence to cecal mucosa | Chen et al. (2006) |
Selective antagonism | At equimolar concentrations in both cecum or colon and phentolamine (alpha-adrenoceptor antagonist), but not propranolol (beta), inhibits NE action. Furthermore, yohimbine (alpha2), but not prazosin (alpha1), inhibits NE action | |
Laterality of drug action | NE at low (µM) concentrations effective only when added to the contraluminal, but not luminal bathing medium, consistent with submucosal localization of adrenergic receptors | Green et al. (2004) |

Fig. 5.2
Photograph of an Ussing chamber containing a porcine intestinal mucosa explant. Arrows indicate the locations of bacterial inoculations and drug additions as performed in many experiments such as those summarized in Table 5.1
5.3.6.1 Role of the ENS and Catecholamines in Bacterial Internalization into the Mucosa of the Small Intestine
The attachment and invasion of enteropathogenic bacteria to the intestinal mucosa appears to be modulated by the ENS. Inhibition of neural conduction by the serosal side addition of the neuronal sodium channel blocker saxitoxin increases internalization of luminally inoculated Salmonella enterica serovar Choleraesuis and EHEC by >6-fold in Peyer’s patch explants from the porcine jejunum. Internalization of a rodent commensal E. coli strain is unaffected by the toxin (Green et al. 2003) and that of S. enterica serovar Typhimurium is decreased by threefold (Brown and Price 2008). Serosal application of the neurotoxin or the local anesthetic lidocaine decreased S. Typhimurium internalization by three- to fourfold in explants of non-Peyer’s patch absorptive mucosa from porcine jejunum. In contrast, electrical stimulation of enteric nerves in this preparation increased S. Typhimurium internalization by 2.5-fold and this effect was inhibited by saxitoxin or lidocaine (Schreiber et al. 2007). Although these neurally mediated effects on Salmonella internalization may appear to be small, it should be emphasized that they were measured over a surface area of 2 cm2 in isolated tissues over a relatively short (90 min) time period. If extrapolated to the large surface area encompassed by the small intestine or even a segment thereof, these changes in Salmonella uptake are likely to be biomedically significant.
The cellular mechanisms underlying these neurally mediated effects on Salmonella internalization in the porcine small intestinal mucosa are undefined at present. There is evidence that they differ for different serovars Choleraesuis and Typhimurium of S. enterica as saxitoxin increases internalization of the former and decreases that of the latter. Moreover, Salmonella internalization is inhibited by the actin polymerization inhibitor cytochalasin D in the nonfollicular absorptive mucosa and monolayers of the porcine enterocyte cell line, IPEC J2, a result that is in agreement with other studies of actin-dependent Salmonella invasion in epithelial preparations (Schreiber et al. 2007; Brown and Price 2007). In contrast, cytochalasin D has no effect on the uptake of S. enterica serovars Cholerasuis and Typhimurium into jejunal Peyer’s patch mucosa explants (Green and Brown 2006; Brown and Price 2008).
Norepinephrine and DA have also been implicated in Salmonella internalization, especially in porcine jejunal Peyer’s patches, where there is strong immunohistochemical evidence for catecholaminergic innervation (Kulkarni-Narla et al. 1999) At these inductive sites for mucosal immunity, nerve fibers immunoreactive for the catecholamine synthetic enzymes tyrosine hydroxylase and dopamine beta-hydroxylase can be seen terminating beneath epithelial cells. Enteric nerves near Peyer’s patch follicles express immunoreactivities for the type 2 vesicular monoamine transporter, which transports catecholamines into synaptic vesicles, and the norepinephrine transporter NET, a target of cocaine action (Kulkarni-Narla et al. 1999; Green et al. 2003).
The serosal application of NE at a bath concentration of 10 µM produced a six- to ninefold increase in luminal S. enterica serovar Choleraesuis and EHEC internalization in porcine Peyer’s patch explants. This effect was not mimicked by luminally-applied NE, but was inhibited in tissues pretreated with the alpha-AR antagonist phentolamine. These results indicate that this NE action is mediated by alpha-ARs which are likely localized to the basolateral aspect of Peyer’s patch epithelial cells (Green et al. 2003). In a study of S. enterica serovar Typhimurium internalization, the serosal administration of DA or the sympathomimetic drugs cocaine and methamphetamine decreased Salmonella recovery from Peyer’s patch explants (Brown and Price 2008). It is not known if these effects of NE and DA extend to species other than swine. Although the underlying cellular mechanisms for them must be investigated further, it is tempting to hypothesize that catecholamines may regulate the sampling function of Peyer’s patches to control the entry or immune processing of pathogenic microbes at these intestinal sites.
5.3.6.2 Catecholamines and EHEC Adherence to the Mucosa of the Large Intestine
When added to the medium bathing the contraluminal surface of cecal explants from mice, NE and DA increase the number of EHEC adhering to the mucosal surface. They do so at 50% effective concentrations (EC50) of 3.8 and 4.2 µM, respectively. The concentrations of NE applied to the basolateral aspect of the intestinal epithelium that are sufficient to promote EHEC adherence are somewhat lower than those necessary to promote epithelial EHEC adherence when incubated directly with the bacterium (Vlisidou et al. 2004; Bansal et al. 2007). The adherence-enhancing actions of NE and DA on the epithelium are inhibited respectively by AR and DR receptor antagonists, a result indicating that they are mediated by specific catecholamine receptors (Chen et al. 2003). This appears to differ from the mechanism by which these catecholamines produce their direct effects on bacterial function (Freestone et al. 2007b).
This phenomenon extends to species other than the mouse. Indeed, in mucosal explants of porcine cecum and colon, NE increases mucosal adherence of EHEC through interactions with alpha 2 -ARs that were characterized by conventional receptor criteria (Table 5.1). Increases in active anion secretion across the porcine colonic mucosa are in comparison mediated by alpha 1 -ARs (Brown and O’Grady 1997). Therefore, it appears that the actions of NE on ion transport and EHEC adherence are not linked through a common cellular mechanism. Alpha 2-ARs are negatively coupled to cyclic AMP production and a concomitant decrease in intracellular protein kinase A activity. In support of this receptor–effector association, the adherence-promoting action of NE in the porcine colonic mucosa is inhibited by the protein kinase A activator Sp-cAMPS and mimicked by the protein kinase A inhibitor Rp-cAMPs (Green et al. 2004). The effects of NE in the mouse and pig cecal mucosae are relatively rapid (≤90 min), and experiments with EHEC eae and EspA deletion mutants strongly suggest that NE and other sympathomimetic drugs enhance early, nonintimate bacterial adherence (Chen et al. 2003, 2006). As with their effects on Salmonella internalization, although the effects of NE and other sympathomimetic drugs on EHEC adherence may appear small (<1.0 log unit increase in the number of adherent bacteria in mucosal explants with an exposed surface area of 1 or 2 cm2), they may assume considerable medical importance when extrapolated over the extensive surface area of the cecal or colonic mucosa (Snipes 1997).
The mechanisms underlying this unique catecholamine action remain to be further defined through the identification of epithelial surface factors that mediate bacterial adherence and the receptor–effector pathways that are linked to their rapid expression. Beta1-integrins are IEC surface receptors implicated in aspects of EHEC adherence (Sinclair et al. 2006). By blocking epithelial beta1-integrins, heparin has been shown to inhibit EHEC adherence to human colonic epithelial cells (Gu et al. 2008). Norepinephrine is known to enhance interactions between blood cells and the vascular endothelium by stimulating the rapid expression of beta1-integrins (Levite et al. 2001; Butta et al. 2004; Delahunty et al. 2006), and it is tempting to speculate that it may similarly do so in promoting IEC interactions with luminal bacteria. In addition to dissecting the cellular and molecular mechanisms underlying this phenomenon, studies of catecholamine action on bacterial adherence in vitro should be extended to investigations of the role of endogenous and exogenous DA and NE in isolated intestinal loops and intact animal models which encompass larger surface areas and have greater translational relevance.
Norepinephrine may play a physiological role in promoting bacterial colonization of the large intestine, perhaps as an element in host defense. This hypothesis is based in part on a finding that NE increases cecal adherence of a non-O157 strain of E. coli, which was isolated from the porcine colonic mucosa (Chen et al. 2006). One interpretation of this result is that the action of NE is not limited to a particular bacterial strain or species. Presumptive NE nerve fibers immunoreactive for dopamine beta-hydroxylase are present throughout the submucosa and appear to terminate near the basal membranes of crypt and surface epithelial cells of the porcine distal colon and cecum (Green et al. 2004; Chen et al. 2006). Drugs capable of inhibiting the degradation (such as the monoamine oxidase inhibitor, pargyline) and neural reuptake (desipramine, cocaine) of NE at neuroepithelial junctions mimic the EHEC adherence-promoting action of NE, and their effects are inhibited by phentolamine (Green et al. 2004; Chen et al. 2006). In the porcine colonic mucosa, dopamine beta-hydroxylase-immunoreactive nerves terminate near IgA-positive B lymphocytes and neighboring IECs immunoreactive for the polymeric immunoglobulin receptor (Schmidt et al. 2007). Norepinephrine stimulates the vectorial secretion of secretory IgA in porcine colonic mucosa explants. This effect has been attributed to an alpha-AR-mediated increase in the luminally directed transport of secretory factor, a component of the polymeric Ig receptor (Schmidt et al. 2007). As noted above, constitutively produced secretory IgA is hypothesized to modulate colonization of the intestinal mucosa by commensal bacteria (Suzuki et al. 2007; Macpherson and Slack 2007).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree