M. Rizwan Sohail, Walter R. Wilson, Larry M. Baddour Keywords angioseal; atrial septal defect; biofilm; cardiac device; cardiac device infection; cardiac resynchronization therapy device; cardiac surgery; cardiac suture line infections; cardiovascular implantable electronic device (CIED); coagulase-negative staphylococci (CoNS); closure device treatment of patent ductus arteriosus; coronary artery stents; coronary stent infection; CRT; Dacron carotid patches; Dacron graft; dialysis graft; hemodialysis graft; hemodialysis prosthetic vascular grafts; implantable cardioverter-defibrillator (ICD); inferior vena cava (IVC) filter; intra-aortic balloon pump (IABP); invasive nonsurgical cardiologic procedures; left ventricular assist device (LVAD); nonvalvular; pacemaker; Perclose; peripheral vascular stents; PM; prosthesis; prosthesis infection; prosthetic vascular graft; PVGI; S. aureus; staphylococci; Staphylococcus aureus; vascular closure devices; vascular graft; vena cava filters; ventricular assist device (VAD); ventricular septal defect
Infections of Nonvalvular Cardiovascular Devices
The rapid evolution of technology, coupled with an aging population with multiple comorbid conditions, has led to the development of several new implantable devices that help to improve or sustain life. However, despite improvements in device manufacturing and availability of experienced operators implanting these devices, infection has remained a major complication of implantable cardiovascular devices. Risk of infection varies greatly (Table 84-1) and depends on the type and size of device, site of implantation, surgical technique used for device implantation, and host factors. Infections that complicate nonvalvular cardiovascular devices are addressed in this chapter. Intravascular catheter-related infections are reviewed elsewhere (see Chapter 302).
TABLE 84-1
Nonvalvular Cardiovascular Device–Related Infections
| TYPE OF DEVICE | RATE OF INFECTION (%) |
| Intracardiac | |
| Permanent pacemaker | 0.13-19.9 |
| Implantable cardioverter-defibrillator | 0.3-3.2 |
| Left ventricular assist devices | 13-80 |
| Ventriculoatrial shunts | 2.4-9.4 |
| Pledgets (cardiac suture line) | Rare |
| Patent ductus arteriosus occlusion devices | Rare |
| Atrial septal defect closure devices | Rare |
| Conduits | Rare |
| Patches | Rare |
| Arterial | |
| Peripheral vascular stents | 0.05-4.0 |
| Vascular grafts, including hemodialysis | 1-6 |
| Intra-aortic balloon pumps | ≤5-27 |
| Angioplasty/angiography-related bacteremias | <1 |
| Vascular closure devices | 0.0-5.1 |
| Coronary artery stents | Rare |
| Patches | 1.8 |
| Venous | |
| Vena caval filters | Rare |
Cardiovascular Implantable Electronic Devices
Permanent pacemaker (PPM), implantable cardioverter-defibrillator (ICD), and cardiac resynchronization therapy (CRT) devices are the three most commonly used cardiovascular implantable electronic devices (CIEDs). Sternotomy or thoracotomy was required for epicardial lead placement for the first generation of CIEDs. In contrast, almost all devices today are implanted percutaneously using transvenous leads. This change has led to a marked reduction in implantation-related morbidity and avoidance of potentially life-threatening infectious complications of major cardiothoracic surgical interventions. However, infection remains a major complication of CIED implantation and is associated with significant morbidity, mortality, and financial cost.1–3
Epidemiology
Reported rates of CIED infection have ranged from 0.13% to 19.9%.4–6 Over the past 2 decades, the rate of CIED implantation has rapidly increased in the United States. In a survey of Nationwide Inpatient Sample (NIS) discharge records from 1993 to 2008,3 the incidence of CIED implantation increased an average of 4.7% annually during the study period. Although the rate of CIED infection remained stable until 2004, it increased significantly from 1.53% in 2004 to 2.41% in 2008. This increase in the rate of device infections coincided with an increase in the number of comorbidities in device recipients. Moreover, this infection rate resulted in significant increases in in-hospital mortality and cost of care. The rate of device infection is several-fold higher for ICDs versus PPMs.7–9 In a separate analysis of a Medicare population,2 patients with CIED infection remained at higher risk of death, even after hospital discharge, compared with device recipients without infection.
Pathogenesis
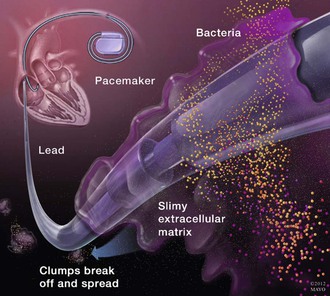
Microbial contamination of a device generator or leads with skin microbiota at the time of implantation is the most likely mechanism for most early-onset (within 6 months of device implantation) CIED infections.11 This likely explains the preponderance of staphylococci as causative pathogens. Colonization and subsequent infection of CIED is facilitated by an ability of these organisms to adhere to device surfaces and produce biofilm.12–14 This interferes with the host immune system’s ability to eliminate infection due to neutrophil dysfunction, poor penetration of antibiotics into the biofilm, and downregulation of metabolic activity in infecting organisms that makes them less susceptible to some antimicrobials, necessitating device removal for the best chance of infection cure (Fig. 84-1). Once a generator or pocket is colonized, bacteria can migrate along the electrode leads and manifest as tunnel infection, bacteremia, or infected vegetations on electrode leads or cardiac valves. However, hematogenous seeding of CIED leads is the predominant mechanism for the majority of late-onset (after 6 months of implantation) device infections, especially when there is no clinical evidence of pocket infection. Up to a third of patients with bloodstream infection due to gram-positive cocci, especially Staphylococcus aureus, in the setting of CIED may have an underlying device infection.15–18 Hematogenous seeding of device leads, however, is exceedingly rare in patients with gram-negative bacteremia that originates from a distant source.19
Risk Factors
Several host- and procedure-related factors predispose to an increased risk of CIED infection (Table 84-2).8,20–26 These factors predispose to device infection by increasing the risk of generator or lead contamination at the time of implantation and/or delay in wound healing at a generator pocket site or increased risk of bacteremia or fungemia from a distant source with hematogenous seeding of device leads.
In a case-control study,8 presence of a permanent central venous catheter, prolonged corticosteroid use, a history of CIED infection, presence of more than two electrode leads, and a history of multiple device-related procedures were associated with increased risk of pacemaker infection. Use of prophylactic antibiotics was associated with a reduced risk of PPM infection in this analysis.8 Operator inexperience has also been linked to an increased risk of device-related complications including infection.22 In another study that was limited to ICD recipients,20 use of epicardial leads and postoperative complications at the generator pocket (delayed wound healing, hematoma formation) were associated with increased risk of early-onset (within 6 months of implantation) device infection, whereas prolonged hospitalization and presence of chronic obstructive pulmonary disease (COPD) were associated with late-onset (6 months or later after device placement) infections. Other comorbidities, including diabetes mellitus, renal failure, and heart failure, have also been linked to an increased risk of CIED infection.24
Risk factors associated with CIED infection were evaluated in another multicenter, prospective investigation21 that included 6319 CIED recipients (5866 PPMs and 453 ICDs). Risk of device infection was significantly higher in patients who had fever within 24 hours of CIED implantation (adjusted odds ratio [OR], 5.83), those with a temporary pacing wire in place before device implantation (adjusted OR, 2.46), and those who underwent an early reintervention (adjusted OR, 15.04) for hematoma evacuation or lead revision. De novo device implantation (adjusted OR, 0.46) and antibiotic prophylaxis (adjusted OR, 0.4) both had protective effects.
Microbiology
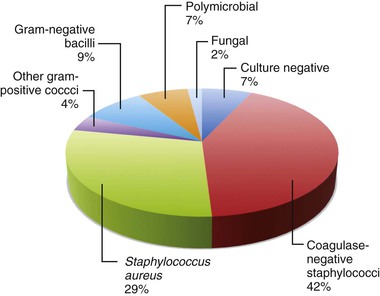
Staphylococci are the predominant pathogens in CIED infections.27,28 In one study from Mayo Clinic,4 coagulase-negative staphylococci (42%), followed by S. aureus (29%), were responsible for more than two thirds of cases of device infection (Fig. 84-2). Early device infections (within 2 weeks of implantation) are primarily caused by S. aureus. Prevalence of methicillin-resistant S. aureus (MRSA) is variable depending on the geographic location of reporting institutions. In the previously referenced study,4 gram-negative bacteria; other gram-positive cocci (including enterococci, streptococci, and micrococci); and fungi (Candida spp. and Aspergillus fumigatus) were isolated in 9%, 4%, and 2% of cases, respectively. Polymicrobial infection may be present in up to 7% of cases and tends to be more common in patients with diabetes mellitus and those receiving corticosteroids.29 Pocket site and blood cultures may be negative in patients who have previously received antibiotics or have low-virulence organisms associated with biofilm formation. Sonication techniques to disrupt biofilms may be a useful tool in the future to maximize the yield of device generator or lead cultures.30,31
Clinical Manifestations
CIED infection typically manifests in three distinct ways.4 The most common presentation is pocket site infection (>60%). Patients generally present with localized inflammatory changes at the generator pocket site, including erythema, pain, swelling, warmth, drainage, or dehiscence of overlying skin. Systemic signs of sepsis or positive blood cultures are present in less than one half of these cases. The second presentation is occult bacteremia or fungemia and no local changes at the pocket site. The third presentation is CIED-related endocarditis (lead or valvular vegetations, or both) in 10% to 23% of cases.32,33 Patients with device-related endocarditis are defined by modified Duke criteria.33–35 When vegetations are visualized on cardiac structures, the tricuspid valve is the most common site of infection (up to 25% of cases with CIED-related endocarditis).33 However, vegetations can develop on the pulmonic or left-sided valves, especially in the setting of S. aureus bacteremia (SAB). Patients with SAB may have radiographic findings of multiple focal pulmonary infiltrates or abscesses due to septic emboli in as many as 40% of the cases.36,37 Patients with CIED-related endocarditis have a higher mortality (14% to 25%) compared with those without it (<5%), even with percutaneous lead extraction.33,35 A small proportion (<5%) of the patients can present with erosion of a device lead or generator in the absence of inflammatory signs at the pocket and negative blood cultures.4 Whether erosion is caused by low-grade infection or purely by mechanical factors may not be entirely clear in all patients.
Diagnosis
A diagnosis of CIED infection is easily discernible when pocket site inflammatory changes are present. However, a diagnosis may be difficult to confirm in cases in which positive blood cultures are the sole manifestation of underlying CIED infection. It is critical that blood cultures be obtained in all patients with suspected CIED infection before starting any empirical antibiotics. If drainage is present, swab specimens should be submitted for cultures. Abnormal laboratory findings (leukocytosis, anemia, high erythrocyte sedimentation rate, or C-reactive protein) are present in 50% or less of cases, and the absence of these findings should not dissuade clinicians from considering a possibility of CIED infection in the appropriate setting.
Positive blood cultures, even in the absence of other manifestations of device infection, should prompt a serious consideration for underlying CIED infection. In patients with SAB, concomitant CIED infection is present in up to 50% of the patients.18,38 Risk is especially high within the first year after device implantation. Of note, local signs or symptoms of pocket or tunnel infection may be absent in more than 50% of these cases. Therefore, we recommend thorough evaluation for underlying CIED lead infection with transesophageal echocardiography (TEE) and possibly an ultrasound scan of the generator pocket (to evaluate for fluid collection) in patients with SAB and the presence of CIED. Although fluid can be present early after surgical implantation of the device generator, its accumulation months to years after device placement would be unusual. Ultrasound-guided diagnostic aspiration could be considered. However, it carries a risk of introducing infection in an otherwise sterile collection during this procedure. Indium-labeled leukocyte or gallium scanning may be helpful in differentiating an inflammatory fluid collection from a noninfected pocket site fluid accumulation. A technetium-labeled white blood cell scan may aid in the diagnosis of CIED infection in patients who present with sepsis and bacteremia but have no inflammatory changes at the generator pocket. In one study that investigated the diagnostic performane of technetium 99–labeled WBC scintigraphy in patients with suspected CIED infection, sensitivity of technetium 99–labeled WBC single-photon emission computed tomography/computed tomography (SPECT/CT) was 94% for both detection and localization of CIED-associated infection. Technetium 99–labeled WBC scintigraphy not only enabled the confirmation of CIED infection but was also helpful in defining of the extent of device involvement and detection of associated complications. Moreover, Tc99 labeled WBC scintigraphy reliably excluded device-associated infection during a febrile episode and sepsis, with 95% negative predictive value.39 In one investigation, positron emission tomography (PET) scan combined with CT was helpful in differentiating between CIED infection and recent postimplant changes.40 In this study, average duration from last device-related intervention and presentation with CIED infection was 11.2 months and majority of the patients presented with pocket infection or erosion. Only eight patients had normal-appearing generator pocket and met Duke criteria for CIED lead endocarditis. Thirty-two of 42 patients with suspected CIED infection had a positive PET/CT. Twenty-four of these patients with positive findings on PET/CT underwent extraction with an excellent correlation with CIED infection. Ten patients, who had negative PET/CT imaging and were managed conservatively with antibiotics alone, without device removal, had no signs of infection relapse after an average follow-up duration of 13 months. Considering the additional cost of nuclear medicine imaging, in our practice we only use it when patients have no inflammatory changes at the generator pocket and TEE is inconclusive in diagnosing or excluding CIED infection.
CIED removal may also be considered in patients who have no identified source of SAB. Failure to remove an infected device is associated with an increased risk of treatment failure with relapsing bacteremia. In patients with no evidence of CIED infection (after a thorough evaluation that includes TEE) at the time of SAB and for whom a decision is made to treat conservatively, there should be close follow-up over the next 12 weeks to monitor for relapsing bacteremia. Surveillance blood cultures after completing 2 to 4 weeks of appropriate parenteral antibiotics should be considered.
Lead tip cultures are frequently used to confirm the diagnosis of CIED-related infective endocarditis.36,37,41 However, most transvenous leads are extracted percutaneously in current practice, and lead tips may be contaminated during removal through the incision of an infected pocket.33 Lead tip cultures are more reliable if they are extracted using a sterile technique (via protected sleeve) or if removed from a different incision than that used at the generator pocket site.
Role of Echocardiography
Echocardiography has been pivotal in securing a diagnosis of CIED-related infective endocarditis and other infectious cardiac complications associated with CIED infection such as valvular vegetations, perivalvular extension of infection, and abscess formation. The diagnostic superiority of transesophageal echocardiogram (TEE) over transthoracic echocardiography (TTE) has been substantiated in multiple studies (reported sensitivity of TEE is >95% compared with <30% of TTE).14,33,42,43 Besides confirming the diagnosis of endocarditis, TEE can assist in making decisions regarding the most appropriate extraction strategy by providing information regarding vegetation size and attachment of device leads to surrounding venous or cardiac structures.
Complications
Complications of CIED infection may include valvular endocarditis (mostly right sided); septic arthritis; spine infection (diskitis, vertebral osteomyelitis, or epidural abscess);44 sternal wound infection; metastatic abscesses (lung, liver, spleen, brain, renal); and thrombosis of a subclavian vein or superior vena cava. In patients with metastatic abscesses or osteomyelitis, it may be difficult to decipher whether an ectopic site is the source of bacteremia with hematogenous seeding of a cardiac device or vice versa.
Management
The primary focus of treatment should be complete removal of the CIED system. Although no prospective, randomized trials have been conducted to evaluate the role of medical (antimicrobial) therapy alone versus a combined medical-surgical treatment approach, data from several retrospective analyses show a clear and clinically important advantage of complete device removal.4,29,45,46 For example, the reported mortality rate of CIED-related endocarditis ranges from 31% to 66% if the infected device is retained compared with 18% or less in patients managed with a combined approach of complete device removal and parenteral antibiotics.33,36,37 Patients with partial device removal (generator only) or those treated conservatively with antibiotics alone have a higher risk of treatment failure or relapse.4,29,47 In one investigation of 416 patients with CIED infection, antimicrobial therapy alone without device removal was associated with a sevenfold increase in 30-day mortality.48 Moreover, immediate device removal, when compared with delayed explantation or no device removal, was associated with a threefold decrease in 1-year mortality in this analysis.
When planning device removal, three factors must be addressed. First, removal of a lead that is embedded in cardiac tissue can be difficult and potentially dangerous. Complications include tamponade due to tearing or perforation of the myocardial wall, laceration of the superior vena cava or tricuspid valve, hemothorax, fracture of lead fragment requiring surgical intervention, and life-threatening arrhythmias.4 However, newer techniques, including the use of a locking stylet, photoablation of fibrous attachments with a laser sheath, and minimally invasive video-assisted thoracoscopic techniques, are less invasive and safer methods of device removal compared with open thoracotomy.4,14
Second, in cases where larger (>10 mm in diameter) vegetations are attached to infected leads, embolic showering of the pulmonary vasculature with percutaneous lead extraction is a concern. However, data from several studies4,33,37,42,49 indicate that risk of clinically significant pulmonary emboli and death with percutaneous extraction is low and does not warrant routine surgical removal of leads via thoracotomy in this patient population. Routine screening for subclinical pulmonary emboli using CT or ventilation-perfusion scan is also not recommended.33 Risks of thoracotomy and need for cardiac bypass (in mostly an older patient population) should be considered before deciding whether a percutaneous or a surgical approach should be used.
Third, the need for ongoing CIED support should be assessed before implanting a new device. The percentage of patients who do not require replacement of a PPM has ranged from 13% to 52%.4,29,36,45 In patients who are dependent on a device, a temporary system can be used while bloodstream infection (BSI) is cleared by antimicrobial therapy before implantation of a new device. However, use of temporary wires may increase the risk of new device infection.21
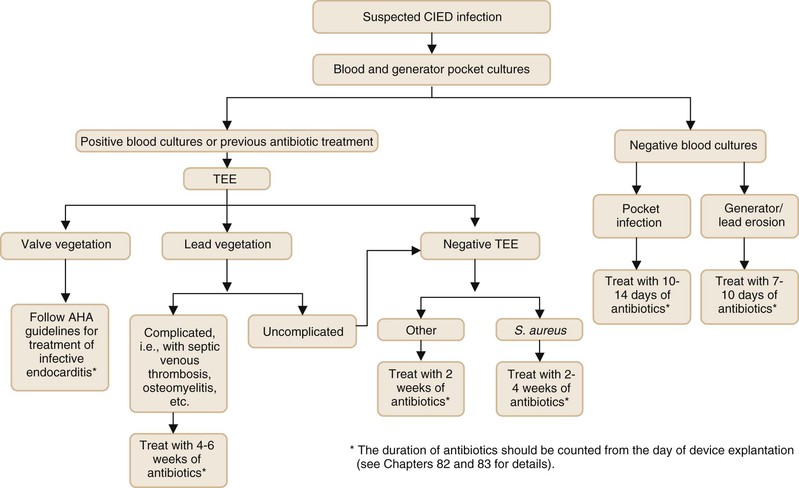
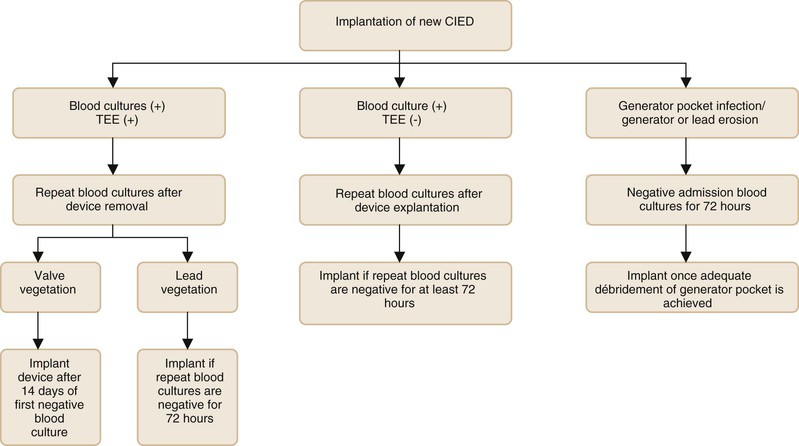
Figures 84-3 and 84-4 summarize the management approach of CIED infection.50,51 We recommend a two-step exchange in most patients (i.e., complete device removal followed by replacement CIED, if needed, in a subsequent intervention). Clearance of BSI, if present, and adequate control of infection at the generator pocket site should be achieved before placement of a new device (see Fig. 84-4). For patients who are bacteremic at initial presentation, blood cultures should be repeated after device explantation. Patients who have persistently positive blood cultures after device removal should be treated for at least 4 weeks with antibiotics even if a TEE is negative for valve vegetations. For patients who present with generator or lead erosion only, in the absence of clinical evidence of pocket infection, the replacement CIED may be placed immediately after removal of the original device (one-step exchange). The new device should be placed on the contralateral side in patients who have clinical or intraoperative findings consistent with generator pocket infection. Cure rates of more than 95% can be achieved.4
The choice and duration of antimicrobial therapy are dictated by pathogen, in vitro antimicrobial susceptibility results, and the presence or absence of concomitant valvular endocarditis. Empirical antibiotics should include coverage for methicillin-resistant S. aureus (MRSA) and coagulase-negative staphylococci. Intravenous administration of antimicrobials is recommended. If an infected CIED cannot be removed, long-term oral suppressive antibiotics52 (for the life of the CIED) should be administered after an appropriate course of initial induction therapy (see Fig. 84-2).
Prevention
Meticulous attention to aseptic technique is key to preventing microbiologic contamination of CIED generators and leads at the time of implantation. Several clinical studies have demonstrated the efficacy of antibiotic prophylaxis before device implantation.8,21,53,54 In a recent large, prospective, randomized, double-blinded, placebo-controlled clinical trial,55 patients undergoing CIED implantation or generator replacement were randomized to either receive single dose of cefazolin or placebo for perioperative prophylaxis. In multivariable analysis, lack of antibiotic prophylaxis was an independent predictor of CIED infection (P = 0.037). For patients who are allergic to β-lactam antibiotics or are colonized with methicillin-resistant S. aureus (MRSA), vancomycin is an acceptable alternative. There are no published data to support the routine use of secondary prophylaxis for patients undergoing dental, respiratory, gastrointestinal, or genitourinary procedures, and the practice is discouraged.14
According to some emerging data, use of an antibiotic (minocycline and rifampin)–impregnated envelope may reduce the risk of CIED pocket infection in high-risk patients.56,57 However, added benefit of using this polymer mesh over standard perioperative prophylaxis has not been demonstrated in randomized, prospective, clinical trials yet.
Left Ventricular Assist Devices
Historically, left ventricular assist devices (LVADs) were used to provide temporary support for patients with end-stage heart failure while waiting heart transplantation (bridge to transplantation). However, with increasing prevalence of advanced heart failure and limited supply of donor organs, LVADs are increasingly being used as long-term myocardial surrogate therapy (so-called destination therapy), especially in patients who are ineligible for a heart transplant.58 With this changing trend, interest is shifting from short-term complications to long-term infection rates and survival. Infection is a major complication of LVAD therapy and was responsible for up to 20% of deaths in one cohort of LVAD recipients.59
LVADs are mechanical pumps that are implanted in the thoracic cavity to assist the pumping action of a failing heart. These devices are connected to the heart via two cannulas, pulling blood from the left ventricle and pumping into the aorta (Fig. 84-5). Most LVADs used in contemporary practice (e.g., HeartMate II; Thoratec Corporation, Pleasanton, CA and Jarvik 2000; Jarvik Heart, Inc., New York) are designed as axial flow pumps to provide continuous flow.60 These devices do not require a mechanical valve, are smaller in size (and therefore less prone to infection), and last longer. These devices are connected to an external battery pack with a driveline. A newer type of LVADs, which employs electromagnetically or hydrodynamically levitated continuous-flow centrifugal pumps (e.g., HeartWare HVAD, Duraheart, Los Angeles) are being tested in clinical trials and have the theoretical advantage of even simpler mechanics with fewer moving parts.
LVAD therapy is associated with significant infection risk, and this risk was highlighted in the REMATCH trial58 that examined the role of long-term LVAD use as destination therapy. In this study, LVAD recipients were more than twice as likely to have a serious adverse event compared with patients in the optimal medical therapy group. Infection and device failure were predominant factors that led to a limited 2-year survival rate of 23%. Within 3 months of LVAD placement, LVAD infection complicated 28% of cases. On the basis of more recent data, LVADs that employ continuous-flow pumps are at lower risk of infection compared with devices with pulsatile flow pumps.61,62
Epidemiology
LVAD therapy is associated with a substantial risk of infectious complications. Patients who receive LVAD therapy tend to have multiple comorbidities, are frequently hospitalized, and usually have multiple other prostheses, such as implanted central venous catheters for infusion of drugs or hemodialysis, cardiovascular implantable electronic devices for cardiac rhythm management, and prosthetic vascular grafts, due to generalized vasculopathy. The risk of infection also varies on the basis of duration of LVAD support,63 with higher rates of infection reported in the destination therapy group compared with patients where LAVD is used as a bridge to transplantation.64 In one review of LVAD-related complications, the incidence of infection was two times higher in patients with an LVAD in place for more than 60 days compared with those with an LVAD for less than 30 days.65
The reported rates of infection in LVAD recipients vary from 13% to 80%14 and depend on multiple factors, including underlying comorbidities, type of device, duration of LVAD support, and study definition for LVAD infection. In a cohort study66 that included patients with HeartMate devices, the overall rate of surgical site infection was 44 per 100 LVAD implantations or 6.2 infections per 1000 device days. Seven of the patients had superficial infection that involved the driveline incision (19.4 infections per 100 LVAD implantations), 3 patients had sternal infections (8.2 infections per 100 LVAD implantations), and 6 had organ space infections that involved either the LVAD or the preperitoneal pocket that contained the pump (16.7 infections per 100 LVAD implantations). Two of the patients also developed mediastinitis.
Risk of LVAD-related BSI was calculated in a retrospective study from Cleveland Clinic.67 An LVAD-related BSI was defined as one in which the same pathogen was cultured from the device and the blood and no other source of bacteremia was identified. Overall, 140 nosocomial BSIs occurred in 104 (49%) of 214 patients (7.9 BSIs per 1000 LVAD days); 53 (38%) episodes were device related. Patients with LVAD-related BSI had a higher mortality rate of 58% compared with 42% in patients with LVAD support but no BSI (P = .07) in this investigation.
Pathogenesis
The impact of LVAD implantation on the immune system is speculated to be one factor that predisposes device recipients to infectious complications.68,69 LVAD implantation can lead to increased susceptibility of circulating CD4 T cells to activation-induced apoptosis, resulting in a progressive decrease in CD4 T-cell count and impaired cell-mediated immunity. This puts LVAD recipients at increased risk of infections, including opportunistic pathogens. In one clinical survey,69 LVAD recipients were at increased risk of developing Candida infections compared with controls (28% vs. 3%; P = .003). Dysregulation of the humoral arm of the immune system has also been reported.
Risk Factors
Duration of LVAD support64 and documented trauma at the driveline exit site are well-known risk factors associated with device infection.70 In another investigation,71 patients who developed LVAD-related BSI had a higher prevalence of diabetes mellitus compared with others (OR, 7.7; 95% confidence interval [CI], 2 to 29.8). Data from INTERMACS, the largest registry of patients who received U.S. Food and Drug Administration–approved mechanical circulatory assist devices, suggest that continuous-flow devices have a lower rate of infectious complications as compared with LVADs with pulsatile pumps.72 Risk factors for LVAD-related infection are summarized in Table 84-3.
TABLE 84-3
Risk Factors for Left Ventricular Assist Device Infection

Clinical Manifestations
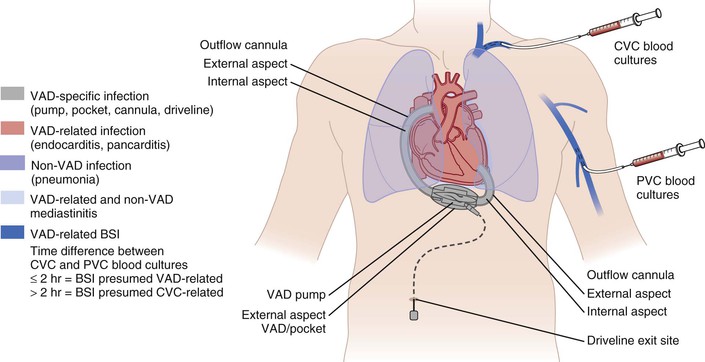
Infectious complications related to LVADs can be broadly categorized into three types (Fig. 84-5): (1) LVAD-specific infections. These infections are related directly to the device hardware and can only occur in patients with LVADs. These include pump and cannula infections, pocket infections, and percutaneous driveline infections; (2) LVAD-related infections. Although these can also occur in patients without LVADs, the presence of an LVAD warrants special management considerations. These include valvular endocarditis, BSIs, and mediastinitis; and (3) non-LVAD infections. These are not related to LVAD placement and may include pneumonia, urinary tract infection, and other remote infections.
The most common presentation of LVAD-specific infection is driveline exit site infection. Local inflammatory changes and purulent drainage are frequently seen. Device pocket infections usually present with frank inflammatory changes in the skin and soft tissues overlying a device pocket. Infection of the valves or other blood-contacting surfaces of the LVAD occurs less often. Clinical manifestations of this form of endocardial infection mirror the manifestations of native or prosthetic valve endocarditis. Fever and other signs of systemic inflammatory response may be apparent. Systemic or pulmonary (for devices that assist the right ventricle) embolic phenomena can be seen. Mechanical dysfunction of the LVAD can occur if an obstructing pannus of infection develops within the internal lumen of the inflow or outflow cannulas that are anastomosed into the apex of the left ventricle and ascending aorta, leading to clinical worsening of cardiac function.
Microbiology
S. aureus and coagulase-negative staphylococci account for more than 50% of cases of LVAD-related infections. Enterococci, Enterobacter spp., and Pseudomonas aeruginosa are other commonly isolated bacterial pathogens in LVAD-related infections.67,71 Because of nosocomial acquisition of these infections, antimicrobial resistance is a common occurrence. Although colonization with Candida species occurs in up to 39% of patients with LVADs, clinical infection is less frequent.73 Use of broad-spectrum antibiotics increases this risk. Candida infection can present as BSI or vegetations on endocardial surfaces that can be large enough to obstruct blood flow or cause peripheral embolization. Eradication of infection is difficult due to adherence of Candida to prosthetic surfaces and resulting biofilm formation.
Management
Limited débridement of an infected driveline exit site or device pocket may be helpful to control localized infection in some patients. Vacuum-assisted closure systems can help to facilitate wound healing.74 Even in patients with LVAD-related BSI, chronic suppressive antimicrobial therapy (after an induction course) may be effective in preventing recurrence of clinical findings of infection. Suppressive antibiotics are continued until the LVAD is removed and cardiac transplantation is performed. However, LVAD removal is required in others to control infection. Of note, LVAD-related infection is not a contraindication to cardiac transplantation.14,71 In fact, for some patients, cardiac transplantation is necessary not only for advanced heart failure but also for removal of the infected device to achieve control of ongoing infection. LVAD-related infection led to significant delays in heart transplantation in one investigation (median duration, 182.8 days vs. 66.3 days for noninfected patients; P < .001).71 Earlier reports suggested that patients with pretransplantation LVAD infection have similar short (<6 months) and long-term (as long as 3 years) survival rates after cardiac transplantation compared with noninfected controls.67,75 However, a subsequent investigation71 showed a prolonged length of hospitalization and increased early mortality in heart transplant recipients with pretransplantation LVAD-related infection. However, long-term survival rates were comparable between the two groups. Invasive vancomycin-resistant Enterococcus faecium (VRE) infection was the driving factor for early post-transplantation mortality in patients with a history of LVAD-related infection (P = .02). In another retrospective analysis,70 late-onset driveline infection was associated with repeated hospitalizations (P < .001) and lower 5-year survival rate (41% vs. 70%, P = .10).
Treatment duration of LVAD-related BSI in patients who are awaiting heart transplantation is unclear. In one investigation of 76 patients with LVADs,71 continuous antimicrobial treatment, from the time of initial diagnosis of BSI until transplantation or death, was associated with a lower rate of relapse compared with a limited antibiotic course of 2 to 6 weeks (P = .003). Relapses were diagnosed a median of 12 days (range, 2 to 89 days) after discontinuation of antimicrobial therapy. All patients who had LVAD-related infection due to S. aureus and were treated with limited courses of antibiotics experienced relapse. There was no difference in the two groups in terms of post-transplantation mortality, length of hospitalization, or 1-year survival.
Prevention
Several strategies have been advocated to reduce the rate of LVAD-related infections.76 Chlorhexidine and silver sulfadiazine coating of the LVAD driveline was shown to reduce early-onset LVAD-related infections in an animal model.77 Elimination of percutaneous drivelines with new generations of totally implantable LVADs, such as the Jarvik 2000 axial flow pump and the LionHeart device (Arrow International, Reading, PA), has been associated with significant reduction in LVAD infection rates.78,79 In addition to advances in technology, we should focus on other preventive measures currently in use, including broad-spectrum antimicrobial administration (to cover resistant gram-positive cocci, gram-negative bacilli, and Candida spp.) at the time of LVAD placement, limiting central venous catheters and other types of temporary percutaneous vascular device use, performing meticulous dressing changes at the driveline exit site, lengthening the driveline subcutaneous tunnel, and immobilizing the driveline at the skin exit site to prevent repeated trauma and skin breakdown.
Prosthetic Vascular Grafts
Despite technical advances in graft design, infection remains a significant complication associated with use of prosthetic vascular grafts and can lead to loss of limb or life. Infections involving the prosthetic vascular grafts can manifest in three distinct ways: (1) perigraft infection or abscess formation, (2) graft exposure due to disruption of the superficial soft tissue layers overlying the prosthesis, and (3) graft erosion or fistula formation involving a mucosal surface. However, these three presentations of graft infection are not mutually exclusive.
Epidemiology
The rate of prosthetic vascular graft infection (PVGI) varies and depends on the anatomic location of the prosthesis. For aortic grafts limited to the abdomen, graft-related infections occur in 1% or less of recipients. The rate of infection is higher (1.5% to 2%) for aortic grafts that extend to the femoral location. Infrainguinal vascular grafts that originate in the groin are at the highest (6%) risk of complicating infection.14,80,81,82,83 Reported morbidity (mostly amputations) and mortality rates for peripheral vascular grafts are 41% and 17%, respectively.84 Prosthetic aortic graft infections have a higher mortality rate (24% to 75%). The average 5-year survival rate for aortic graft infections is only 50%.80,85
Pathogenesis
Several distinct routes of microbial contamination are recognized in vascular graft infections. Microbial seeding of a graft at the time of implantation or in the immediate postoperative period accounts for most of the graft infections. Vascular graft contamination can also occur if there is infection in a contiguous anatomic area. Bacterial colonization of a thrombus (13%) in an aneurysmal sac or in atherosclerotic plaques (40%) in arterial walls is an example of adjacent site of infection.85,86 Superficial surgical site infection can also lead to graft infection. Subsequent manipulation of an implanted graft by either surgical or percutaneous procedures can predispose to graft infection. Finally, an anatomically remote site of infection can contribute indirectly to vascular prosthetic graft infection by causing BSI and secondary graft contamination. This risk of hematogenous seeding is highest in the early postoperative period and decreases over time because of partial endothelialization of the graft.86
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree