INFECTIONS DUE TO NONTUBERCULOUS MYCOBACTERIA
JEANA L. BENWILL AND RICHARD J. WALLACE, JR.
Mycobacteria other than Mycobacterium tuberculosis have established themselves as human pathogens. By 1960, most of the common species of nontuberculous mycobacteria (NTM) had been described, including Mycobacterium kansasii, Mycobacterium avium, Mycobacterium intracellulare, and Mycobacterium fortuitum (1). More than 140 species have now been recognized and characterized in the genus Mycobacterium (2–5), more than 70 of them in the past 20 years (4,5). Much of this taxonomic explosion relates to the use and availability of 16S ribosomal (rRNA) gene sequencing, 65 kDa heat shock protein (hsp65), β-subunit of RNA polymerase (rpoB), and large public DNA databases (2–5).
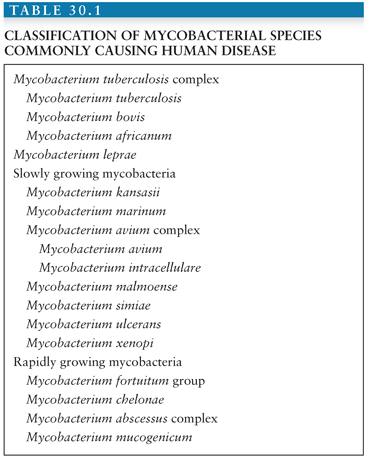
Two general groups of NTM can be delineated on the basis of microbiologic, clinical, and epidemiologic characteristics: the slowly growing and rapidly growing mycobacteria (RGM). Characteristics of the species included in these groups are shown in Table 30.1 (see later discussion). The incidence of disease due to NTM has increased substantially in the past 20 years, in part because of better knowledge of its diseases, in part because of improved laboratory techniques, and in part because of the pandemic of human immunodeficiency virus (HIV). The most commonly recovered species or species complex is M. avium complex (MAC), which commonly caused disseminated disease in patients with advanced HIV infection before the advent of effective antiretroviral therapy (ART). Among the slowly growing mycobacteria: MAC, M. kansasii, M. genavense, M. malmoense, and M. simiae have been identified with central nervous system (CNS) disease. In addition to the slowly growing NTM, the RGM organisms that have been associated with CNS disease have been M. fortuitum group (primarily M. fortuitum) and the M. abscessus complex. This chapter summarizes the epidemiology, diagnosis, and treatment of each of the NTM reported to cause CNS disease.
GENERAL OVERVIEW
NTM are generally free-living organisms that are ubiquitous in the environment. They have been recovered from surface water, household water and biofilms, soil, and domestic and wild animals (1–6). Studies have demonstrated that mycobacteria easily become aerosolized from aqueous sources and that more easily aerosolized strains also more commonly cause pulmonary infections.
During the 1950s, the ability of NTM to cause human disease became increasingly clear as a consequence of several landmark publications. In 1959, Runyon (7) proposed a classification system that divided human isolates of NTM into four groups on the basis of growth rates, colony morphology, and pigmentation in the presence and absence of light. This allowed mycobacterial laboratories to more readily identify individual species of NTM, allowing clearer characterization of distinct diseases and syndromes associated with these organisms. In 1979, Wolinsky (1) published an exhaustive review of the clinical and laboratory features of these organisms. This provided one of the first reference sources for physicians to review the clinical significance of the wide variety of species grouped as NTM and the clinical disease syndromes they produce. The number of cases of NTM disease has dramatically increased, as has knowledge about these mycobacteria, in the more than 30 years since this publication appeared. Subsequent major reviews that have updated newer species since the study by Wolinsky (1) in 1959 were by Wayne and Sramek (2) in 1992, Brown-Elliott and Wallace (3) in 2002, and Tortoli (4,5) in 2003 and 2006.
The frequency of disease due to the different species of NTM is unknown. By the early 1990s, laboratories informally reported that more than 50% of evaluated mycobacterial species were NTM. Much of this increase is related to the high incidence of disseminated MAC disease in patients with acquired immunodeficiency syndrome (AIDS). Among 41,439 cases of AIDS reported to the Centers for Disease Control and Prevention (CDC) from 1981 to 1987, disseminated NTM infection occurred in 5.5%, and 96% of these were due to MAC (8). As a consequence, CNS infections due to NTM have increased in frequency.
Prior to describing NTM infections of the CNS in patients with HIV, it may be useful to briefly review disseminated NTM infection in these patients. With the exception of rare cases that might occur by direct extension or from trauma, infection of the CNS is likely to occur from hematogenous dissemination. Therefore, these patients may approximate the patient population from which CNS disease arises. Horsburgh and Selik (8) summarized the epidemiology of disseminated NTM infection in the pre-ART era in the United States from 1981 to 1987 based on data reported to the CDC. As stated previously, disseminated NTM infection was reported in 5.5% (2,269 cases) of patients with AIDS over this 7-year period and 96% of these were MAC. The remaining 4% included M. kansasii (2.9%), M. gordonae (0.6%), and M. fortuitum complex (0.3%). The number of patients with NTM disease increased in parallel with the incidence of AIDS, and the overall proportion in the pre-ART era remained nearly constant at 5.5%. Significant regional variations in this figure, from 3.9% to 7.8%, were noted. NTM disease occurs primarily in patients with AIDS, generally in patients with fewer than 50 CD4+ cells/µL (9). Patients with disseminated NTM infection had significantly shorter survival (median, 7.4 months) than patients with AIDS without disseminated NTM infection (median, 13.3 months). Disseminated NTM infection is diagnosed after AIDS in nearly one half (47.2%) of patients. In short, the early AIDS epidemic has been accompanied by an epidemic of disseminated NTM disease as severely immunocompromised patients were exposed to these ubiquitous but relatively less virulent organisms. With the advent of ART, however, the recovery rate of MAC and other NTM in patients with AIDS has fallen dramatically since the mid to late 1990s.
In the absence of HIV infection, on the other hand, the distribution of infections due to the different NTM species was reported by Swiss investigators who reviewed all 513 HIV-negative patients from whom NTM were isolated at the University of Zurich from 1983 to 1988 (9). In this study, 34 patients were thought to have clinical disease due to these infections, 21 had pulmonary disease, 10 had soft tissue disease, and 1 had disseminated disease. There were no instances of CNS involvement. The most common isolates were MAC in 16 patients and M. kansasii in 9 patients. M. terrae complex, M. fortuitum group, M. marinum, and M. malmoense were also involved.
NTM disease in children before the AIDS era was reviewed by Lincoln and Gilbert (10). In contrast to adults, cervical lymphadenitis due to M. scrofulaceum and MAC, as well as cutaneous disease due to M. marinum and M. ulcerans, predominated. Twelve cases of disseminated disease were reported. Six patients were reported with definite CNS disease and one additional case was highly probable. Of these seven patients, M. kansasii was the pathogen in three patients (one was a dual infection with M. tuberculosis), scotochromogens were reported in two patients, and MAC in two patients. Three patients were Asian and two patients each were from Europe and the United States. Cerebrospinal fluid (CSF) results reported in five of seven patients were typical of mycobacterial infection, that is, lymphocytic pleocytosis, elevated protein level, and depressed glucose levels. One definite case, however, had a neutrophilic pleocytosis. Treatment was not detailed and four patients died. A 2-year prospective surveillance study of children in Netherlands with NTM demonstrated that MAC was the most common pathogen, but no disseminated or CNS disease was reported (11). Jensen et al. (12) conducted a retrospective review of mycobacterial disease in HIV-infected children in Spain from 1997 to 2008. Of 1,307 HIV-infected children, 42 had mycobacterial disease of which 22 had NTM. Nine had disseminated disease, but CNS involvement was not reported. Overall, a reduction of mycobacterial infection decreased from 5.9 to 1.6 events per 1,000 HIV-infected children-year. NTM disease rates were 2.3, 3.4, and 0.3 per 1,000 HIV-infected children-year for 1997 to 1999, 2000 to 2002, and 2003 to 2008, respectively. The authors attribute the increase of NTM disease during the 2000 to 2003 period to increased awareness and diagnostic consideration of NTM (12).
The review by Wolinsky (1) serves as a convenient and authoritative starting point for a discussion of NTM infections of the CNS prior to 1979. One case each of meningitis due to M. kansasii, MAC, and M. gordonae were described. The patient with M. kansasii was a 34-year-old woman who died of disseminated infection. The patient with MAC was a 13-year-old boy with disseminated disease who had MAC isolated from CSF and had granulomatous meningitis on postmortem examination. The patient with M. gordonae infection was a hydrocephalic infant who had undergone multiple shunt procedures with infection of the peritoneal fluid and CSF. Wolinsky (1) also mentioned one case of MAC meningitis from his own experience. In addition, he noted two instances in which other pathogens were also present: M. kansasii was isolated from the meninges at postmortem examination in a 2-year-old with miliary and meningeal M. tuberculosis, and M. intracellulare was isolated from the CSF of a 46-year-old woman with cryptococcal meningitis (a subsequent smear of the CSF for acid-fast bacilli [AFB] was positive, but cultures were negative with the patient receiving chemotherapy). Because two pathogens were isolated, the role of NTM in these patients’ diseases was difficult to determine.
The following sections of this chapter review the epidemiology, microbiology, and chemotherapy of the NTM implicated in CNS disease and summarizes reported cases to date. The chapter is organized by species of mycobacterium divided into slowly and rapidly growing species. Several case series described more than one species. Investigators from Brooklyn, New York described 11 years of experience at Kings County Hospital with NTM from 1980 through 1990 infections of the CNS in patients with AIDS (13). Sixteen CSF cultures were positive for NTM. Fifteen grew MAC and one grew M. fortuitum group. Three patients concurrently had CNS toxoplasmosis. Ten of the fifteen died during hospitalization. Japanese workers accumulated 109 cases of NTM disease by nationwide investigation from 1955 to 1965; 6 involved the CNS, but the NTM isolates were not speciated (14). A report from Malaysia identified a 9-month-old admitted with meningitis and CSF from which AFB with yellow-orange colonies were cultured from a cisternal puncture but not from a lumbar puncture (15). Wolinsky’s view of these reports raised the important issue of acceptable criteria for the diagnosis and reporting of CNS disease due to NTM. Given a compatible clinical situation, isolation of multiple colonies of NTM in pure culture from repeated specimens of CNS tissue or fluid would constitute strong evidence for NTM as a cause of the patient’s CNS disease (16). Clinical and microbiologic response to specific therapy and the absence of other pathogens would virtually seal the diagnosis.
SLOWLY GROWING NONTUBERCULOUS MYCOBACTERIA
Mycobacterium avium Complex
Epidemiology
MAC includes ubiquitous, free-living organisms (1). MAC isolates are readily recovered from natural reservoirs including soil and water, and organisms similar to those causing human disease have been recovered from aerosols near natural waters. Recent environmental studies have shown that the major reservoir for M. avium is household water and pipe biofilms but that M. intracellulare is absent and presumably acquired from environmental exposure outside the household (17). The geographic distribution of the organism, however, is not uniform. A survey of skin-test reactivity within the United States to an antigen prepared from MAC was published in 1969 (18). The skin-test reagent, called PPD-B or Battey antigen (Battey State Hospital, Rome, Georgia), was applied to approximately 275,000 naval recruits who had lived their entire lives in a single county. Skin-test reactivity was most common among recruits from the southeastern and Gulf Coast states. In these areas, more than 70% of individuals had been exposed to or infected with MAC or an antigenically similar organism. MAC lung disease appears to be relatively more prevalent in these same areas.
The incidence of MAC infections in selected groups of HIV-infected patients before the advent of effective ART was measured by Nightingale et al. (9). They followed 1,006 patients at one institution during a 3-year period with monthly blood cultures from the date of AIDS diagnosis and determined the incidence of MAC bacteremia to be 21% at 1 year and 43% at 2 years (9). The incidence of MAC bacteremia was inversely related to CD4+ lymphocyte counts, occurring in only 3% of patients with CD4+ lymphocytes of 100 to 199 cells/µL increasing progressively to 39% for patients with fewer than 10 CD4+ lymphocytes/µL.
Pathogenesis
MAC is likely to disseminate to the CNS hematogenously from a respiratory or gastrointestinal source. In rare cases, CNS disease may occur following trauma, direct extension from an adjacent focus of infection such as the paranasal or mastoid sinuses and with implantable foreign material (19–22).
Early experience with MAC disease in HIV-infected patients indicated that heavy bacteremia, widespread disease, and a high tissue burden of organisms were typical. Subsequent study suggests that these early observations focused on patients with relatively far advanced disease. Torriani et al. (23) studied 44 patients with MAC bacteremia who eventually had complete autopsies over 5 years in California. No tissue involvement could be identified in 30% of patients, whereas 70% of patients had one to nine different tissue sites involved (median of four). CNS involvement was seen in one patient who had both cerebral and spinal cord infection with MAC. The likelihood of detectable tissue involvement increased directly in proportion to the length of survival after the first positive blood culture: 43% in patients dying within 2 months, 72% among patients dying in 2 to 10 months, and 90% among patients dying after 10 months. This suggested that mycobacteremia preceded disseminated MAC disease. These workers hypothesized that mycobacteria infect a mucosal surface (gut or lung), multiply locally, and eventually enter the bloodstream and disseminate, seeding other organs and tissues. CNS involvement is likely to be a natural consequence of this pathogenic sequence.
Some understanding of the pathogenesis of CNS infection by MAC in immunocompromised patients is provided by a murine model reported by Wu et al. (24). Following intravenous administration of M. avium, numerous mycobacteria were present in macrophages within granulomatous lesions in the brain by 6 months. Interestingly, none of the mice exhibited clinical signs of meningitis or encephalitis, a finding similar to many HIV-infected patients with CNS involvement with M. avium.
Mycobacterium avium Complex Infection of the Central Nervous System in HIV-Infected Patients
CNS disease due to MAC in HIV-infected patients has been documented through case reports, case series, and reviews of medical records associated with laboratory isolates of MAC (Table 30.2). Interestingly, of the five patients in the initial description of AIDS from Los Angeles (25), all five eventually developed disseminated MAC disease and two of the five had CNS disease due to MAC (26).
Hawkins et al. (27) reported the experience of the Memorial Sloan-Kettering Cancer Center with disseminated MAC infection in patients with AIDS from 1981 to 1984. Of 366 patients with AIDS, 67 (18.3%) were diagnosed with disseminated MAC infection. CNS involvement was not identified before death in any of these patients. Autopsies were performed in 45 of these patients, and 12 (39%) of 31 whose brain tissue was cultured had positive cultures for MAC from brain tissue. In another report from the same institution focusing on the neuropathology of AIDS, Snider et al. (28) described 50 patients (of 160 patients with AIDS from 1980 to 1983) who developed neurologic dysfunction. Twenty patients had complete autopsies. MAC was cultured from the brains of two patients with disseminated MAC disease, and AFB were seen microscopically in the brain tissue in one of these. AFB were seen in brain tissue of a third patient with disseminated MAC disease, but cultures were negative. These patients had impaired cognition, psychomotor retardation, fevers, sweats, anorexia, malaise, and gastrointestinal complaints. CSF showed a mild pleocytosis (7 to 36 white blood cells [WBCs]/µL, 90% polymorphonuclear [PMN] in one patient, and 70% lymphocytes in a second) but normal protein and glucose concentrations. Pathologically, two had gray and white matter changes of subacute encephalitis with nodular collections of microglial cells, without inflammatory changes and reactive astrocytosis and small foci of demyelination in the white matter.
Levy and Bredesen (29) reviewed the neurologic manifestations of 1,286 patients with AIDS in San Francisco, of whom 474 had diseases affecting the CNS. Included were four patients with MAC infection of the CNS. Computed tomographic (CT) scan of the head revealed single hypodense lesions in two cases, a single contrast-enhancing lesion in one, and no abnormalities in one. Biopsy of the contrast-enhancing lesion revealed toxoplasmosis and lymphoma, in addition to MAC infection. In the CSF, there was a mild pleocytosis (12 to 15 WBCs/µL) and mild elevations of protein but normal glucose values. CSF culture grew MAC in two patients. Brain biopsy yielded MAC in one patient, and postmortem examination identified MAC in the CNS in the fourth patient. In reviewing the published literature to that point, the authors identified 14 cases of CNS infection with MAC. Most patients had disseminated MAC infection before presenting with diffuse encephalitis. Meningitis, cranial neuropathy, and peripheral neuropathy were also reported in association with MAC infection of the CNS. Survival was uniformly poor.
In these severely immunocompromised patients, more than one opportunistic pathogen may be isolated from the CNS especially in the era of more sensitive tests like polymerase chain reaction (PCR) assays. Two AIDS patients were reported by Sharma et al. (32) in which one patient had altered sensorium, fever, and meningismus who in the CSF had 20 WBCs/µL (100% lymphocytes) and mild elevation of protein with normal glucose. CSF culture and multiplex PCR grew M. avium and M. tuberculosis. The second patient had prior pulmonary tuberculosis (TB) and had severe headache. CSF contained 110 WBCs/µL (70% lymphocytes), 600 mg/dL protein, and glucose 63 mg/dL. Cultures of CSF were negative for M. avium and M. tuberculosis, but multiplex PCR was positive for both species. In addition, CSF cryptococcal antigen was positive.
At Kings County Hospital in New York, Jacob et al. (13) reviewed all positive CSF cultures for NTM over 11 years, from 1980 to 1990. Of the 16 patients with positive cultures, 14 occurred from 1987 to 1990, 15 were MAC, and 1 was M. fortuitum group (see later discussion). Twelve patients had a previous diagnosis of AIDS and the remaining four had at least one major risk factor for HIV infection. All 15 patients with MAC disease had widespread dissemination, and in 3 patients, autopsies demonstrated extensive MAC disease of CNS, bone marrow, liver, gastrointestinal tract, and lymph nodes, but no other CNS disease.
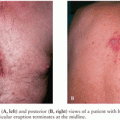
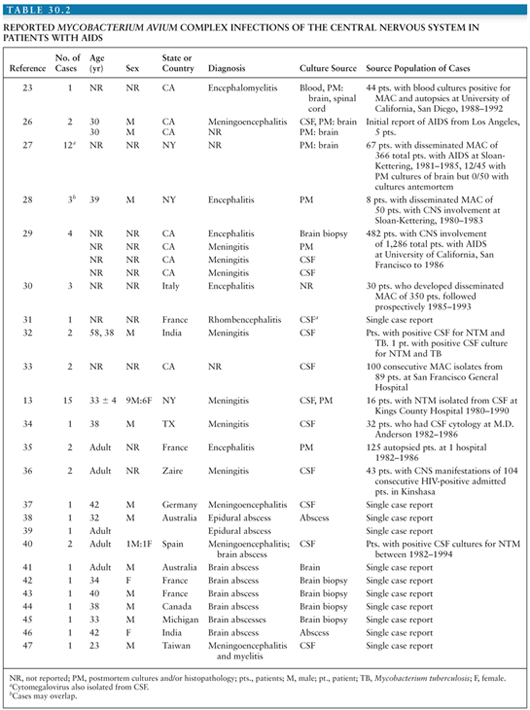
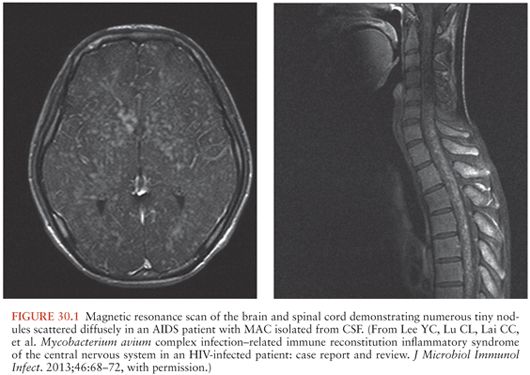
The number of additional cases of CNS disease due to MAC over the years has declined dramatically with better ART and the decline in cases of disseminated MAC (33–46). However, MAC involving the CNS has been increasingly reported with immune reconstitution (41,42,44,47). Lee et al. (47) reported an interesting case of diffuse nodules of the brain and spinal cord (Fig. 30.1) of a patient with AIDS whose treatment for disseminated MAC had been discontinued after immune reconstitution. MAC was isolated from blood and CSF cultures and responded well with reinitiation of MAC drug therapy. Most cases in the literature describe one to three brain lesions, but Verma and Dhamija (45) reported a case of disseminated M. avium-intracellulare infection in an AIDS patient with multiple brain lesions (Fig. 30.2).
Mycobacterium avium Complex Infection of the Central Nervous System Not Associated with HIV Infection
MAC infection of the CNS has also been reported in the absence of HIV infection, both in patients with another immunocompromising process and in patients without identifiable immunologic deficits (Table 30.3). The frequency with which this occurs is difficult to determine because it is relatively rare, generally not reported, and no surveillance system for NTM infection exists. At Mt. Sinai Hospital in New York, mycobacteria were recovered from CSF specimens from 21 patients between 1970 and 1983 (16). Three isolates were NTM and 19 were M. tuberculosis. Two patients had high colony counts of MAC recovered on both inoculated slants. The third had a single colony of M. gordonae, probably a contaminant, isolated from otherwise normal CSF. The first patient was previously healthy and presented with meningitis clinically. Her CSF had 471 WBCs/µL (84% lymphocytes), 234 mg/dL of protein, and 41 mg/dL of glucose. Both MAC and M. tuberculosis were isolated from the CSF. The second patient had chronic myelogenous leukemia (CML) in blast crisis and MAC was isolated in pure culture with high colony counts on both slants.
These cases raise the critical issues of interpreting positive CSF cultures of NTM and reasonable criteria to diagnose NTM infection of the CNS. These authors suggest that to ascribe CNS disease to NTM, multiple colonies of the NTM should be demonstrated on repeated cultures of CSF in the absence of other pathogens. None of these three patients met these criteria, but the high colony counts and pure culture of MAC from the single CSF specimen of the second patient make contamination unlikely. However, these criteria may focus too narrowly on CSF alone as a specimen source. For example, isolation of MAC from a biopsy of brain, meninges, aspirate of a brain abscess, or from a postmortem specimen in an appropriate clinical setting would be strong microbiologic evidence of MAC disease. In addition, these criteria do not allow for the possibility of dual infection with NTM and another pathogen. A case report illustrates these points (48). A 48-year-old woman presented with fever, a shift in her leukocyte differential toward immature forms, and a posterior fossa mass lesion. A ventriculoatrial shunt was placed and CSF from this procedure contained 32 mg/dL of glucose, 38 mg/dL of protein, and 83 WBCs/µL (all mononuclear). Two days later, CSF obtained by lumbar puncture (opening pressure 300 mm) had 14 mg/dL of glucose, 107 mg/dL of protein, and 44 WBCs/µL (75% mononuclear). India ink and culture demonstrated Cryptococcus neoformans. The patient was treated with amphotericin B and 5-fluorocytosine and gradually improved. After 6 weeks, CSF from the original lumbar puncture grew M. intracellulare. Lumbar puncture was repeated and several AFB were seen on direct smear, but no further cultures grew the organism. Isoniazid and rifampin were added to her therapeutic regimen and she eventually recovered. She was free of relapse at a 3-year follow-up but had significant neurologic sequelae. The role of M. intracellulare in producing disease in this patient was difficult to determine, but based on careful evaluation of the circumstances, the authors concluded that the organism was unlikely to be a contaminant.
Another case of MAC infection of the CNS in a previously healthy person was reported by Uldry et al. (49). A 31-year-old woman presented with a 2-year history of progressive headaches that suddenly became worse associated with lethargy, confusion, vomiting, and a grand mal seizure. On examination, the patient had depressed consciousness, a stiff neck, decorticate posturing, and bilateral flexor-plantar reflexes. CT of the head showed hydrocephalus due to compression of the fourth ventricle and meningeal enhancement with contrast. A ventriculoatrial shunt was placed and the CSF contained 453 WBCs/µL (80% lymphocytes). The patient was treated with isoniazid, rifampin, and pyrazinamide for TB and steroids for sarcoidosis, but all cultures were negative. Immunologic evaluation was negative. The patient improved clinically, and repeated immunologic parameters, HIV serology, and chest radiography remained normal. Over the subsequent 2 years, however, the patient fluctuated clinically, the CSF profile remained abnormal, and cultures were repeatedly negative. After 2 years, headache recurred and a left hemiparesis developed. Repeated CT scans showed a right temporal mass with surrounding edema. Craniotomy revealed a hemorrhagic necrotic mass, which on microscopic examination had granulomas, multinucleated giant cells, and few AFB. The tissue grew MAC. This case is noteworthy because it illustrates the difficulty of diagnosing and treating MAC infection of the CNS. However, an alternative explanation is possible, based on the long duration of disease before the diagnosis of MAC. The patient may have had an undiagnosed cause for her CNS disease initially and then developed a MAC cerebral abscess as a consequence of chronic steroid therapy.
Additional cases of CNS infection due to MAC have been reported in the context of disseminated MAC disease by workers at National Jewish Hospital (Denver, Colorado)—in a review of 13 cases of disseminated MAC infection in HIV-negative patients from 1940 through 1984 (50). The criteria for disseminated disease by which they selected cases required isolation of MAC from at least one nonpulmonary site and microbiologic or histopathologic evidence of granulomatous disease at a second anatomically distinct site. By this case definition, one patient with meningeal involvement was identified. No details were provided. It is now presumed that all these patients had a genetic abnormality that predisposed them to NTM disease.
One case of disseminated MAC infection with CNS involvement was reported by Japanese workers in a patient with pulmonary alveolar proteinosis and CML (51). A 47-year-old woman with CML who had been treated with busulfan for 2 years developed a recurrent cough and had an abnormal chest radiogram. AFB smears of expectorated sputum were positive. Transbronchial lung biopsy revealed pulmonary alveolar proteinosis. Cultures of sputum, bone marrow, and CSF grew MAC.
Four interesting cases of brain abscess mimicking spindle cell tumors have been described. Di Patre et al. (52) reported a 50-year-old patient with systemic lupus erythematosus who presented with a dural-based meningioma-like mass in the right frontal lobe. The mass resembled a meningioma with fascicular arrangement of spindle cells without caseation or giant cells but by AFB stains consisted of large numbers of AFB in histiocytes (52). A very similar case was reported by Morrison et al. (53) of a 38-year-old man with sarcoidosis who also presented with a spindle cell pseudotumor. It had minimal necrosis but was loaded with AFB. Arkun et al. (54) reported a 52-year-old with history of lung carcinoma, left upper lobectomy, and radiotherapy with complex, multiloculated, ring-enchancing cystic lesion of the left tentorium with compression of the fourth ventricule resulting in hydrocephalus. The tissue consisted of spindle cells arranged in bands, fascicles, and ill-defined nodules with a single giant cell. Ziehl-Neelsen and Fite staining was positive, and MAC was identified by DNA probe (54). Sadek et al. (55) described a case with underlying sarcoidosis with headache and word-finding difficulties with a left frontal ring-enchancing lesion. Tissue revealed multiple foci of spindle cell pseudotumor formation with large number of AFB. Similar “pseudotumors” have been described with M. tuberculosis in immunosuppressed patients, but these are some of the first to be described due to NTM.
Gyure et al. (56) presented the case of a patient with underlying Hodgkin disease who presented with signs of meningoencephalitis that included seizures, confusion, nuchal rigidity, and extensor-plantar responses. The patient died and at autopsy was found to have focal aggregates of lymphocytes, macrophages, and AFB located predominantly in a perivascular location. Cultures of CSF and brain were positive for MAC. Okada and Yoshioka (57) reported a case of acute disseminated encephalomyelitis associated with meningitis in a 73-year-old female who had fever, meningism, disorientation, left eye eversion, muscle weakness, and ataxic gait. Magnetic resonance imaging (MRI) showed multifocal and asymmetric increased T2 signals of the white and cortical gray–white matter junction of cerebral hemispheres, cerebellum, and brainstem. CSF contained WBCs of 1,295/µL (60% PMNs) and protein of 60 mg/dL with normal glucose. M. intracellulare was isolated from CSF by PCR. The patient was treated with rifampicin, streptomycin, clarithromycin, and steroids with resolution of symptoms.
Immune defects involving interleukins (IL) 10 and 12 and tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) are risk factors for pulmonary and disseminated NTM. Browne et al. (58) reported adult-onset immunodeficiency in Thai and Taiwanese patients associated with autoantibodies to IFN-γ. One hundred five patients had NTM isolated, with 52 having disseminated disease, but no details of CNS involvement were reported. Two cases of immunodeficiency with CNS NTM involvement have been reported. Dickerman et al. (59) reported a case of a 38-year-old male with sarcoidosis who underwent MRI due to possible seizure activity which demonstrated right frontal, left parietal, and right cerebellar lesions. M. avium was cultured from resected frontal and parietal lesions. Despite therapy, the cerebellar lesion increased in size and total resection of the cerebellar mass was performed. Histopathology showed encapsulated abscess with mixture of plasma cells, lymphocytes, and macrophages with AFB. The patient was found to have low concentrations of IFN-γ and TNF-α. The case described by Sadek et al. (55) as previously discussed on further analysis showed TNF-α and IL-12 defect in response to MAC antigens but high levels of IFN-γ. These cases demonstrate the complex and fascinating IL, TNF, and IFN immune response in NTM disease.
Laboratory
MAC is readily identified in the laboratory by acid-fast smear and culture using techniques for CSF and tissue standardized for TB. The organisms grow well in broth medium and on Middlebrook 7H10 agar, typically producing small, flat transparent smooth colonies on agar that often have a pale yellow color. Their colony morphology readily distinguishes them from M. tuberculosis. Commercial nucleic acid probes (AccuProbe, Gen-Probe, Inc.) are available that identify MAC isolates with greater than 99% accuracy 1 day after the colonies have grown, a technique used in most laboratories. M. avium and M. intracellulare are separate species, but their separation has no clinical value for the individual patient with lung disease and, hence, is generally not done. Specific DNA probes that recognize only M. avium or M. intracellulare are commercially available, however, MAC is also readily identified by high-performance liquid chromatography (HPLC) by analysis of mycolic acid patterns, a technique used by many large state laboratories and the CDC.
Antimicrobial susceptibility testing of NTM based on the American Thoracic Society (ATS) and the 2011 Clinical and Laboratory Standards Institute (CLSI) guidelines currently recommend reporting primary susceptibility to only clarithromycin with secondary testing of linezolid and moxifloxacin (60,61). It recognized that the only drug for MAC for which susceptibility testing has been shown to be predictive of clinical outcome was clarithromycin. Studies have shown that untreated strains of MAC were all clarithromycin susceptible, with minimum inhibitory concentrations (MICs) of less than 8 µg/mL, whereas strains that relapsed or failed therapy had one of two mutations in the 23S rRNA gene, with MICs generally more than 32 µg/mL. Susceptibility to other drugs such as ethambutol, rifampin, rifabutin, streptomycin, and amikacin provided no information about clinical responses to therapy. However, recently, Brown-Elliott et al. (62) proposed amikacin MIC breakpoints reporting 96.2% of clinical isolates had MICs of less than 32 µg/mL and prolonged exposure correlated with development of 16S rRNA mutation with MICs more than 64 µg/mL.
Treatment
Therapy of disease due to MAC, like susceptibility testing, is more difficult and more controversial than that for M. tuberculosis. There have been few prospective controlled treatment trials (63–67). Treatment of CNS disease must be extrapolated from cumulative experience and data on treatment of MAC lung disease in HIV-negative patients and disseminated disease in patients with AIDS. Recommendations for therapy generally follow ATS guidelines last updated in 2007 (60). The regimen recommended by the ATS for the treatment of MAC lung disease is a three-drug regimen. The daily regimen consists of clarithromycin (500 mg twice daily) or azithromycin (250 mg daily), rifampin (600 mg), and ethambutol (15 mg/kg daily). The same three drugs can also be given intermittently. The doses then are clarithromycin at 1,000 mg or azithromycin 500 mg, ethambutol at 25 mg/kg, and rifampin at 600 mg given three times weekly (Monday, Wednesday, and Friday). Rifabutin at 150 mg can be substituted for rifampin in the daily regimen, and at 300 mg in the three-times-weekly regimen for severe disease. Aminoglycoside therapy with either streptomycin or amikacin is usually reserved for cavitary, extensive disease or macrolide-resistant cases. Patients requiring long-term parenteral therapy should receive a dose of 8 to 10 mg/kg two to three times per week.
These macrolide-based regimens appear to have good activity in the therapy of disseminated MAC disease in patients with AIDS (68–71) and in MAC lung disease in HIV-negative patients (60,63–65). Their usefulness and role in the therapy of MAC disease of the CNS have not been studied, but they offer exciting potential for therapy of a very difficult disease. Treatment of MAC lung disease is continued until cultures remain consistently negative for at least 12 months.
For the treatment of MAC in AIDS, a joint panel between the CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America published recommendations (72). The basic principles for therapy included the necessity of regimens containing at least two drugs, one of which should be either clarithromycin or azithromycin. Most commonly, ethambutol and either rifampin or rifabutin would be the second and third drugs. Clofazimine was once considered among the possible agents to treat these infections. However, studies now show increased mortality when this drug is used in this setting, so it should not be used for treatment of disseminated MAC. Treatment should be continued for at least 12 months and discontinuing treatment is based on the absence of ongoing MAC disease and appropriate and sustained immune response due to ART.
Treatment of CNS infection is complicated by the lack of therapeutic data and by the preference for parenteral therapy in critically ill patients with altered mental status. Clarithromycin, rifampin, and ethambutol penetrate relatively well into the CNS. With inflamed meninges, the aminoglycosides and the newer quinolones penetrate into the CNS to a limited extent. Intrathecal therapy with aminoglycosides has been well described in other conditions, especially meningitis due to gram-negative bacteria. At present, the standard agents for treatment of pulmonary or disseminated MAC disease would appear to be the best available agents for MAC CNS disease.
Mycobacterium kansasii
The major reservoir for M. kansasii is likely commercial or household water, and clinical disease predominates along the southeastern and southern coastal states and the central plains states. Unlike other NTM, M. kansasii has never been found in soil or natural water supplies but has been recovered consistently from tap water in cities where M. kansasii infection is endemic. Previous studies in Texas show that M. kansasii disease is concentrated in urban areas, supporting a possible association between clinical disease and potable water supplies. M. kansasii infection causes pulmonary nodular disease in patients with bronchiectasis, fibrocavitary pulmonary disease resembling TB, and, rarely, disseminated disease in immunocompromised hosts including patients with AIDS (1,8). The most common extrapulmonary sites are lymph nodes, bone marrow, bone, joints, and skin. Only 10 cases of CNS disease have been described, 7 of whom occurred in patients with AIDS.
Mycobacterium kansasii Infections of the Central Nervous System
The 10 cases of CNS disease due to M. kansasii that have been reported are listed in Table 30.4. The first description of M. kansasii disease in the CNS was reported by Wood et al. (73) in 1956 in the context of disseminated M. kansasii infection and was just discussed. In the second report of M. kansasii disease of the CNS in the literature (1966), both M. kansasii and M. tuberculosis were isolated from a 2-year-old girl with meningitis (74). The patient presented with signs of meningitis and her father was subsequently discovered to have active pulmonary TB. Lumbar puncture on two occasions had pleocytosis (200 to 313 WBCs/µL, with a lymphocytic predominance), elevated protein level (59 mg/dL), and depressed glucose levels (22 to 27 mg/dL). The second specimen had one AFB on direct microscopy and grew M. tuberculosis. Despite treatment with isoniazid and streptomycin, the patient died. At autopsy, the CSF grew M. tuberculosis, but the leptomeninges grew M. kansasii in pure culture. This highly unusual report, the first of its kind, raises the possibility of mixed infection by M. tuberculosis and M. kansasii.
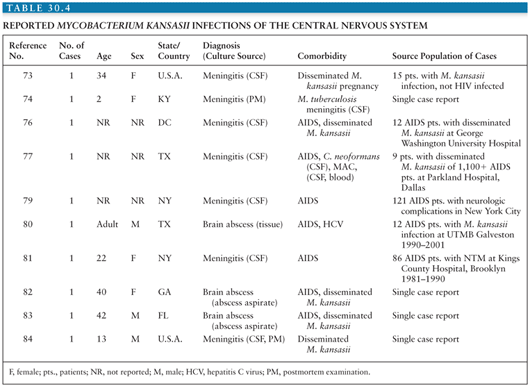
In patients with advanced HIV infection, M. kansasii often produces disseminated infection. Among patients with AIDS reported to the CDC from 1981 to 1987, disseminated M. kansasii was reported in 0.44% of patients from areas endemic for M. kansasii and in 0.08% in patients from nonendemic areas (8). Several retrospective studies have examined disseminated M. kansasii infection in HIV-infected patients (75–80). In two of these reports, M. kansasii was isolated from CSF in one patient each, but few clinical details were provided (79,80).
A report from New York reported 121 patients with AIDS of whom one had M. kansasii isolated from the CSF (79). Symptoms included fever, weight loss, hypoadrenalism, and hematemesis. Lumbar puncture was performed before treatment and three times over the next month because of neurologic deterioration. All CSF specimens had normal cell counts, protein values, and glucose values, and negative cultures until the fourth specimen grew M. kansasii several weeks after the patients’ death. Smith et al. (80) reported a retrospective review of the pathologic features of M. kansasii in patients with AIDS during 1990 to 2001. Twelve patients were identified with only one patient having CNS involvement. Little details were provided, but brain biopsy revealed granulomatous inflammation with AFB and the patient recovered on therapy.
The sixth case of M. kansasii CNS disease was reported by Shafer and Sierra (81) in 1992. They summarized the experience with NTM (excluding MAC and M. gordonae) over a 10-year period (1981 to 1990) at Kings County Hospital in Brooklyn, New York. Seven patients had M. kansasii. Six of the seven M. kansasii isolates were from respiratory specimens. The seventh was isolated from the CSF of a 22-year-old HIV-infected Haitian woman who had a brain lesion visualized by contrast-enhanced CT scan but no other details of this case were provided.
In 1992, Gordon and Blumberg (82) reported a 40-year-old woman with a 5-day history of right-sided headaches and left-sided weakness. Physical examination disclosed fever, oral thrush, right Horner syndrome, and gait ataxia. CD4+ lymphocyte count was 118 cells/µL. CT scan of the head revealed two ring-enhancing lesions in the right frontal lobe and thalamus. Fine-needle aspiration of the right frontal lobe mass revealed focal necrosis and perivascular inflammation, but no organisms were visible microscopically. Cultures grew only M. kansasii. The patient survived 20 weeks after the initiation of treatment.
A mass lesion with associated meningitis due to M. kansasii infection was reported from Florida in 1993 (83). A 42-year-old man with AIDS presented with 2 weeks of headaches, 1 week of confusion and fever, and 1 day of loss of balance. CT of the head revealed a 4-cm mass in the right occipitoparietal region, which enhanced with intravenous contrast. CSF contained no cells, no organisms microscopically, and normal glucose level, but protein level was 690 mg/dL. Aspiration of the intracranial mass yielded pus, which was smear positive for AFB. Blood, sputum, and CSF cultures grew M. kansasii and PCR of the abscess aspirate was positive for M. kansasii. Treatment with isoniazid, rifampin, and ethambutol led to resolution of symptoms and negative blood cultures within 1 month and clearing of the lesion on CT by 3 months.
Thus, disseminated M. kansasii disease occurs in a small but consistent fraction of patients with AIDS, and CNS involvement occurs rarely. The majority of published CNS infections with M. kansasii have occurred in HIV patients. CNS disease may be a manifestation of hematogenous spread of this microorganism.
Laboratory
M. kansasii is seen on smear and recovered in culture by techniques designed for M. tuberculosis. It grows readily in broth, as well as on Middlebrook 7H10 agar and Lowenstein-Jensen agar. The organism typically produces rough large colonies that turn bright yellow with exposure to light (photochromogen). A species-specific DNA probe for M. kansasii has been described and is commercially available (AccuProbe, Gen-Probe, Inc.). The organism is readily identified by biochemical tests as well as by HPLC. On acid-fast smears, M. kansasii typically appears as a large, long bacillus that has unusual beading when stained with Ziehl-Neelsen or Kinyoun stains. This often allows an initial suspicion that one is dealing with this organism, as opposed to M. tuberculosis.
Current 2011 CLSI recommendations for susceptibility testing of M. kansasii are for rifampin and clarithromycin only given the concentration problems with isoniazid and to do additional testing for other drugs such as amikacin, ethambutol, ciprofloxacin, moxifloxacin, linezolid, rifabutin, and sulfonamides only if the isolate is rifampin resistant (61).
Treatment
Treatment of M. kansasii disease of the CNS is extrapolated from the treatment of M. kansasii lung disease in HIV-negative patients and from the case reports summarized herein. Treatment of M. kansasii was dramatically improved by the additions of rifampin and ethambutol to the pharmacopeia, as the success rate for regimens containing these two drugs approach 100% (84,85). The current 2007 ATS recommendation for treatment of pulmonary disease due to M. kansasii includes isoniazid (300 mg per day), ethambutol (15 mg/kg per day), and rifampin (600 mg per day) for a duration that includes 12 months of negative cultures (60).
However, the British Thoracic Society reported 155 patients who were treated with ethambutol and rifampicin (86). Sputum conversion was reported in 99.4% of patients. In addition, Griffith et al. (87) reported 15 patients treated with standard MAC therapy three times weekly. The sputum conversion and long-term success rates were excellent with no treatment failures or relapses, but the study was too small to change the current ATS recommendations. Thus, clarithromycin in combination with rifampin and ethambutol is believed to be the best regimen based on excellent in vitro activity, minimal toxicity, clinical performance against other NTM, and anecdotal experience (78,88,89). For patients with HIV disease on ART regimens for which rifampicins are contraindicated, a macrolide may substitute for the rifampicin if the ART regimen cannot be adjusted (63). Tompkins and Witzig (90) retrospectively reviewed M. kansasii infection in 137 HIV patients grouped pre-ART era and ART era. Clarithromycin-treated patients survived a median of 8 months (2 vs. 10 months) longer compared to those treated without clarithromycin.
In place of isoniazid, which has marginal activity, sulfamethoxazole-containing regimens have been used successfully in the treatment of rifampin-resistant disease, but these studies antedated the availability of clarithromycin (91,92). In a study of 40 patients, Ahn et al. (84) demonstrated that the addition of streptomycin at 1 g twice weekly for the first 3 months to a 12-month regimen of the recommended three-drug daily combination resulted in a long-term success rate of 97%.
Treatment for CNS disease should be based on optimal regimens for this infection at other body sites where sufficient numbers of cases provide a more solid understanding of the chemotherapy of this disease, combined with pharmacologic principles of the treatment of CNS infections. One additional drug that offers potential for treatment of CNS disease due to M. kansasii is linezolid (Zyvox). All isolates of M. kansasii have MICs of less than 4 µg/mL (peak serum levels are 15 to 20 µg/mL) (93) and the drug has excellent CNS penetration. Because of the large number of drugs available for M. kansasii and the cost of linezolid, there is no reported experience with other clinical forms of M. kansasii or with CNS disease. Linezolid has proven effective for the treatment of Mycobacterium chelonae (94) and Nocardia species (95), including patients with brain abscesses (95).
Mycobacterium simiae
This species of mycobacteria was first reported in 1965 when it was isolated from Macacus rhesus monkeys (96). M. simiae are slow-growing organisms that may produce pigmented and nonpigmented colonies. It has been rarely associated with human disease but has been described to cause lung disease in the setting of structural lung disease (bronchiectasis). M. simiae is usually reported from Israel and the southwestern United States based on clinical samples (60). The epidemiologic source of M. simiae is not well established but has been reported in several pseudo-outbreaks in the southwestern United States attributed to hospital water and has been recovered from tap water sources derived from large aquifers (97). Four cases of CNS infection of M. simiae have been reported. Valero et al. (98) reported 137 clinical isolates of M. simiae in 75 patients in San Antonio, Texas over an 11-year period (1983 to 1993). One hundred twenty-eight (93%) isolates were from respiratory sources, four blood, one skin, one urine, one lymph node, and one bone marrow. Only one case involved the CNS, a 38-year-old man with AIDS who presented with fever, night sweats, and falls. CT of the head revealed three brain lesions with brain biopsy reported as B-cell lymphoma that was AFB stain positive with no granulomas. Tissue culture grew M. simiae, but the patient refused therapy and 10 weeks after brain biopsy died.
The second case of M. simiae occurred in a 40-year-old man with AIDS who developed right hemiparesis, progressive truncal ataxia, vertigo, and horizontal binocular diplopia with fever and weight loss (99). MRI demonstrated multiple enhancing isointense nodular lesions at the left cerebral peduncle and medulla involving the leptomeninges. Lumbar puncture on two occasions revealed 10 to 20 mononuclear cells/µL, glucose of 47 to 72 mg/dL, and protein of 49 to 135 mg/dL. Partial surgical resection of the medullary lesion revealed dense proliferation of spindle cells with histiocytes, lymphocytes, and mononuclear cells. Ziehl-Neelsen staining was AFB positive and tissue culture grew M. simiae that was confirmed using 16S rRNA PCR gene sequencing. M. haemophilum was also isolated from blood cultures, and the authors attribute the negative tissue culture for M. haemophilum due to lack of hemin or ferric ammonium citrate in culture medium that is essential for its growth (61). He received isoniazid, rifampin, pyrazinamide, ethambutol, and clarithromycin for treatment and improved with only residual neurologic deficits. This is the fifth case of CNS NTM infection forming spindle cell pseudotumors (52–55,99).
Balkis et al. (100) reported the third case of M. simiae in an 83-year-old HIV-negative man who developed fever, confusion, and agitation 2 days after surgery for a right hip fracture secondary to a fall who had complained of “feeling ill” for several weeks prior to the fall. Bilateral interstitial infiltrates were seen on chest radiography. His level of consciousness deteriorated along with respiratory failure requiring intubation. Brain CT scan showed small vessel ischemic disease and CSF specimen showed 1 WBC/µL, normal glucose and protein levels. Bronchoalveolar lavage was performed and 1 week later grew M. simiae in culture. One week after lumbar puncture, CSF culture grew M. simiae. Both isolates were identified using 16S rRNA gene sequencing. The patient developed cardiopulmonary arrest and died. The authors contend that because cultures were recovered from two different sterile sites and no other mycobacteria was isolated in any other culture during hospitalization, M. simiae was a true pathogen.
The final case of M. simiae was reported in a 39-year-old with a 4-month history of fever, night sweats, and weight loss who was diagnosed with AIDS (101). Physical exam revealed hepatosplenomegaly, and liver biopsy showed AFB with minimal disruption of the liver tissue and was treated empirically for TB with rifampicin, isoniazid, ethambutol, and pyrazinamide. CSF specimen showed no WBCs, normal protein, and glucose. After hospital discharge, blood and CSF culture grew M. simiae and antimycobacterial therapy was changed to moxifloxacin, rifabutin, ethambutol, and azithromycin and ART was started 10 days later. Three months after ART, fever and malaise returned with negative microbiologic workup, and immune reconstitution inflammatory syndrome was diagnosed and resolved with steroid therapy.
M. simiae is highly drug resistant, including ethambutol and rifabutin. The best antimycobacterial drugs are the macrolides (clarithromycin or azithromycin), trimethoprim-sulfamethoxazole (TMP-SMX), moxifloxacin, and amikacin (102).
Mycobacterium gordonae
M. gordonae organisms are ubiquitous in the environment, but their recovery has been most closely identified with water. M. gordonae are slow-growing, rarely pathogenic organisms that are occasionally encountered in the microbiology laboratory and usually considered an environmental contaminant. Weinberger et al. (103) reviewed all reported cases of disease due to M. gordonae to 1992 and identified 23 cases, of which 13 had sufficient information to be judged definite. Eight had pulmonary disease, seven had soft tissue infection of an extremity, five had disseminated disease, three had peritonitis, and one had corneal infection. The CNS was involved in two cases, both of whom involved CSF shunts (see following section). In most of these patients, the infection was treated with three antimycobacterial drugs including isoniazid, rifampin, ethambutol, and/or an aminoglycoside. Overall, 6 of 24 patients died. These cases and all reported cases of M. gordonae infection involving the CNS were in the premacrolide era, and no isolates were confirmed by molecular methods.
The first case was a hydrocephalic child who had two ventriculoatrial shunts placed at 18 days and 7 months of age (104). The child had an elevated CSF protein level (106 mg/dL) and increased intracranial pressure necessitating a ventriculoperitoneal (VP) shunt. The patient underwent several shunt revisions complicated with the development of ascites. CSF specimens grew M. gordonae, and shunt valve was “loaded” with AFB. The meningitis and ascites resolved with shunt removal and antimycobacterial therapy. The second case of M. gordonae meningitis occurred in a 23-year-old woman, who underwent a VP shunt following excision of a cerebellar medulloblastoma 9 years prior, who presented with fever, hematuria, and progressive renal insufficiency (105). Nine months before admission, she had been placed on prednisone for presumed hypersensitivity pneumonitis and hepatitis. Cultures of lung biopsy obtained 9 months prior revealed 2+ growth of M. gordonae. CSF was tapped from the shunt and showed numerous AFB. CSF analysis showed normal glucose and slightly elevated protein. M. gordonae were cultured from CSF, urine, renal biopsy, and the shunt that had been removed.
Although one cannot generalize from two reported cases, it remains prudent to consider a mycobacterial etiology in chronically febrile patients who have had shunting procedures including infection of the shunt itself. Treatment with antimycobacterial agents and removal of the foreign body was effective in these two cases.
Mycobacterium genavense
This species of mycobacteria was first reported by Bottger et al. (106) in 1992 who described it in 18 patients with AIDS presenting with fever, diarrhea, and marked weight loss. Two subsequent reports on M. genavense described similar symptoms in patients with AIDS, with a 68% mortality by 12 months (107,108).
In terms of CNS disease, two cases have been reported. Berman et al. (109) reported a 50-year-old man with AIDS. The patient was admitted with a 1-month history of memory problems, confusion, and episodic tremor of the right arm, as well as a 2-week history of right-sided weakness and paresthesias. The patient had mild right-sided motor and sensory deficits and slurred speech. CT revealed cerebral edema and a mass effect in the left parietal region. The patient was treated empirically for toxoplasmosis with pyrimethamine and sulfadiazine but had a grand mal seizure after 1 week. MRI at that time showed a left parietal mass abutting the dura with meningeal enhancement and a large zone of surrounding edema. CT-guided needle biopsy revealed a patchy lymphocytic infiltrate without granuloma formation but with clusters of AFB on Ziehl-Neelsen stain. Treatment was started with five drugs to cover M. tuberculosis and MAC, and then was changed to clarithromycin, ciprofloxacin, and ethambutol when the organism was identified as M. genavense. The patient did well clinically and repeat imaging demonstrated complete resolution at 12 months.
Uchino et al. (110) reported a 50-year-old man with primary immunodeficiency who presented with dysarthria, aphasia, and right hemiparesis. MRI revealed multiple intracranial masses in the subcortical regions. CSF cultures were negative for NTM. Brain biopsy was perfomed, which confirmed M. genavense infection. These reports, as well as the previous reports on M. genavense, describe significant difficulties culturing this organism using standard techniques for mycobacteria. Identification to the species level, in most cases, requires molecular methods.
Mycobacterium terrae Complex
The M. terrae complex initially included only M. terrae and M. nonchromogenicum. The species has now been expanded to multiple other species, with the most common isolate being M. arupense. The M. terrae complex are rare, slowly growing nonpigmented mycobacteria that are present in tap water presumed to be nonpathogenic in the lung but are a recognized cause of posttraumatic tenosynovitis and local osteomyelitis of the extremities. Two cases involving the CNS have been reported (111,112). Woods and Washington (111) reviewed the microbiology and clinical aspects of NTM and described the Cleveland Clinic Foundation’s experience with NTM, from 1982 to 1985. Of 140 patients with mycobacteria isolated from clinical specimens, 93 were NTM, primarily MAC (124 specimens). M. gordonae (75 specimens), M. kansasii (59 specimens), and M. fortuitum group (58 specimens) accounted for most of the remainder. Whereas most of these isolates were from respiratory tract specimens, one patient who had an acoustic neuroma removed several months previously developed meningitis due to M. terrae complex. Details of the case were not published. Lai et al. (112) described a 65-year-old HIV-negative man who had radiotherapy for nasopharyngeal carcinoma 6 years prior to presentation. He was admitted with a 2-month history of headache and 5-day history of fever, nausea, and poor appetite. Physical exam revealed meningismus and CT disclosed dilated ventricles with diminished sulci. Two lumbar punctures were performed revealing pleocytosis, 140 to 488 WBCs/µL (77% to 81% PMNs), elevated protein of 131 to 267 mg/dL, and glucose 11 to 37 mg/dL. Microbiologic CSF analysis showed negative Gram stain and culture, AFB stain, and cryptococcal antigen. Fever and headache persisted, and MRI revealed leptomeningeal enhancement of the right temporal region and premedullary areas of the brain stem. Empirical therapy for TB meningitis was initiated, but neurologic status worsened to coma. Repeat CT demonstrated hydrocephalus necessitating external ventricular drainage. Five weeks after CSF AFB cultures were obtained, both yielded M. nonchromogenicum.
Mycobacterium malmoense
M. malmoense is a slow-growing pigmented species first recovered in Malmo, Sweden. It can cause a variety of diseases such as chronic pulmonary disease or cervical lymphadenitis in children. It is second only to MAC in many areas of northern Europe as a cause of NTM disease and is rarely seen in the United States. Two cases with CNS disease have been reported. Florakis et al. (113) described a patient with chronic mastoiditis who was found to have pituitary insufficiency secondary to mycobacterial infection due to M. malmoense. The disease was proven by surgical biopsy and 16S rRNA gene sequencing. The second case was a 49-year-old woman with a 6-month history of deteriorating vision, anorexia, anxiety, and poor memory with a change in behavior manifested as disinhibition and aggression (114). Physical exam revealed poor cognitive performance, bilateral papilledema, and general hyperreflexia and extensor plantar reflexes. CSF analysis revealed pleocytosis, 37 WBCs/µL (monocytes), elevated protein of 949 mg/dL, and 1:3 CSF:serum glucose ratio with negative AFB stain. MRI demonstrated periventricular white matter changes and enhancement in the basal ganglia. CSF culture grew M. malmoense and M. interjectum based on the Hain PCR method. Empirical therapy with rifampicin, isoniazid, ethambutol, and pyrazinamide with steroids was initiated. Over the next 3 months, the clinical course worsened with development of myoclonus, vision loss, sleep–wake cycle disturbance, and agitation. Repeat CSF analysis revealed elevated protein of 541 mg/dL and bilateral optic atrophy by funduscopic examination. MRI demonstrated development of secondary white matter changes (Fig. 30.3). After 8 weeks of treatment, encephalopathy improved, enabling the patient to be discharged home. Specific antimycobacterial therapy was not described further.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree