Key points
- •
Uterine leiomyomata are common benign neoplasms that may cause symptoms related either to tissue bulk or bleeding for many women.
- •
Triage to the ideal treatment depends not only on fibroid characteristics and location within the uterus but also the patient’s fertility goals, nature of presenting symptoms, and consideration of potential complications.
- •
In addition to watchful waiting and traditional treatment, including medication for symptomatic relief, hormonal therapy, and surgical excision, patients now have the option of minimally invasive, image-guided procedures: uterine artery embolization (UAE) and many forms of thermal ablation (radiofrequency ablation and high-intensity focused ultrasound [HIFU]).
- •
UAE, associated with significant fibroid volume reduction and symptom relief, as well as less major adverse events and a shorter recovery as compared with hysterectomy, is also shown to lead to the need for reintervention in almost one third of patients, negating the cost-effectiveness at 5 years posttreatment.
- •
HIFU, especially when guided by MR imaging, has shown to be clinically effective in reducing fibroid volume and reducing symptoms, with fewer major complications and less lifetime total costs compared to currently available treatments.
- •
Clinical outcomes for minimally invasive, image-guided procedures are improving with improved technical skill and greater treatment volumes, but randomized trials analyzing longer-term outcomes (>5 years) and postprocedure fertility outcomes have not been reported to date.
Introduction
Uterine leiomyomas (fibroids) are benign, hormonally driven, well-encapsulated neoplasms originating from uterine smooth muscle, affecting an estimated 20%–35% of all reproductive-age women; some studies report that a cumulative lifetime incidence of leiomyomas occurs in 70% of U.S. white and 80% of African American women. Risk factors include African or African American background, family history, early menarche, nulliparity, obesity, hypertension, and polycystic ovary syndrome. Although the majority of women with uterine leiomyomas are asymptomatic, approximately 20%–30% of women have related symptoms, typically presenting in the fifth decade of life and lasting into menopause, with subsequent decrease in symptoms. Uterine fibroid-related symptoms are secondary to either mass effect (bulk) or excessive endometrial effect (bleeding). Bulk-related symptoms include back and pelvic pain, pelvic pressure, urinary frequency, dyspareunia, and defecation disorders. Bleeding-related symptoms include menorrhagia, metrorrhagia, menometrorrhagia, and dysmenorrhea, and the associated anemia. Submucosal fibroids, or those that significantly distort the endometrial cavity, are also thought to contribute to mass-related infertility. Leiomyoma-related symptoms, among the most common of all gynecologic disorders, are thought to be responsible for more than 360,000 hysterectomies in the United States annually, with an overall estimated annual cost of care exceeding $2 billion.
Imaging characteristics of uterine leiomyomas
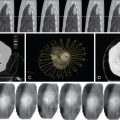
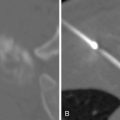
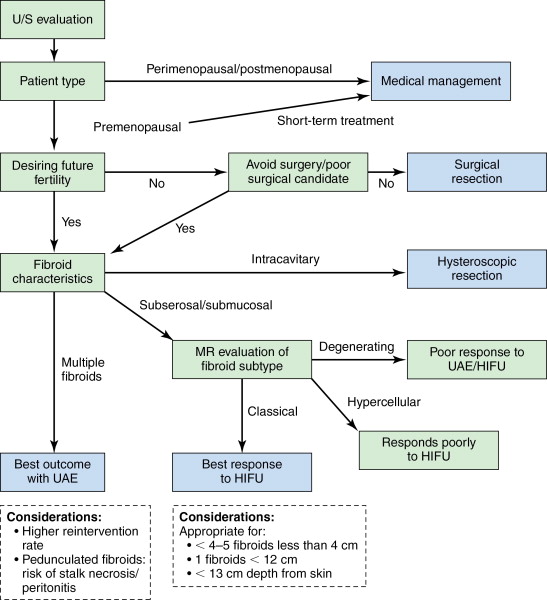
Leiomyomas are readily detectable on pelvic ultrasonography, typically appearing as solid, hypoechoic masses originating from the uterine myometrium with varying degrees of echogenicity depending on fibrous, calcified, and degenerating components. There is no interruption of the endometrium (unless submucosal in location, which may cause adjacent cavity distortion), differentiating these lesions from endometrial polyps or more concerning endometrial carcinoma. Although ultrasonography most commonly identifies fibroids, MR imaging offers superior soft tissue contrast and tissue characterization of individual fibroids, multiplanar imaging, and characterization of perfusion, which helps triage patients to one or more therapeutic options, including surgical resection, uterine artery embolization (UAE), and high-intensity focused ultrasound (HIFU) ablation. It is also superior in diagnosing concomitant adenomyosis, which may contribute to UAE treatment failure. Based on MR imaging and enhancement patterns, individual leiomyomas can be classified as classical, hypercellular, or degenerating. Classical fibroids appear hypointense on T2-weighted sequences and enhance on gadolinium-enhanced T1-weighted sequences ( Figure 19-1 , A ). Hypercellular fibroids, which are T2 hyperintense, enhance avidly on gadolinium-enhanced T1-weighted sequences. Degenerating fibroids have variable T1 and T2 signal and do not exhibit contrast enhancement. The tissue characterization of these different subtypes of fibroids can help determine the best choice of treatment, as classical fibroids have been found to respond better to HIFU than hypercellular fibroids, while degenerating fibroids respond poorly to both HIFU and UAE.
Location of uterine leiomyomas
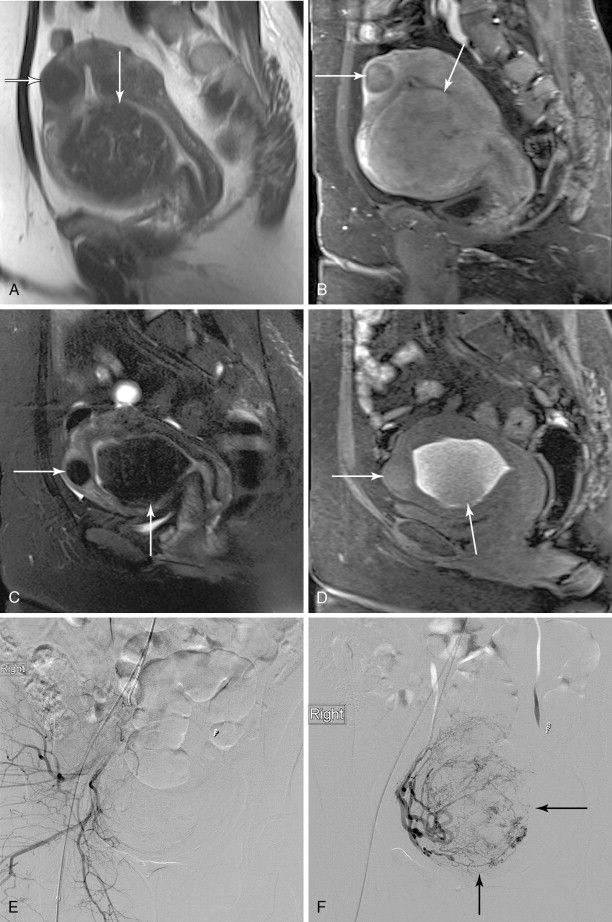
Leiomyomata are also described in terms of their location in the uterus, and these subtypes can display varying responses to specific treatments as well. Intramural leiomyomas, completely contained within the uterine wall, are most common and mostly asymptomatic, but if large, may cause bulk symptoms. Subserosal lesions are found along the outer contour of the uterus, and when large, can also cause bulk symptoms. Fibroids abutting or extending into the endometrial cavity are termed submucosal, and even small lesions can cause uterine bleeding. This subtype can potentially become intracavitary following UAE, predisposing to fibroid expulsion and postprocedure complications, the risk for which can best be gauged by preprocedure magnetic resonance imaging (MRI), with assessment of fibroid size to endometrial interface. Intracavitary leiomyomas grow into the endometrial cavity on a fibrovascular stalk and commonly result in bleeding symptoms. Those growing outside the uterus on a fibrovascular stalk are termed pedunculated and can cause mass effect on adjacent organs, and voiding or defecation symptoms.
Traditional treatment of uterine leiomyoma
Traditional options for therapy of uterine leiomyoma-related symptoms may be divided into medical, surgical, or watchful waiting/alternative therapy. Medical treatments, like nonsteroidal anti-inflammatory drugs (NSAIDs) or contraceptive steroids, or alternative therapies, such as acupuncture or herbal therapies, may be effective in the short term or for bridging perimenopausal patients whose fibroids may still spontaneously involute, but most symptomatic patients progress over time, requiring more durable treatment. Hormone therapy, namely gonadotrophin-releasing hormone (GnRH) agonists, are also used and act by suppressing circulating estradiol and progesterone levels via the pituitary–ovarian axis. This results in significant fibroid shrinkage, but long-term use of these compounds is not recommended because of the side effects of bone loss. Surgical therapy, including laparoscopic, hysteroscopic, robotic-assisted, or open hysterectomy, is the standard of care and is considered the definitive therapy for fibroid-related bulk symptoms, bleeding, and infertility. Although these are extremely common and safe surgical procedures, there is a major complication rate (such as hemorrhage requiring transfusion, urinary tract, and bowel damage) of 8.9% for abdominal, 14% for vaginal, and 19% for laparoscopic hysterectomy (the latter including the risk of conversion to laparotomy). Removal of the uterus and surrounding attachments also has been thought to increase a woman’s postmenopausal risk of pelvic floor disorders (pelvic prolapse and laxity). , For women who desire the option of future fertility or who wish to retain their uterus, open or laparoscopic surgical resection of individual leiomyomas (myomectomy) is performed. Although generally safe, the most frequent complications include perioperative blood loss requiring transfusion, fever, and ileus. There is a postoperative recurrence of up to 51% over time, and 10%–25% of patients will require a major surgery after the first myomectomy.
Minimally invasive treatments
Uterine artery embolization
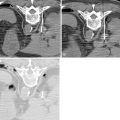
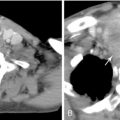
UAE, also called uterine fibroid embolization (UFE), is the most widely available nonsurgical treatment option for moderate and large symptomatic uterine leiomyomas, and was first developed in the early 1970s for control of postpartum hemorrhage. The procedure itself typically lasts from 50 to 75 minutes in 90% of patients. With slightly differing techniques between institutions, the goal of therapy is the same: to occlude the uterine artery branches that supply only the fibroid tumors, causing cutoff of blood flow and subsequent infarction, while sparing normal myometrial vessels through extensive collateral blood supply ( Figure 19-1 , B ). Utilizing local anesthesia and conscious sedation, vascular access is obtained with a wire via a unilateral femoral artery approach, which is advanced up to the aortic bifurcation; a small visceral catheter, typically 4–5 French (Fr), is placed over the wire to select the contralateral internal iliac artery. Digital angiography is then performed in the posterior–anterior projection to identify the origin of the uterine artery, which usually arises as a first or second branch of the anterior division of the internal iliac artery, and is usually dilated in the presence of a uterine fibroid tumor ( Figure 19-2 ). Using a coaxial microcatheter, 3 Fr in size, the portion distal to the cervicovaginal branch, supplying the fibroid of interest, is then selectively catheterized. Embolic particles, most commonly trisacryl microspheres or polyvinyl alcohol (PVA) microspheres, from 350 to 700μm in size, are injected; depending on the size of the leiomyoma, anywhere from 100 to 1500 mg may be used. The contralateral uterine artery is catheterized in similar fashion and embolized, with the procedure deemed complete when no arterial flow is visualized in the perifibroid arterial plexus, with slow antegrade flow maintained in the main uterine artery ( Figure 19-3 ).
Thermal ablation
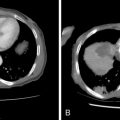
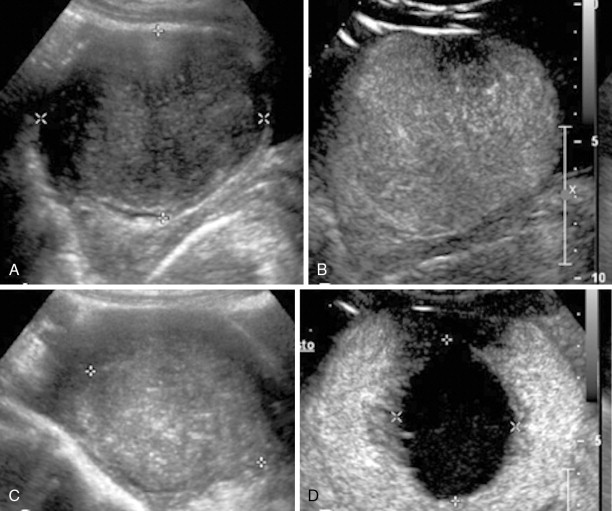
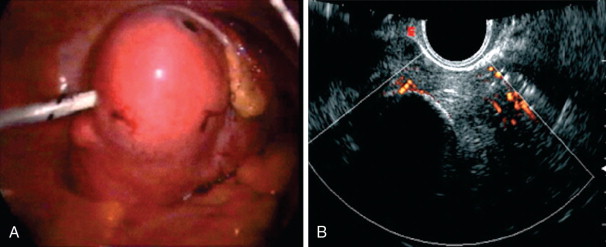
More recently, uterine fibroids are being treated by thermal ablation, one of the newest and least invasive treatment option for uterine fibroids, particularly in those who wish to retain the option of future fertility. Thermal ablation was initially performed using a neodymium-doped yttrium aluminum garnet (Nd:YAG) laser to deliver energy to fibroids via laparoscopy or hysteroscopy, but now can be performed by electrosurgical radiofrequency ablation (RFA), in which needle electrodes deliver heat energy. Exposure to temperatures exceeding 60°C instantaneously and irreversibly injures soft tissue through protein denaturation and coagulative necrosis ( Figure 19-4 ). Unlike UAE, which results in tissue infarction with cell membrane disruption and spillage of intracellular contents, hyperthermic ablation allows for thermal fixation, which preserves cellular architecture. Coagulated lesions shrink in size from water volume loss and inflammatory response reabsorption, and the consistency changes from very firm to soft and gel-like, which may also contribute to the resolution of symptoms. A similar concept is ablation by freezing the fibroid tissue, or cryomyolysis, which utilizes gas-cooled probes to deliver high-pressure argon gas, causing irreversible cell injury at a temperature of –20° C and forming elliptical “ice balls” in the affected tissue ( Figure 19-5 ), promoting vascular thrombosis that ultimately necroses the fibroid.
Varying techniques are used for needle-based thermal ablation among institutions, including needle electrode design (bipolar vs. monopolar, single tine vs. multiple tines), mode of deployment (transabdominally, transcervically, transvaginally), the modality used for real-time visualization (laparoscopy, sonography), and the hardware/software monitoring energy delivery to tissue. Typically, a 15-gauge needle electrode is inserted with the tip positioned at the center of the target myoma, with manually deployable prongs designed to laterally bracket the target tissue in order to produce a spherical area of coagulative necrosis. The needle electrode is connected to a generator that displays the temperature of the electrode tip, as well as tissue impedance characteristics and ablation time, and allows for cauterization of the needle track on completion via modification of the power settings. The desired fibroid tissue damage induced by these procedures is monitored by real-time imaging, namely, ultrasonography or MR. Although empirically more costly, MR-guided ablation has several theoretical advantages over ultrasound-guided ablation: MRI provides much higher, near-real-time, multiplanar unenhanced soft tissue contrast and spatial resolution and provides near-real-time, MR-based thermal mapping of the ablated tissue and surrounding nontarget soft tissues. This real-time feedback of thermal gradient enables a complete ablation of the targeted site that avoids adjacent tissues and allows for the evaluation of thermal sites posttreatment.
High-intensity focused ultrasound
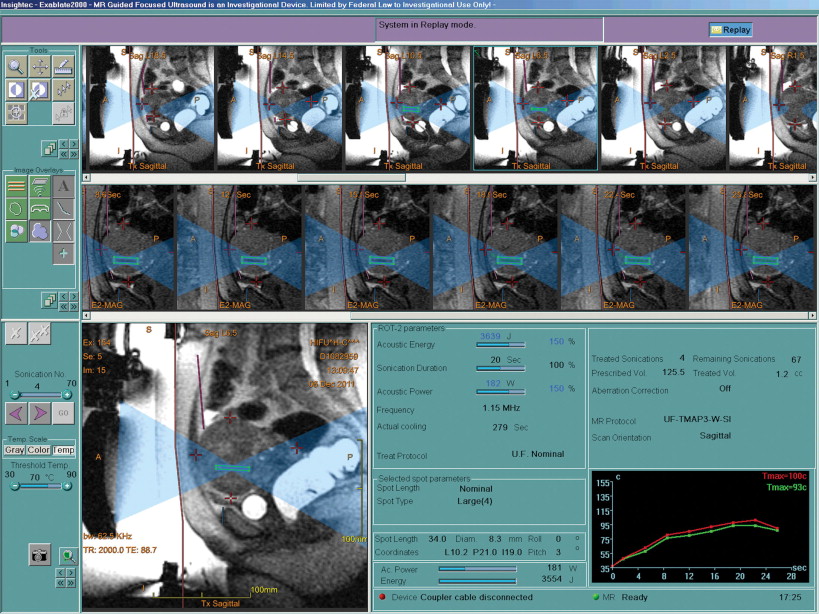
HIFU is another mode of thermal ablation, delivering a noninvasive, targeted dose of low-frequency, high-intensity cyclic acoustic pressure to cause focal thermal toxicity and coagulation necrosis. Unlike thermal or cryoablation, HIFU obviates the need for (and potential complications of) direct access to the fibroid tissue. HIFU can treat a variety of disorders, including solid tumors, such as uterine leiomyomas and malignant tumors in liver, bone, pancreas, and prostate. For the current first-generation technology, with motion correction in development, minimally mobile lesions like uterine fibroids present an ideal target. HIFU devices are available from several manufacturers, combining the therapeutic HIFU transducer with either MR guidance (ExAblate, InSightec, Hiafa, Israel; Sonalleve, Philips, Best, Netherlands) or ultrasound guidance (Seapostar, Chongqing Haifu [HIFU] Technology, Co. Ltd., Chongqing, China). The Insightec Exablate 2000, known widely by its marketing name, MR guided Focused Ultrasound Surgery (MRgFUS), received approval from the United States Food and Drug Administration (FDA) in 2004 for the noninvasive treatment of uterine leiomyomas.
MRgFUS incorporates a HIFU transducer unit and is built into a table designed to fit into a gantry in a 1.5- or 3.0-Tesla magnet. InSightec’s ExAblate system 2000 is compatible exclusively with General Electric Healthcare’s standard Signa line of 1.5-T MR machines. Potential risks from the procedure include bowel, skin, or nerve injury and possible deep venous thrombosis (DVT). Patients are placed prone on the table with the lower abdomen and pelvis on a degassed water bath over the transducer, with a coupling gel pad placed in between. The anterior pelvic wall is shaved to the symphysis pubis to minimize the chance of air bubble entrapment by hair in the water bath. A Foley catheter is always placed to control bladder distension (bladder fill) as necessary for optimal uterus position. In selected instances, sonographic gel, water, or air may be needed to fill the rectum (rectal fill) via a small-gauge rectal tube to displace the uterus anteriorly and move the bowel out of the treatment field. Manual displacement of the bowel via suprapubic massage can also be performed for optimal positioning of the fibroid. A pre-treatment MR of the pelvis is performed with T2-weighted coronal, sagittal, and axial sequences, which serves as the template scan to map the procedure and select for individual leiomyomas. The treatment volume of the target lesions is then calculated by dedicated software from the measured circumference. Imaging can then display near-real-time temperature and phase maps using the receiver channels built into the ExAblate table. The bowel, pubic bone, lumbosacral spine, and nerves are all demarcated to prevent unintended target sonication.
On the ExAblate system, transducer power ranges from 0 to 3000 W/cm 2 , with an ultrasound frequency of 1.0–1.3 MHz. Over an approximately 20-second period, energy deposition can vary between 2000 and 4000 J into the target site per each sonication. The target site is ellipsoid with dimensions of 8–40 mm parallel to the sonication beam and 1–10 mm perpendicular to the beam direction at the 17 cm focal distance from the therapeutic transducer (up to 13 cm deep to the skin within the patient’s pelvis). Newer devices will allow for deeper pelvic penetration by physical movement of the transducer. Low-power test sonications are delivered to the target lesions to ensure precise aim and to correct for sonication-related distortion in the coronal and sagittal planes. High-power treatment sonications, lasting from 15 to 20 seconds, are then delivered to the selected treatment volume, followed by a cooling duration of 45–90 seconds to minimize risk of skin burns.
With the currently available system, up to approximately 120 sonications may be needed to ablate a given volume in the maximum 3-hour period. Temperature at the target sonication is noninvasively derived by phase changes on MR imaging every 3–4 seconds, creating an MR thermal map and allowing for adjustments to the therapeutic energy and sonication time. Postprocedure dynamic contrast-enhanced MRI can then assess nonperfused tissue. Patients are monitored for 1–3 hours posttreatment for recovery from conscious sedation and then discharged home. Normal activities may be resumed almost immediately, typically the following day, with a small subset needing a more prolonged recovery.
Other gynecological applications of HIFU
Adenomyosis is the ectopic location of endometrial and fibrous stroma in the myometrium, and is a common, often misdiagnosed, gynecologic disorder, with symptoms mimicking those of uterine fibroids (menometrorrhagia, menstrual cramps, dyspareunia, and dysmenorrhea). Medical therapies can provide symptomatic relief, but they are short-lived; currently, the definitive therapy for uterine adenomyosis is hysterectomy, with minor surgical procedures, including endomyometrial ablation, laparoscopic myometrial electrocoagulation, and adenomyoma excision, used with varying degrees of success. The use of HIFU for treatment of adenomyosis, though not approved by the U.S. FDA, has shown potential as an effective modality in China; multiple studies have reported reductions in mean uterine volumes up to 25% after 12 months posttreatment, with significant symptomatic improvement and without serious adverse effects.
Cervical ectopy, or ectropion, is a normal condition that results from extension of the endocervical columnar epithelium out over a portion of the visible ectocervix, and is associated with infection, typically chlamydial cervicitis; the subsequent inflammation and repair results in reactive changes, which are characterized by epithelial disorganization and nuclear atypia. , For chronic cervicitis refractory to medical management, current surgical options, such as cryocautery, electrocoagulation, and laser therapy, are available to mitigate symptoms and to prevent neoplastic transformation, but are destructive procedures that induce necrosis of treated tissue and subsequent cervical scarring, stenosis, and, hence, secondary infertility and cervical-origin dystocia. HIFU as a treatment option for cervical ectopy offers several advantages, primarily that sonication may be precisely delivered at a target depth without collateral damage to interposed, nontarget tissues in the path of the ultrasound beam. Infected tissue is treated through inactivation of pathogenic microorganisms and their metabolic products, thus improving local irritation without tissue loss. Subsequently, the cervix is preserved, with function and elasticity of the cervix not affected. One study found no differences in the symptomatic cure rate (97% vs. 98%) or success rate in treatment (95% vs. 96%) of the ectopic areas between the ultrasonography- and laser-treated groups, respectively, but the rate of side effects (including vaginal reactive discharge and colporrhagia) was much lower in the ultrasonography group (8% vs. 45%, respectively).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree