- Hypertension is up to twice as common in people with diabetes as in the general population, affecting 10–30% of people with type 1 diabetes mellitus (T1DM) and 60–80% of those with type 2 diabetes (T2DM).
- Hypertension is associated with insulin resistance and other features of the metabolic syndrome. Insulin resistance could raise blood pressure (BP) by loss of insulin’s normal vasodilator activity or through effects of the accompanying hyperinsulinemia.
- BP rises during the early microalbuminuric phase of diabetic nephropathy, especially in young patients with T1DM.
- Hypertension worsens both macrovascular and microvascular complications in diabetes. The effects of BP on the risk of fatal coronary heart disease are 2–5 times greater than in people without diabetes. The risks of nephropathy and end-stage renal failure are also increased 2–3 times by hypertension.
- All people with diabetes should be carefully screened for hypertension and evidence of hypertensive tissue damage at diagnosis and at least annually thereafter. Treatment is required for values that consistently exceed 130–140/80–85mmHg – lower than the World Health Organization/International Society of Hypertension thresholds defined for hypertension in the general population. The blood lipid profile should also be checked.
- The treatment of hypertension begins with lifestyle management, including reduced dietary fat and salt intakes, weight loss for obese patients, smoking cessation and increased regular physical activity. These measures can lower BP by up to 11/8mmHg.
- First-Line antihypertensive drugs suitable for use in patients with diabetes are diuretics, such as low dose bendroflumethiazide (bendrofluazide); or cardioselective beta-blockers, calcium-channel antagonists (CCAs), angiotensin-converting enzyme (ACE) inhibitors and angiotensin II type 1 (AT1) receptor blockers (ARBs). Subjects of African decent tend to have low renin hypertension, and may not respond to beta-blockers or ACE inhibitors. Drugs can be selected for their beneficial effects on coexistent problems, e.g. angina or arrhythmia (beta-blockers, CCAs), heart failure (ACE inhibitors, certain beta-blockers), previous myocardial infarction (ACE inhibitors, beta-blockers) or nephropathy (ACE inhibitors, ARBs).
- Over two-thirds of people with diabetes need combinations of two or more antihypertensive drugs to control hypertension. Effective combinations include beta-blocker plus CCAs; ACE inhibitor/ARB plus diuretic (non – potassium – sparing); and CCA plus ACE inhibitor/ARB.
Introduction
Hypertension often accompanies diabetes mellitus, both type 1 (T1DM) and type 2 (T2DM). The association between the two conditions has long been recognized. In 1923, the Swedish physician Eskil Kylin described a syndrome of diabetes, hypertension and hyperuricemia [1], which are now regarded as aspects of the broader “metabolic syndrome” that has been linked to insulin resistance (IR) [2,3]. The relationship between diabetes and hypertension is complex. Both are common and so are likely to be associated by chance, but in some instances, they may have a common cause; moreover, hypertension can develop as a consequence of diabetic nephropathy, while some drugs used to treat hypertension can induce diabetes in susceptible subjects.
Hypertension is important because, like diabetes, it is a major cardiovascular risk factor and one that synergizes with the deleterious effects of diabetes. It is also a risk factor for microvascular complications: nephropathy and retinopathy. The management of hypertension in diabetes has been widely debated, and there is still a need to agree on treatment targets and strategies. During the last decade, several well-constructed trials have added considerably to the evidence base [4–8], demonstrating convincingly the benefits of lowering blood pressure (BP), but also highlighting how difficult this can be to achieve in practice.
Size of the problem
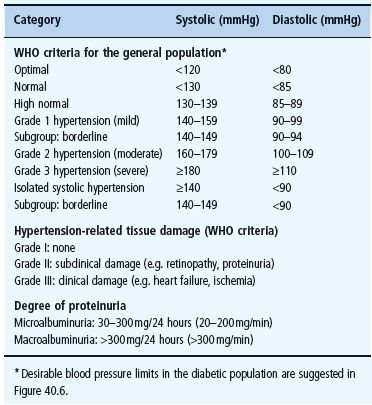
Hypertension is widely defined according to the World Health Organization/International Society of Hypertension (WHO/ISH) criteria (Table 40.1). People with diabetes are still at risk of macrovascular and microvascular complications at BP levels below these thresholds, and the treatment target range is therefore lower (130–140/80–85 mmHg).
Table 40.1 Criteria for hypertension and related tissue damage, defined by the World Health Organization (WHO) and the International Society for Hypertension, 1999 [33].
Overall, hypertension (according to the WHO criteria) is up to twice as common in people with diabetes as in the general population [9]. In white Europeans, 10–30% of subjects with T1DM and 60–80% of those with newly diagnosed T2DM are hypertensive [10]. There are racial and ethnic differences in the prevalence of hypertension, which presumably are at least partly genetically determined: for example, hypertension (and macrovascular disease) is less frequent among the Pima Indians and Mexican-Americans [11]. Impaired glucose tolerance (IGT) is also associated with hypertension (20–40% of cases), perhaps reflecting the common origins of these aspects of the metabolic syndrome [12].
There is evidence that the true prevalence of hypertension is increasing in the diabetic population (especially T2DM) after allowing for the greater number of cases identified through improved screening and the lowering of thresholds for treatment of BP [13]. The causes probably include the rising prevalence of obesity and longer survival of older people with diabetes.
Causes of hypertension in diabetes
Associations between hypertension and diabetes are listed in Table 40.2. Essential hypertension and isolated systolic hypertension are both common in the non-diabetic population (especially in the elderly). It is estimated that essential hypertension accounts for about 10% of cases in people with diabetes. Other important causes are the hypertension that coexists with IR, obesity and IGT in the metabolic syndrome, and hypertension secondary to diabetic nephropathy, as discussed in detail below.
Table 40.2 Associations between hypertension and diabetes.
| Hypertension associated with T2DM (insulin resistance syndrome X, metabolic syndrome) |
| Hypertension associated with nephropathy in T1DM |
| Coincidental hypertension in patients with diabetes |
| Essential hypertension |
| Isolated systolic hypertension |
| Renal scarring (e.g. from recurrent pyelonephritis) |
| Diabetogenic antihypertensive drugs |
| Potassium – losing diuretics (chlorthalidone, high – dose thiazides) |
| Beta – blockers (high dose) |
| Combined diuretics and beta – blockers |
| Drugs causing obesity, hypertension and glucose intolerance |
| Glucocorticoids |
| Combined oral contraceptive pills |
| Antipsychotics |
| Endocrine disorders causing hypertension and glucose intolerance |
| Acromegaly |
| Cushing syndrome |
| Conn syndrome |
| Pheochromocytoma |
Hypertension in the metabolic syndrome
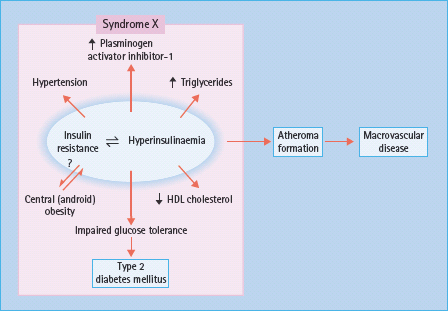
This syndrome consists of IR, IGT (including T2DM), a characteristic dyslipidemia – hypertriglyceridemia, low high-density lipoprotein (HDL) cholesterol, and raised low density lipoprotein (LDL), with an excess of small dense LDL particles – truncal obesity, procoagulant changes (raised plasminogen activator inhibitor 1 and fibrinogen levels) and hyperuricemia [2,14,15]. As these abnormalities are all risk factors for atherogenesis, the syndrome is completed by a marked tendency to vascular aging leading to macrovascular disease, especially coronary heart disease (CHD) and stroke (Figure 40.1). As discussed in Chapter 11, IR has been proposed by Reaven [2], DeFronzo and Ferrannini [14] and others [15] to be a fundamental cause of hypertension and cardiovascular disease (CVD) as well as T2DM. IR is partly genetically determined, and acquired factors such as obesity, physical inactivity and perhaps malnutrition in utero and during early infancy may also contribute [16]. In support of the latter, family studies have revealed a correlation between the BP of the mother and her offspring that appears to be non-hereditary in origin; early growth retardation is suggested to program abnormal development of the vasculature as well as the tissues that regulate glucose homeostasis.
IR is closely associated with high BP in both humans and animals. Experimental induction of IR (e.g. feeding rats with fructose) is accompanied by a rise in BP. More persuasively, an inverse relationship has been demonstrated in humans between BP and insulin sensitivity [17] (Figure 40.2). Various mechanisms have been proposed to explain how IR and/or the hyperinsulinemia that accompanies it could increase BP (Figure 40.3). First, there is some evidence that insulin is an endothelium-dependent vasodilator, releasing nitric oxide (NO) from the endothelium, which relaxes vascular smooth muscle [18,19]; blunting of this effect, caused by insensitivity to the action of insulin on the endothelium as well as on metabolically important tissues, could contribute to the increased peripheral resistance that is the hallmark of hypertension in obesity and T2DM. Impaired endothelium-mediated vasodilatation is associated with IR states and may have a key role in the initiation and progression of atherosclerosis [20].
Figure 40.2 Hypertension is associated with insulin resistance. Insulin sensitivity, measured as the metabolic clearance rate (MCR) of glucose during an insulin clamp study, is inversely related to the mean 24-hour systolic and ambulatory blood pressure. Reproduced from Pinkney et at. [17], with permission from the Editor.
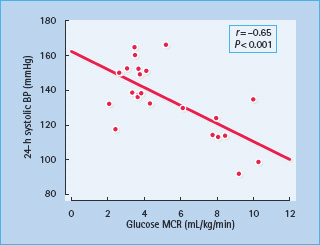
Figure 40.3 Possible mechanisms of hypertension in conditions of insulin resistance. NO, nitric oxide.
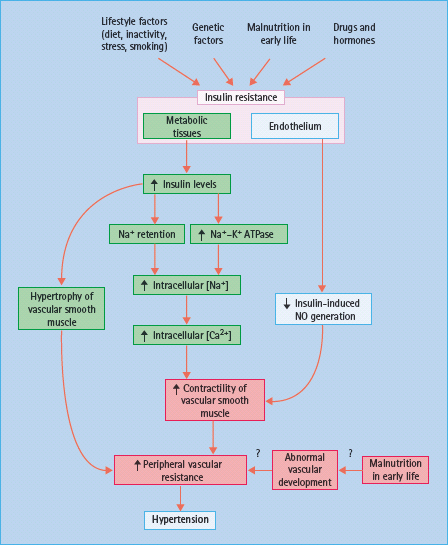
By contrast, insulin also has several actions that tend to raise BP, and there is some evidence that these are accentuated in IR states, presumably because sensitivity is preserved to the effects of the raised insulin levels. Insulin acts on the distal renal tubule to retain Na+ ions and water [20,21], an effect that still operates in IR subjects [22], and so could contribute to the rise in total body Na+ content that occurs in obesity and T2DM [23]. Insulin also stimulates the cell membrane Na+ – K+ ATPase, which would raise intracellular Na+ concentrations in vascular smooth muscle and, by increasing systolic Ca2+ levels, would enhance contractility and increase peripheral resistance [22,23]. Through its effects on the CNS, insulin may stimulate the sympathetic outflow. Theoretically, this could also increase BP, although direct evidence in humans is lacking [22,24]. Finally, insulin may stimulate the proliferation of vascular smooth muscle cells, which could lead to medial hypertrophy and increased peripheral resistance [22,25].
Hypertension and diabetic nephropathy
This association is most obvious in young patients with T1DM, in whom the presence of hypertension is strikingly related to renal damage and even minor degrees of proteinuria. BP begins to rise when the urinary albumin excretion (UAE) enters the microalbuminuric range (>30mg/24 hours) and is usually over the WHO threshold when UAE reaches the macroalbuminuric stage (>300mg/24 hours) [26]. The association may be partly genetically determined: subjects with diabetes and microalbu-minuria commonly have parents with hypertension and may also inherit overactivity of the cell-membrane Na+–H+ pump (indicated by increased Na+–Li+ counter-transport in red blood cells), which would tend to raise intracellular Na+ concentrations and thus increase vascular smooth muscle tone [27].
The basic mechanisms of hypertension include decreased Na+ excretion with Na+ and water retention. Peripheral resistance is increased, to which raised intracellular Na+ will contribute. The role of the renin angiotensin aldosterone system (RAS) is uncertain, as both increased and decreased activity has been reported [28,29]. These discrepancies may be explained by differences in diet, treatment, metabolic control and the type and duration of diabetes. Na+ retention and hypertension would be predicted to suppress the RAS, while renin levels may be influenced by other complications of diabetes: renal tubular acidosis type 4 causes hyporeninemic hypoaldosteronism and neuropathy can also lower plasma renin, while renin may be raised in retinopathy and advanced nephropathy. Patients with microalbuminuria who are insulin-resistant appear to be particularly susceptible to hypertension [30].
Impact of hypertension in diabetes
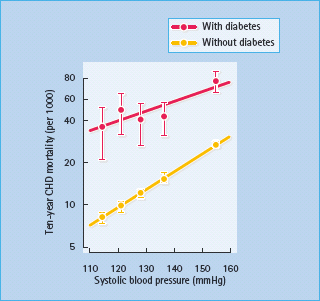
A large proportion of hypertensive people with diabetes show signs of cardiovascular aging and target-organ damage [10]. Hypertension, as an independent risk factor for atherogenesis, synergizes with the effects of diabetes and significantly increases the development and progression of CHD, cerebrovascular and peripheral vascular disease. Overall, the effects of hypertension on deaths from CHD are increased by 2–5 times in people with diabetes, with the greatest increase occurring at the lowest BP levels (Figure 40.4).
Figure 40.4 Synergistic effects of diabetes and hypertension on deaths from coronary heart disease (CHD). Data from 342 815 people without diabetes and 5163 people with diabetes aged 35–57 years, free from myocardial infarction at entry. Reproduced with permission from O. Vaccaro, paper presented at the 26th Annual Meeting of the European Diabetes Epidemiology Group, Lund, 1991.
The deleterious effects of hypertension on left ventricular function are also accentuated by the presence of diabetes. These include impaired left ventricular relaxation [31] and increased left ventricular mass [32], the latter being an independent predictor of premature death from CHD.
Hypertension also predisposes to the development of certain microvascular complications, particularly nephropathy and end-stage renal failure (ESRF), for which the risk is increased by 2–3 times (see Chapter 37). Hypertension is also a risk factor for retinopathy, as has been confirmed by the beneficial effects of improved BP control in patients with T2DM, reported by the UK Prospective Diabetes Study (UKPDS) [4].
Screening for hypertension in diabetes
As the two conditions are so commonly associated, people with diabetes must be regularly screened for hypertension and vice versa. Hypertensive patients, especially if obese or receiving treatment with potentially diabetogenic drugs, should be screened for diabetes at diagnosis and during follow-up. Should hyperglycemia be detected, potentially diabetogenic antihypertensive drugs should be reduced or changed to others or used in combinations that do not impair glucose tolerance, and normoglycemia can then often be restored.
All people with diabetes should have their BP checked at diagnosis and at least annually thereafter. This is especially important in those with other cardiovascular risk factors, such as nephropathy (which is associated with a substantial increase in the cardiovascular mortality rate), obesity, dyslipidemia, smoking or poor glycemic control.
Measurement of blood pressure
BP should be measured with the patient in the supine or sitting position, with an accurate sphygmomanometer and a cuff of appropriate size (i.e. wider for obese subjects with an arm circumference of >32cm). Systolic and diastolic BP should be recorded, to the nearest 2 mmHg if using a manual sphygmoma-nometer, from phases I and V (i.e. appearance and final disappearance of the sounds of Korotkoff). Usual precautions should be taken to ensure reliability and avoid “white coat” stress effects which can acutely raise BP. Conditions should be quiet and relaxed, and at least two readings should be taken initially and then repeated at intervals over weeks or months to determine the subject’s typical values and any trend to change. Office BP could be complemented by repeated home BP recordings.
BP should also be checked with the patient in the upright position (1 minute after standing), because there may be a significant postural fall (>20 mmHg systolic) in patients with diabetic auto-nomic neuropathy, the elderly or those treated with vasodilators or diuretics. Marked postural hypotension, which can coexist with supine hypertension, may indicate the need to change or reduce antihypertensive medication, especially if symptoms are provoked.
Ambulatory BP monitoring over 24 hours may be useful in some cases to exclude “white coat” effects, and in patients with early nephropathy who have nearly normal BP during the day, but who may be at risk of hypertensive tissue damage because they fail to show the physiologic BP dip during sleep [33].
Diagnosis of hypertension in diabetes
The criteria issued in 1999 by WHO and ISH [34] define hypertension as an office BP exceeding 140/90 mmHg (Korotkoff I-V), and borderline hypertension as being below these limits but above 130 mmHg systolic and/or 85 mmHg diastolic (Figure 40.5) [34]. Established hypertension is diagnosed when readings consistently exceed 140/90 mmHg over several weeks, or when the BP is very high (diastolic BP >110 mmHg), or when there are clinical signs of tissue organ damage from long-standing hypertension.
Figure 40.5 Blood pressure treatment targets suggested for subjects with diabetes, compared with the World Health Organization/International Society of Hypertension definition of hypertension and borderline hypertension [34]. ALFEDIAM, Association de langue française pour l’étude du diabète et des maladies metaboliques; ADA, American Diabetes Association; ESC, European Society of Cardiology; EASD, European Association for the Study of Diabetes; ESH, Europe Society of Hypertension.
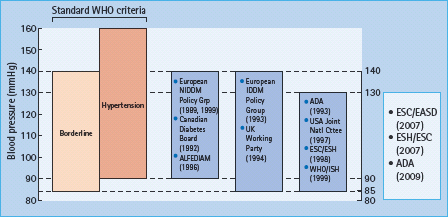
It is clear from numerous epidemiologic studies that the WHO/ ISH threshold is too high in people with diabetes because of their additional risk of both macrovascular and microvascular disease, and that there are definite benefits from treating microalbuminuric subjects whose diastolic BP is <90mmHg [35]. Various other expert bodies have suggested alternative, generally lower target levels (Figure 40.5). A consensus would be to aim for a BP of less than 130–140mmHg systolic and below 80–85 mmHg diastolic, and to treat any subject whose BP is consistently above one or both of these thresholds.
Investigation of hypertension in diabetes
Initial investigation of the hypertensive patient with diabetes aims to exclude rare causes of secondary hypertension (Table 40.2), to assess the extent of tissue organ damage caused by hypertension and diabetes (Table 40.1) and to identify other potentially treatable risk factors for vascular disease. The major points in the medical history and examination are shown in Table 40.3.
Table 40.3 Investigation of the patient with diabetes and hypertension.
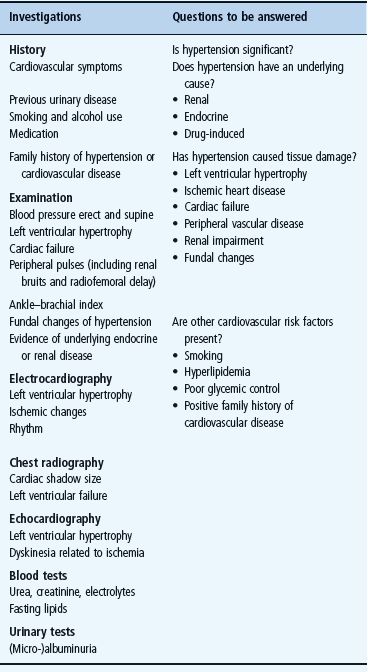
- Cardiac function. A standard 12-lead electrocardiogram may show obvious ischemia, arrhythmia or left ventricular hypertrophy; the latter is more accurately demonstrated by echocardiography, which will also reveal left ventricular dysfunction and decreased ejection fraction. Exercise testing (or stress-echo) testing and 24-hour Holter monitoring may also be appropriate.
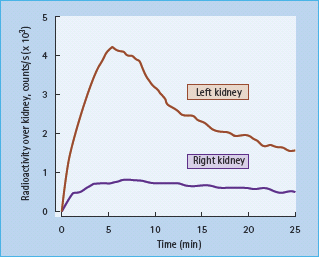
- Renal function. A fresh urine sample should be tested for microalbuminuria (see Chapter 37) and another examined microscopically for red and white blood cells, casts, and other signs of renal disease. Microscopic hematuria can occasionally occur in patients with T1DM (particularly children) in the apparent absence of significant renal dysfunction, but coexistent renal disease must always be excluded. Serum urea, creatinine and electrolytes should be checked. If the serum creatinine concentration is raised, measurement of the glomerular filtration rate (GFR) should be considered, ideally using a specific clearance method such as chromium ethylenediamine tetra-acetic acid (Cr-EDTA), iohexol or cystatin C. Further specialist investigations that may be needed include an isotope renogram and other tests for renal artery stenosis (Figure 40.6). This complication of renal arterial atherosclerosis may affect up to 20% of older patients with T2DM and, if bilateral, can lead to severe and sometimes permanent renal impairment if angiotensin-converting enzyme (ACE) inhibitors are given.
Figure 40.6 Renal artery stenosis affecting the right kidney, in a patient with diabetes and hypertension. Uptake of the isotope on this side is markedly reduced and delayed.
- Lipid profile. Fasting serum lipid concentrations should be checked. If total cholesterol or triglyceride levels are found to be elevated after repeated measurements, further investigation of lipoprotein subclasses – very low density lipoprotein (VLDL), LDL, HDL, as well as the apo-B:apo-A1 lipoprotein ratio – is recommended. Treatment for hyperlipidemia should be considered if the total cholesterol is > 4.5 mmol/L, the LDL cholesterol level is >2.5 mmol/L or the LDL: HDL cholesterol ratio is >4 [36]. This is discussed in more detail in the second half of this chapter.
Other forms of secondary hypertension may be indicated by clinical findings of endocrine or renal disease, significant hypokalemia (plasma potassium <3.5 mmol/L without previous diuretic treatment), failure of hypertension to respond to standard treatment or a sudden decline in GFR after starting treatment with ACE inhibitors (suggestive of renal artery stenosis).
Management of hypertension in diabetes
Strict BP control is the primary goal of treatment. In recent years, target treatment levels have declined progressively to the current recommendation of a mean office BP less than 130–140/80–85 mmHg, for all patients who can tolerate this without side effects such as orthostatic reactions or compromising arterial circulation in critical vascular beds. Recent observations indicate that subgroups of susceptible patients might exist who will not tolerate a dramatic BP reduction below 130 mmHg systolic BP and so caution should be exercised.
Management begins with lifestyle modification, but few patients respond to this alone, and most will require more than one antihypertensive drug to control BP adequately [4,5].
Non-pharmacologic treatment
The treatment of hypertension in patients with diabetes must be based on structured lifestyle intervention. This means weight reduction or weight stabilization in the obese, sodium restriction, diet modification and regular physical exercise (moderate intensity, 40–60 minutes, 2–3 times weekly). Dietary intake of saturated fat has been associated with impaired in insulin sensitivity and should therefore be reduced [37]. Alcohol should be restricted to 2–3 units/day in men and 2 units/day in women, but omitted altogether if hypertension proves difficult to control.
Smoking causes an acute increase in blood pressure and greater variability overall [38]. Smoking cessation is especially important, as smoking not only accelerates the progression of atherosclerosis and vascular aging, but also impairs insulin sensitivity [39] and worsens albuminuria [40]. Treatment with nicotine supplementation for 4–6 weeks (chewing gum or patches), bupropion or varenicline may be useful.
When adopted in full by the patient, lifestyle modification can be extremely effective. The above measures can lower systolic and diastolic BP by 11 and 8 mmHg, respectively [41] – as much as many antihypertensive drugs – and sometimes enough to obviate the need for drug therapy. Weight reduction in obese patients can similarly reduce BP.
Antihypertensive drug therapy
Numerous drugs are available to lower BP, but some are better suited than others to the particular needs of subjects with diabetes because of their favorable or neutral effects on glucose metabolism and other factors. Most patients (at least two-thirds) will require combinations of antihypertensive drugs to control BP – an average of around three different drugs in two large studies [4,5]. Accordingly, the clinician must be able to use a wide variety of antihypertensive drugs and to choose combinations that exploit pharmacologic synergy. Combination therapy usually means that lower dosages of individual drugs can often be used, thus reducing the risk of their adverse effects.
Diuretics
Diuretics are often effective antihypertensive agents for people with diabetes, in whom the total body sodium load is increased and the extracellular fluid volume expanded [42]; however, diuretics that increase urinary potassium and magnesium losses can worsen hyperglycemia, as insulin secretion is impaired by potassium depletion, and insulin sensitivity in peripheral tissues may also be decreased [43]. The use of high-dose thiazide diuretics – equivalent to ≥5 mg/day bendroflumethiazide (bendrofluazide) – is reported to increase the risk of hypertensive patients developing diabetes by up to threefold; this does not seem to occur with low dosages (up to 2.5 mg/day bendroflumethiazide) [44]. Potassium depletion is particularly severe with high-dose chlortalidone (chlorthalidone), less with furosemide (frusemide) and bendroflumethiazide and apparently negligible with indapamide. This mechanism is irrelevant to C-peptide-negative subjects with T1DM who are totally dependent on exogenous insulin. Thiazides may also aggravate dyslipidemia [45], although low dosages probably carry a small risk. Thiazides have also been associated with gout and impotence and are generally avoided in middle-aged men with diabetes and hyperuricemia or erectile dysfunction; nevertheless some evidence suggests that the risk of erectile failure may have been overstated. Diuretics may precipitate hyperosmolar hyperglycemia syndrome and should be avoided or used at the lowest effective dose in patients with a history of this complication.
Diuretics have been shown to prevent CVD successfully in elderly subjects with T2DM and systolic hypertension [46], but one observational study suggested that the use of diuretics increased cardiovascular mortality in hypertensive patients with T2DM who were still hyperglycemic in spite of treatment [47]. Overall, these drugs are effective and safe when used appropriately in patients with diabetes.
Diuretics suitable for use in diabetic hypertension include furosemide, bendroflumethiazide (≤2.5 mg/day), hydrochloro-thiazide, spironolactone and indapamide. Low dosages should be used, sometimes in combination with potassium supplements or potassium-sparing drugs, such as amiloride. If ineffective, diuretics should be combined with another first-line drug (e.g. an ACE inhibitor or an angiotensin II-receptor antagonist [ARB]), rather than given at increased dosage. Spironolactone is best not combined with an ACE inhibitor, as this increases the risk of hyper-kalemia. Furosemide is useful in patients with renal impairment (serum creatinine >150μmol/L) or edema.
Serum urea, creatinine and potassium should be checked when starting diuretic therapy and every 6–12 months thereafter, as dangerous disturbances in plasma potassium levels can develop, especially in patients with diabetes and renal impairment.
β-Adrenergic blocking agents
Beta-blockers may significantly lower BP levels in patients with diabetes and hypertension, even though renin release (a major target for these drugs) is commonly reduced in diabetes because of Na+ and fluid retention. These drugs are often ineffective in Afro-Caribbean patients, who commonly have low renin hypertension. Other mechanisms of action that reduce BP include reductions in heart rate and cardiac output via interaction with β1 and β2-receptors in the myocardium and in the vessel wall.
Like diuretics, beta-receptor blockers may aggravate both hyperglycemia and dyslipidemia [48]. These effects depend on both the dosage and the degree of selectivity of the individual drug. The hyperglycemic effect is attributed to inhibition of β2-adrenergic-mediated insulin release and decreased insulin action in peripheral tissues; the long-term risks of a person without diabetes developing the disease may be increased by sixfold [49] and even more if given together with thiazides. Some studies suggest that the hazards of both hyperglycemia and hyperlipidemia have been exaggerated and may be both dose-dependent and secondary to weight gain [50]. The metabolic side effects of beta-blockers can be reduced by using low dosages combined with other agents, particularly dihydropyridine calcium channel antagonists (CCAs), or by intensifying non -pharmacologic efforts to decrease weight and improve physical activity.
Beta-blockers have other side effects relevant to diabetes. They may interfere with the counter-regulatory effects of catecholamines released during hypoglycemia, thereby blunting manifestations such as tachycardia and tremor and delaying recovery from hypoglycemia [51]. In clinical practice, however, this rarely presents a serious problem, especially when cardioselective β1– blockers are used. Beta-blockers may also aggravate impotence, and are generally contraindicated in second- or third-degree atrioventricular (AV) heart block, severe peripheral vascular disease, asthma and chronic airway obstruction. Recent studies have shown that certain beta-blockers such as metoprolol and carvedilol [52,53] can be used favorably in cardiac failure in patients with diabetes, as shown in the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF) study, in which 25% of the patients had diabetes [52].
Atenolol is a commonly used drug, as it is cardioselective and water soluble, which reduces CNS side effects and renders its metabolism and dosage more predictable. It is mostly effective as a single daily dose, which probably encourages compliance. In the UKPDS, its effect was comparable to that of the ACE inhibitor, captopril [54]; however, it should be kept in mind that the stroke preventive effect of atenolol is 16% less than other antihy-pertensive drugs, based on data from meta-analyses. Metoprolol is an alternative, in moderate dosages. Both non-selective and selective beta-blockers are effective in the secondary prevention of myocardial infarction (MI) after an initial event in patients with diabetes [55]. Metoprolol or carvedilol may be indicated in patients who also have heart failure [52,53]. and beta-blockers in general are useful in patients who also have angina or tachyarrhythmias.
Calcium-channel antagonists
These useful vasodilator agents do not generally worsen metabolic control when used at conventional dosages, although sporadic cases of hyperglycemia have been reported after starting a calcium-channel antagonist (CCA) of the dihydropyridine class [56]. This may be caused by inhibition of insulin secretion (a calcium-dependent process) in susceptible patients, or a compensatory sympathetic nervous activation, which antagonizes both insulin secretion and action, following vasodilatation.
CCAs have a slight negative inotropic effect and are contrain-dicated in significant cardiac failure; they often cause mild ankle edema, but this is caused by relaxation of the peripheral precapillary sphincters and raised capillary pressure rather than to right ventricular failure. Because of their potent vasodilator properties, these drugs can cause postural hypotension and can aggravate that brought about by autonomic neuropathy. Non-dihydropyridine CCAs (e.g. verapamil) reduce proteinuria in diabetic nephropathy, but this effect is not seen with dihydropyridine derivatives such as nifedipine, amlodipine, felodipine and isradipine [57].
Because of their other cardiac actions, these drugs are particularly indicated in hypertensive patients who also have angina (e.g. sustained-release nifedipine and diltiazem) or supraventricular tachycardia (e.g. verapamil). Their vasodilator properties may also be beneficial in peripheral vascular disease. CCAs are ideally combined with selective β1-blockers, but the specific combination of verapamil and beta-blockers (especially together with digoxin) must be avoided because of the risk of conduction block and asystole. Overall, CCAs appear less or similarly cardioprotective but better at preventing stroke than either beta-blockers or thiazide diuretics [58,59].
Amlodipine given once daily is an evidence-based and convenient preparation for general use, and felodipine, isradipine and sustained-release nifedipine are suitable alternatives.
Angiotensin-converting enzyme inhibitors
ACE inhibitors may be used in diabetic hypertension, even in cases where the general RAS is not activated as the drugs may interfere with local angiotensin action in specific target tissues. When used alone, however, these agents have a limited hypotensive action in many black patients, who tend to have suppressed RAS activity.
ACE inhibitors have no adverse metabolic effects and may even improve insulin sensitivity [60]; hypoglycemia has rarely been reported [61]. These drugs are particularly beneficial in diabetic nephropathy by reducing albuminuria and possibly delaying progression of renal damage [62]. Their antiproteinuric effect may be caused specifically by relaxation of the efferent arterioles in the glomerulus, which are highly sensitive to vasoconstriction by angiotensin II, thus reducing the intraglomerular hypertension that is postulated to favor albumin filtration; however, the importance of this mechanism remains controversial [63]. ACE inhibitors are also indicated in cardiac failure, in combination with relatively low dosages of diuretics.
A dry cough is reported by 10–15% of patients treated with ACE inhibitors, because these drugs also interfere with the breakdown of kinins in the bronchial epithelium. Changing to another ACE inhibitor or an ARB may avoid this problem. ACE inhibitors occasionally precipitate acute renal failure, particularly in the elderly and in subjects taking non-steroidal anti–nflammatory drugs (NSAIDs), or who have bilateral renal artery stenosis. Other side effects (rashes, neutropenia, taste disturbance) are unusual with the low dosages currently recommended, but become more prominent in renal failure. Because ACE inhibitors cause potassium retention, they should not generally be taken concurrently with potassium-sparing diuretics (spironolactone and amiloride) or potassium supplements. Serum creatinine and potassium levels should be monitored regularly, especially in patients with renal failure or type 4 renal tubular acidosis, in whom hyperkalemia can rapidly reach dangerous levels.
Ramipril, enalapril, captopril, lisinopril and perindopril are all established ACE inhibitors that are suitable for use in people with diabetes; enalapril, lisinopril, perindopril and ramipril are given once daily for hypertension. The first dose of an ACE inhibitor should be small and taken just before bedtime to minimize postural hypotension, which may be marked in subjects receiving diuretics or on a strict sodium-restricted diet. The same problem may arise in patients with autonomic neuropathy. ACE inhibitors are now recommended in patients with left ventricular dysfunction following MI (see Chapter 41). Ramipril has been shown to prevent cardiovascular morbidity and mortality in high-risk patients with diabetes, with or without pre-existing ischemic heart disease [64].
Angiotensin II type 1 receptor blockers
This promising new class includes losartan, irbesartan, valsartan, candesartan and telmisartan, which act on the AT1 receptor to decrease BP. They are metabolically neutral [65] and, unlike the ACE inhibitors, do not cause cough. They are effective antihypertensive drugs in people with diabetes [66] and have been shown to slow the progression of nephropathy in patients with diabetes and varying degrees of albuminuria (in the RENAAL, IDNT and PRIME-2 studies) [67–69]. Losartan has also been shown (in a subgroup of the LIFE study) to be better than atenolol in reducing both cardiovascular endpoints (by 25%) and total mortality (by 40%) in high-risk patients with T2DM with hypertension and left ventricular hypertrophy [70]. Interestingly, the combination of an ACE inhibitor (lisinopril) with an AT1-antagonist (candesartan) was more effective than either agent alone in lowering BP and UAE in patients with T2DM [71]; however, in the recent ONTARGET study, no extra benefits were recorded for the combination of telmisartan and ramipril on cardiovascular endpoints compared to monotherapy [72].
α1-Adrenoceptor antagonists
α1-Blockers can lower BP effectively and also improve dyslipidemia and insulin sensitivity. Doxazosin is normally well tolerated, especially in combination therapy; side effects include nasal congestion and postural hypotension. Doxazosin has been reported to be inferior to the diuretic chlortalidone in the prevention of stroke and heart failure [73].
Treatment strategies
In general, lifestyle modification should be tried initially for 3 months or so. If moderate hypertension (diastolic BP >100 mmHg, or systolic BP >160 mmHg) or signs of hypertensive tissue damage are present, then drug therapy should be started at the outset. Initially, monotherapy with one of the first-line drugs suggested above should be used, the choice being influenced by other factors such as coexistence of angina, heart failure or nephropathy. All drug treatment should aim for being evidence-based and cost-effective in the individual patient.
Hypertension in T1DM
ACE inhibitors are especially suitable if the patient has albuminu-ria or more advanced stages of diabetic nephropathy. Diuretics, β1-selective blockers and CCAs are equally valid alternatives with regard to BP reduction.
If renal function is moderately impaired (serum creatinine values >150 μmol/L), thiazide diuretics become less effective, and furosemide or other loop diuretics should be used instead; however, in established ESRF (serum creatinine >500 μmol/L) furosemide may be toxic, and dialysis must be started. In some patients, hypoglycemia attacks may be masked by use of beta – blockers.
Hypertension in T2DM
BP control is generally more important than the choice of individual drugs. First-line agents, according to evidence from clinical studies, are ACE inhibitors, ARBs, beta-blockers, low-dose thiazide diuretics (in the elderly), furosemide and CCAs [4–8].
Ramipril has evidence-based support for its use in patients with T2DM because of their high cardiovascular risk [64]. Beta-blockers (in combination with low-dose aspirin) are indicated as secondary prevention for patients who have had a MI, as long as no serious contraindications are present. Low doses of thiazide diuretics are useful in elderly patients with diabetes, as this class of drugs has proven efficacy in preventing stroke and all-cause mortality in elderly hypertensive patients [8].
α1-Blockers may be used as part of combination therapy, especially in patients with dyslipidemia (high triglycerides and low HDL cholesterol levels) and prostatic hyperplasia. Indapamide is well tolerated and has no metabolic side effects. Spironolactone may also be of value [74], especially for elderly obese female patients with hypertension and hypervolemia with a low renin profile.
Combination therapy
Combination therapy is needed in most people with diabetes (especially those with T2DM) to achieve satisfactory BP control [4,5]. It is often better to use low dose combinations than to increase dosages of single agents, as side effects are commonly dose-dependent. As already mentioned, potassium-sparing agents (spironolactone and amiloride) should not be combined with an ACE inhibitor, because of the increased risk for hyperkalemia.
Certain combinations of antihypertensive drugs have proved very safe and effective in low to moderate doses, e.g. ACE inhibitor plus diuretic, for example in the ADVANCE study [75]; CCA plus ACE inhibitor, for example in the ACCOMPLISH study [76]; selective β1-blocker plus CCA; or β1-blocker plus α1-blocker. In some high risk patients a combination treatment could also be considered as initial therapy.
Special considerations in ethnic groups
Hypertension in diabetes represents a serious medical problem in many ethnic groups, such as African-Americans [77]. In non-white European patients, beta-blockers and ACE inhibitors are often less effective at lowering BP because the RAS is already underactive. Diuretics and CCAs are often drugs to be preferred, particularly in African-Americans [78].
Outcome of treating hypertension in diabetes
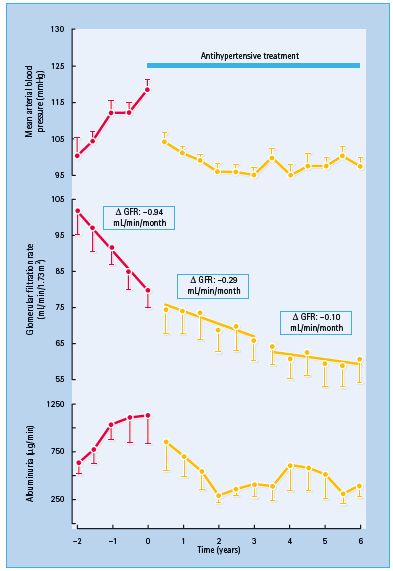
It has long been recognized that effective treatment of hypertension can slow the progression of diabetic nephropathy, lowering UAE and decreasing the rate of fall of the GFR [79] (Figure 40.7).
Figure 40.7 Treating hypertension slows the progression of diabetic nephropathy. Lowering blood pressure signifi cantly decreased the rate of decline in the glomerular fi ltration rate (GFR) and urinary albumin excretion. Reproduced from Parving et al. [79] , with permission.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree