- Managing type 2 diabetes has become more complex as pharmacotherapy has expanded. Clinicians have more pharmaceutical agents targeted to hyperglycemia and obesity than before, but a relentlessly progressive disorder to overcome.
- Clinical trials continue to show that glycemic control is critical to reduce microvascular complications and in the long-term also cardiovascular events. Nonetheless, poor glycemic control, hypoglycemia and obesity remain stubborn barriers for clinicians.
- Few comparative data direct us in the best use of multiple drug therapies for the management of type 2 diabetes and its co-morbidities. One is guided by good clinical studies, the potential of therapeutic agents to reverse pathophysiology and ultimately the proper use of insulin which is eventually needed in most patients to replace the loss of (β-cell function.
Introduction
The epidemic of type 2 diabetes mellitus (T2DM) presents the clinician as well as society with complex challenges in designing both prevention and treatment strategies for T2DM. T2DM is a worldwide epidemic with a global prevalence in 2009 of 285 million people, expected to increase to 435 million by 2030 [1]. The Centers for Disease Control and Prevention (CDC) estimates that in 2007 nearly 24 million people in the USA have diabetes [2], mostly T2DM. At least 57 million people have pre-diabetes (impaired fasting glucose or impaired glucose tolerance), providing an expanding pool of new patients with T2DM. Current prevalence represents a 90% increase in the new diagnosis of diabetes over the last decade [3] and more rapid increases in many parts of the world. This increasing prevalence of diabetes relates both to environmental and genetic factors, each of which may influence insulin sensitivity (e.g. insulin resistance) and insulin secretion capacity. Insulin resistance begins in early life and is fueled by obesity and a sedentary lifestyle which contribute to alterations in glucose homeostasis and to abnormal lipid and protein metabolism.
Relative insulin deficiency is the defining metabolic difference between obesity and the development of hyperglycemia with progression to T2DM. An inexorable progression of diabetes appears related to worsening insulin deficiency [4,5]. Most people therefore have gradually increasing needs for additional therapy. Combinations of therapy such as adopting a therapeutic lifestyle change, as well as oral and injectable medicines, are required to keep the glycemia under control. The failure to advance therapy at an early sign of therapeutic failure and, in particular, a reluctance to advance to insulin underlies the less than optimum control for many patients with diabetes.
Algorithms for management
Several approaches to the best management of T2DM have been proposed. All algorithms begin with therapeutic lifestyle change and are generally focused primarily upon controlling hyperglycemia. Consensus statements from the American Diabetes Association (ADA) have been recently revised in conjunction with the European Association for the Study of Diabetes (EASD) [6]. The most recent algorithm (Figure 31.1) moves away from the focus on glucose as the sole goal of therapy and tries to develop evidence-directed therapy to treating diabetes by normalizing hyperglycemia and minimizing cardiometabolic risk. The US Food and Drug Administration (FDA) has recently advised that new medicines for glycemic control in diabetes be routinely evaluated early for their effects upon cardiovascular disease. By doing so, they recognize atherosclerosis as the primary cause of cost and mortality in diabetes.
Figure 31.1 The ADA/EASD consensus statement on the recommended strategy to advance therapies to control hyperglycemia in diabetes. The top portion focuses on traditional well-validated therapies buttressed by large studies and long experience (metformin, sulfonylureas, insulin). The lower portion focuses on more recent emerging therapies that have less follow-up and validation in clinical trials (thiazolidinediones or glitazones as they are often called; e.g. pioglitazone, GLP-1 agonists such as exenatide). According to the authors, the amylin agonists, α-glucosidase inhibitors, glinides and DPP-4 inhibitors were not included in the two tiers of preferred agents in this algorithm, owing to their lower or equivalent overall glucose-lowering effectiveness compared with the first- and second-tier agents and/or to their limited clinical data or relative expense. However, they may be appropriate choices in selected patients. Similarly, colesevelam is not mentioned in this algorithm but may be appropriate for selected patients. Rosiglitazone is omitted from the algorithm because of unsettled concerns raised about cardiovascular side effects. Reproduced from the updated American Diabetes Association/ European Association for the Study of Diabetes consensus statement [6].
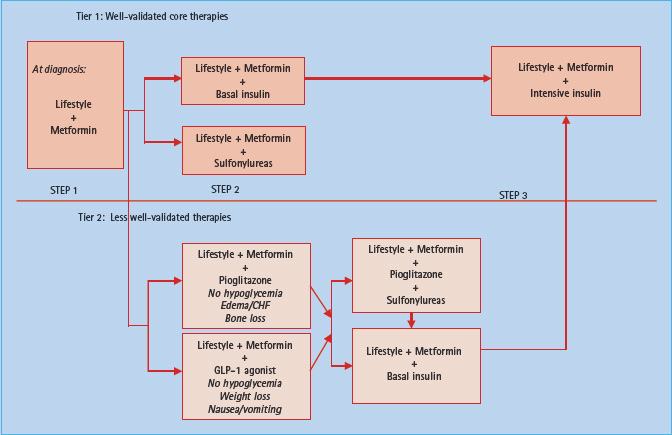
The current consensus algorithm (Figure 31.1) moves quickly from metformin treatment failure to the addition of sulfonylurea or insulin. Newer classes of drugs (e.g. incretin mimetics, pioglitazone) are recommended later or as a secondary consideration because of the lack of compelling data to suggest their superiority compared to existing treatments and associated concerns for unintentional ill effects such as long bone fractures or increase in congestive heart failure (glitazones) or pancreatitis (exenatide). Ultimately, the treatment pathway must incorporate patient individualization, clinical acumen, and the broad experience gained from clinical trials.
Goals of treatment
Treatment goals for patients with diabetes have changed considerably over the past two decades. The Diabetes Control and Complications Trial (DCCT) [7] confirmed the concept of the relationship of glycemic control to microvascular complications in type 1 diabetes. Similar results were found for T2DM in the landmark UK Prospective Diabetes Study (UKPDS) [8,9]. Long-term analysis of subjects participating in these trials at 10 years following a period of improved glycemic control found cardiovascular risk reduction with insulin, metformin and sulfonylureas [10,11] and for T2DM also a mortality advantage.
In assigning patients to a treatment plan, consideration must be given to treatment goals. The ADA continues to recommend HbA1c of <7% (<53 mmol/mol) as a general glycemic goal and individualization is recommended. For properly selected patients, either less tight control or alternatively more rigorous normalization of glycemia down to 6% (42 mmol/mol) HbA1c can be recommended, if the latter can be achieved without hypoglycemia problems. Using similar data sources, the International Diabetes Federation (IDF) and American Association of Clinical Endocrinologists (AACE) suggest ≤6.5% (48 mmol/mol) as a general goal. All authorities recommend the need for individualization of goals based on co-morbidities and vulnerability to hypoglycemia among other criteria. The ACCORD trial hypothesized that close to normal glucose control (HbA1c 6% or less [<42 mmol/mol]) would improve cardiovascular endpoints. Recent analysis of the ACCORD trial [12] cautions the provider against attempting to normalize glycemia in those with characteristics similar to this patient cohort (e.g. older and those with existing cardiovascular disease), as the intensively treated group had a 22% increase in mortality primarily from fatal cardiovascular events; very possibly, although unproven, this may have been as a result of hypoglycemia. This poses a dilemma to the practitioner: how best to normalize glycemia but avoid hypoglycemia. This practical consideration suggests newer therapies with a relatively low hypoglycemia risk should be considered for certain patient groups. Additionally, strategies to recognize and prevent hypoglycemia risk in patients at high risk should be adopted routinely. These include the need for self-monitored blood glucose (SMBG) tests, good communication and education about how to treat and adjust therapy should hypoglycemia become frequent or severe, especially if hypoglycemia unawareness occurs.
Ultimately, patients with T2DM require progression of therapy from combination oral therapy to combination injectable therapy (basal bolus insulin regimens, incretin insulin combinations). Initiating combination therapy early minimizes side effects while maximizing clinical effectiveness, decreasing pill counts and cost while facilitating compliance. New ACCE guidelines use all drugs and tailor their number and starting insulin to the baseline HbA1c [13].
Lifestyle advice for diabetes and pre-diabetes
Lifestyle management is one of the most challenging barriers to successful diabetes control. Deriving recommendations for eating, physical activity and minimizing psychologic stressors is frequently frustrating for both the provider and patient [14]. The assumption that complex patterns of lifestyle behavior can be altered by dispensing general advice in the course of a busy medical visit is flawed, but unfortunately a reality for many providers. It should be replaced by an agreement that the patient is responsible for assisting in their disease management and is the only source of key information needed to manage their illness [15]. One strategy is to make patients more aware of their own lifestyle patterns. Developing a baseline for eating and exercise (type, frequency, duration, barriers) is as integral as obtaining initial laboratory testing in patients with T2DM to compile a treatment plan based on physiology as well as the psychology of the patient. Obtaining a narrow focus on a few behavioral objectives can increase success. Approaches we often use for patients are:
- Keep an eating behavior diary over the next week; and
- Wear a pedometer and keep track of your baseline steps for the next week.
The information almost always contains some surprises for patients but it helps them to identify personal behaviors and to focus and motivate them to take the lead in setting meaningful objectives.
The role of the physician is critical in emphasizing the importance of lifestyle change to the patient, initiating a process of working on lifestyle change, and reinforcing, and refining the education to achieve incremental obtainable objectives. Use of motivational interviewing and assessment of readiness to change are important aspects of evaluation of the patient by both the medical provider and diabetes educator. It is our practice to recommend strongly diabetes education for every patient who is willing to do so and to reinforce the recommendation for lifestyle change at any point when additional therapy needs occur. A useful acronym that we have used to help trainees remember how to assist patients in making lifestyle change is FIRM [15]. This stands for negotiating Few changes, typically 1–3 at any clinical visit; those changes should be Individualized by the patient’s selection of what aspects of behavior change to embark on; the changes should be Realistic and therefore likely to succeed by setting moderate, achievable goals within a specific timeframe; and be Measureable and monitored through patient record-keeping to be shared with the provider or diabetes educator at the next visit.
Therapy for obesity as a treatment for T2DM
Because the vast majority of people with T2DM are overweight or obese, a serious consideration is the use of weight loss and increasing physical activity strategies as a useful treatment to gain control of glycemia and favorably influence multiple cardiovascular risk factors as well, 16,17]. Prevention and/or treatment plans for T2DM could also legitimately consider use of pharmacotherapy for obesity. Successful weight loss of 10% or less has repeatedly been shown to improve glycemic control (HbA1c reductions 0.30–0.5% [3–6 mmol/mol] and sometimes much more) as well as favorable improvements in lipids, hypertension as well as other cardiac risk factors. Currently, three drugs (phentermine, orlistat and sibutramine) are approved for management of obesity in the USA. Rimonabant was approved for use in several countries outside the USA, but recent withdrawal of support by the European Medicines Agency has ended its possibility of wider use. Other drugs of the endocannabinoid receptor blocker class have also been discontinued from clinical development. Phentermine is only approved for short-term use and is the least well-studied and is less commonly used and not generally recommended.
Orlistat is currently approved for use in the management of obesity. Orlistat functions as a gastric and pancreatic lipase inhibitor. When given at a dosage of 120 mg three times daily with meals, approximately 20–30% of ingested fat is passed into the feces. Large amounts of fat in the gastrointestinal tract result in the most difficult side effect to manage, namely anal leakage of oil to post-prandial diarrhea which may be abrupt. Attention to this expected effect as well as dietary counseling or use of daily or twice daily dosing can minimize patient’s gastrointestinal accidents and may enhance patient adherence to low fat dietary regimens. No long-term malabsorption of fat-soluble vitamins has been seen but replacement with a multivitamin including vitamin D is recommended during therapy. A lower dosage (60 mg) over-the-counter form is now available.
Orlistat has been critically studied both in the prevention [18] and in the treatment of T2DM in patients receiving metformin or insulin [19,20]. In the XENDOS trial, 3305 obese subjects at risk for T2DM were randomized to therapeutic lifestyle change or therapeutic lifestyle change plus orlistat 120 mg three times daily over a study period of 4 years. The subset of subjects with impaired glucose tolerance at entry receiving orlistat enjoyed a 37% relative risk reduction in development of T2DM at study end. Favorable lipid effects were also seen.
In obese subjects with T2DM failing metformin monotherapy [19], the addition of orlistat to the treatment plan lowered HbA1c by 0.35% (4 mmol/mol) and improved lipid as well as blood pressure control. Similarly, benefits were also seen in insulin-treated patients given orlistat for 1 year [20] with improved glycemia (HbA1c –0.62 ± 0.08 for orlistat-treated vs –0.27 ± 0.08% given placebo; P < 0.002, 7 vs 3 mmol/mol) and improved lipids despite lowered diabetes medicine doses.
Sibutramine enhances satiety and diminishes appetite primarily through central pathways, involving the inhibition of synaptic reuptake of norepinephrine, serotonin and to a lesser extent dopamine.* Doses approved for use in the management of obesity range 5–15 mg, with greatest efficacy at the cost of highest frequency of side effects seen at the 15 mg dosage. Doses up to 20 mg have been used in the clinical trial setting with a similar observation. A recent meta-analysis studied eight of 30 available clinical trials using sibutramine in the management of diabetes [21]. The authors included studies using sibutramine in combinations with sulfonylureas, metformin or a hypo-caloric diet. In general, treatment effects were associated with a 5.5-kg loss in body weight versus an approximate 1-kg weight gain in comparator groups. On average, HbA1c improved 0.28% (3 mmol/mol) with variability among the different studies included. Additional positive effects were noted in reducing triglycerides and raising high density lipoprotein (HDL) without any adverse effects on blood pressure. Small increases in heart rate were noted. The presence of hypertension, especially if not ideally controlled, should be considered a contraindication to use of sibutramine, especially given how sensitive patients with diabetes are to the adverse impact of modest blood pressure changes and the effects of blood pressure upon diabetes complications.
Rimonabant, an endocannabinoid CB1 receptor blocker, has recently been removed from the European market because of concerns over exacerbating mood disorders (depression). Ongoing clinical trials using rimonabant have also been halted, raising questions over both the utility and availability of drugs acting on the endocannabinoid system. Despite its removal from studies and failure to stay widely marketed, there are studies finding a substantial antihyperglycemia effect similar to that of some approved diabetes pills. Such work validates the notion that treatment directed toward obesity have glycemic effects that are similar to some of the already approved pharmacotherapy choices for diabetes. Safety concerns regarding depression appear unlikely to allow this type of medication to be marketed in the future.
Bariatric surgery for appropriate patients (typically BMI >40 kg/m2 or >35 kg/m2 with co-morbidities) who have failed successful lifestyle interventions and/or pharmacotherapy for obesity is an increasingly utilized option. Improved safety and standardization combined with expanding data for the surgical management of obesity with favorable effects including longstanding remissions of T2DM in most patients make this an increasingly employed procedure [22,23]. The physiology behind such benefits remains poorly understood. Paradigms for identifying patients best suited for surgical treatment of cardiometabolic risk are evolving. Typically, many patients who are candidates for bariatric surgery are already on a combination of therapies. Following surgery, most patients who have been on insulin will be able to stop unless there is a very long history of diabetes. During the hypocaloric diet employed in preparation for such surgery, significant decreases in the need for insulin and sometimes other diabetes medications occur. For those who undergo Roux-en-Y procedures it is common to have a rapid reduction of insulin requirements within days to weeks. Decreasing insulin dosage by half or more is frequently necessary to avoid hypoglycemia.
Combination pharmacotherapy for T2DM
Therapies for T2DM can be divided into drugs facilitating supply of endogenous insulin (secretagogues, incretins) or those enhancing insulin actions (biguanides, thiazolidinediones, incretins, α-glucosidase inhibitors, amylin analogs, colesevelam). Successful treatment of T2DM combines approaches to lifestyle modification frequently in conjunction with use of pharmacologic therapy.
We conclude from data found in the UKPDS that insulin secretory loss starts on average 10 years before the formal diagnosis of T2DM, followed by insulin deficiency on average 10–12 years after diagnosis [8]. The dual aspects of T2DM, insulin resistance and insulin deficiency, are important factors in therapy selection and the subsequent response to therapy. This chapter provides an update on combinations of therapies for T2DM, presents evidence and opinions on sequences of medications, reviews new insights into diabetes and obesity treatments, and briefly describes some new medications emerging for the treatment of T2DM.
Need for combination therapy
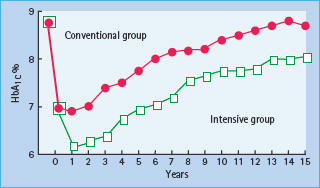
The management of T2DM, as with any chronic illness, requires individual assessment and involvement of patient with their care plans. Perhaps the original “combination therapy” for T2DM remains advice on flexible approaches to healthy eating styles coupled with encouragement and support to improve physical activity with consideration of the use of early pharmacologic treatment interventions in most patients. Most patients with T2DM eventually require combination pharmacologic therapy; some at the time of initial diagnosis, especially if they have a markedly elevated HbA1c. Data from the UKPDS [5] showed that about 50% of patients required combination therapy within 3 years of diagnosis, and approximately 75% at 9 years after diagnosis (Figure 31.2).
Figure 31.2 This figure, which is adapted from the UK Prospective Diabetes Study comparing conventional policy to intensive policy with sulfonylureas or insulin, shows the progressive rise of median HbA1c that presumably is related to the progressive insulin secretory defect present in type 2 diabetes mellitus. A noteworthy aspect of this figure is the decline in HbA1c from nearly 9% (75 mmol/mol) to around 7% (53 mmol/mol) in the first 3 months after initial evaluation. During this period, subjects in this study had visits with educators and dietitians helping them to improve lifestyle.
Choice of initial therapy
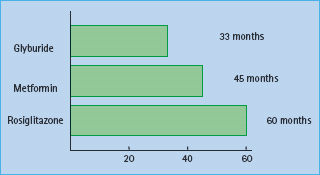
No therapy has rigorously been proven to alter the natural history of progressive β-cell decline and the ultimate need for combination treatment. Nonetheless, randomization to rosiglitazone in the ADOPT trial [24] was associated with longer monotherapy success for a new diagnosis of T2DM (60 months) versus metformin (45 months) or glyburide (glibenclamide) (33 months). The data from this study (Figure 31.3) found that at the 4-year evaluation, 40% of the 1456 patients in the rosiglitazone group had a glycated hemoglobin level of less than 7% (<53 mmol/mol), compared with 36% of the 1454 patients in the metformin group (P = 0.03) and 26% of the 1441 patients in the glyburide group (P < 0.001). Thus, even with some superior durability of treatment-related glycemic response, it is clear that most patients with T2DM will require combination therapy within the first 3–6 years after medication is begun.
Figure 31.3 The ADOPT study examined the duration of maintenance of fasting glycemia in patients with diabetes on three monotherapies: glyburide (glibenclamide), metformin and rosiglitazone. As shown in this figure, rosiglitazone was superior to metformin which was in turn superior to glyburide in efficacy of maintenance of HbA1c. Nonetheless, at 4 years most patients in each of the three groups did not maintain their HbA1c goals and needed to progress to combination therapy. The analysis shown reflects time until mean HbA1c within treatment group exceeds 7% (>53 mmol/mol) based on a repeated measures mixed model. Reproduced from Kahn et al. N Engl J Med 2006; 355:2427–2443.
Rationale for combination therapy
In the context of at least two major physiologic defects in T2DM, insulin resistance and progressive insulin secretory failure, combining treatments with complementary actions can be a logical approach. Several distinct advantages of combining agents can be distinguished.
Efficacy
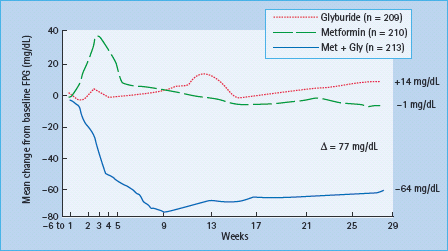
The first rationale for combination therapy (either with oral agents alone, with oral agents and insulin or oral agents with other injectable medicine such as incretin mimetics) is its superior efficacy. One principle that emerges from randomized controlled trials of antidiabetic therapies is that switching from one medication to another does not work as well as adding on or combining therapies. Figure 31.4 shows a classic study of combination oral agent therapy that illustrates this point [25]. Patients with inadequate glycemic control on maximal doses of glyburide were randomized to continuation of that monotherapy, to metformin monotherapy gradually titrated to maximal doses (850 mg orally three times a day), or to a combination of glyburide and metformin. Neither monotherapy resulted in any significant improvement in fasting plasma glucose (FPG), but combination therapy with an insulin secretagogue and metformin showed a dramatic improvement. Similarly, studies with other combination therapies showed no benefit of switching to a new agent class, but greater glucose-lowering efficacy through combining it with an agent with a different mechanism. A final benefit of combination treatment may add efficacy by using some agents that treat preprandial hyperglycemia and others that treat post- prandial hyperglycemia. As with basal and bolus insulin, it may be important to use both approaches in balance.
Figure 31.4 As shown by DeFronzo et al. [25] in subjects failing maximal dose glyburide (dotted line), continuation of the “failing” secretagogue led to gradually worse hyperglycemia. Likewise, a switch from glyburide to metformin (dashed line) showed little glycemic improvement. A worsening of hyperglycemia with increased fasting plasma glucose (FPG) was seen in the metformin group after glyburide had been stopped and before full dose titration of metformin had occurred. This study exemplifies the general principle that switching makes little sense as the combination of therapy (solid line) substantially improves FPG which is not observed with either monotherapy.
Tolerability and convenience
Most side effects of glycemic medications are dose-related. For example, hypoglycemia is a side effect of insulin or insulin secre-tagogues, gastrointestinal side effects are common with metformin, incretin mimetics and α-glucosidase inhibitors, and fluid retention or weight gain may occur with the thiazolidinediones. In contrast to some of the adverse effects of diabetes agents noted above, the efficacy of oral agents in glucose lowering is often not linear with increasing dose. Thus, with dose escalation one may increase undesirable side effects while gaining little in efficacy. One option is to utilize lower doses of two complementary medications, which can minimize side effects while achieving equal or better glycemic control. This principle has been tested directly for the combination of glyburide and metformin [25].
Using combinations of oral agents may seem more complex than monotherapy, but in some cases their convenience can be enhanced. Combining a single dose of a long-acting sulfonylurea, such as glimepiride, with one or two tablets of metformin, may have greater benefit than three or four tablets of metformin alone. Metformin–secretagogue (metformin–glyburide, metformin–glipizide, metformin–repaglinide) combination pills, have been introduced. Similarly, metformin–thiazolidinedione (metformin–rosiglitazone, metformin–pioglitazone) combination pills are also available. Recently, thiazolidinediones–secretagogue (rosiglitazone–glimepiride, pioglitazone–glimepiride) combination pills have become available. Some newer agents such as sit-agliptin have been approved for use when combined with metformin and are also available as a combination pill.
The trend to use combination pills will likely increase in the future. Formulations of two agents in a single pill with dual actions may appeal to many patients and practitioners. While separate titration of agents may be desirable for many patients, for others a case can be made for combination preparations. Side effects are often characteristic of a particular agent (e.g. sulfonylureas with hypoglycemia, metformin with gastrointestinal side effects, glitazones with fluid retention) and thus can be commonly attributed correctly to the specific agent in a combination pill. Occasionally, rarer or idiosyncratic side effects could be less readily attributed to the specific agent. This tactic may prove especially attractive for patients who must take not only two or more agents for glycemic control, but also other medications for manage blood pressure, lipid abnormalities, heart disease and other problems.
Secretagogues
Whether rapid or longer-acting, secretagogues work well with both metformin [25] and thiazolidinediones [26]. Their use with basal insulin therapy, such as evening NPH or glargine [27] is also evidence-based. Use of secretagogues with a mixture of evening basal plus mealtime insulin reduces insulin requirements and prevents interim hyperglycemia during insulin dose titration [28], however, their use with two or more mealtime insulin doses is considered superfluous, and might increase the risk of hypoglycemia. Insulin secretagogues work by binding to sulfonylurea receptors (SUR), which in the pancreas are of the SUR1A subtype. SUR are linked to potassium inward rectifier (Kir 6.2) channels. When Kir channels are closed by secretagogue binding to SUR, calcium channels are opened and insulin is released. The Diabetes mellitus, Insulin Glucose infusion in Acute Myocardial Infarction 1 (DIGAMI-1) study [29] showed that, compared with usual care, insulin treatment reduced mortality from acute myocardial infarction in diabetes mellitus; thus, it seems reasonable to avoid secretagogues, particularly glyburide, in patients with active ischemic heart disease (acute coronary syndromes or the presence of stable angina). DIGAMI-2 [30] did not confirm the mortality differential found in DIGAMI-1. The DIGAMI-2 study was limited by the inability to achieve targeted glycemic goals (5–7 mmol/L) and a small separation of glycemic control between treatment groups. Nevertheless, the investigators confirmed the epidemiologic relationship between hyperglycemia and cardiac mortality. Taken together, this information suggests cardiac ischemia with hyperglycemia may still be best managed with insulin.
The primary side effect of secretagogues, used alone or in combination, is hypoglycemia. Avoidance of hypoglycemia, particularly in older patients or patients with cardiovascular disease as recently highlighted by the ACCORD trial [12], restricts overuse of this class. For a few patients hypoglycemia occurs overnight. Chlorpropamide and glyburide are the secretagogues most likely to cause hypoglycemia although it may occur with any of the sulfonylureas. Nonetheless, daytime hypoglycemia, most commonly in the mid afternoon, with once daily morning dosing is a more common timing of hypoglycemia. It should lead to advice not to skip or delay lunch and may occasionally require a snack when patients are physically active in the middle of the day.
Loss of early prandial insulin release is thought to be an early event in the development of T2DM [31]. Epidemiologic studies suggest that post-prandial hyperglycemia or impaired glucose tolerance independently predicts risk for cardiovascular disease in patients with diabetes mellitus and normal fasting glycemia [32,33]. Several antidiabetes agents specifically target post-prandial hyperglycemia. These agents include the rapid-acting insulin analogs aspart, lispro and glulisine; α-glucosidase inhibitors; rapid-acting insulin secretagogues and incretins. Table 31.1 lists commonly used medication, their efficacy and their preprandial and post-prandial effects and side effects of different agents.
Table 31.1 Diabetes drugs with efficacy, preprandial and post-prandial effects and side effects.
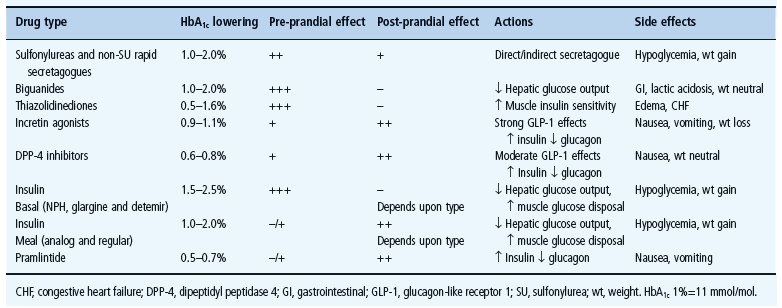
Rapid-acting secretagogues (glinides)
The meglitinide repaglinide and the phenylalanine derivative nateglinide have theoretical advantages, with rapid prandial insulin release potentially mimicking normal physiology. Some facilitation of prandial insulin release, however, does occur with long-acting secretagogues. The differential effect on early insulin secretion with rapid-acting secretagogues compared to sulfonylureas has not been consistently seen in all studies. Carroll et al. [34] failed to identify a substantial and consistent prandial glycemic benefit of rapid-acting secretagogues in comparison to extended-or immediate-release glipizide. Rapid-acting secretagogues are considered most appropriate for sulfonylurea-intolerant patients, patients with erratic food intake and patients in whom there is a demonstrated individual benefit. The absence of data showing improved clinical outcome, greater cost, more frequent dosing and no superiority in HbA1c reduction limits enthusiasm for their wider use. Moreover, the rapid kinetics of nateglinide may explain its somewhat reduced overall antihyperglycemic efficacy. Secretagogues will retain their role in restoring insulin secretory deficits, especially in leaner patients, alone and in combination.
Biguanides
The antihyperglycemic effect of metformin is largely brought about by suppression of hepatic glucose release, thus reducing insulin resistance in the liver. Metformin may accomplish this by activation of the enzyme adenosine 5’-monophosphate-activated protein kinase (AMPK), which acts as a “fuel sensor.” Although not approved by the FDA for the treatment of the metabolic syndrome or intermediate hyperglycemia, some clinicians have taken advantage of the insights from the Diabetes Prevention Program (DPP) [35]; in patients with impaired glucose tolerance, there was a delay in the onset of T2DM through the use of intensive lifestyle modification (58% reduction), metformin (31% reduction) and troglitazone (early DPP data suggest a benefit, but this study arm was discontinued because of the hepatotoxicity observed). Thus, in patients with pre-diabetes or the metabolic syndrome, metformin is sometimes used in combination with intensive lifestyle modification for prevention of T2DM.
Metformin is usually the preferred initial treatment selection in most T2DM. Data, again from the UKPDS, demonstrate that metformin as monotherapy in overweight patients reduced mortality, myocardial infarction and weight gain [9]. Long-term follow-up from the UKPDS suggests this cardiovascular and mortality benefit persists [11]. The use of metformin in combination with secretagogues in T2DM is very common and two combination pills are available. One formulation combines metformin with glyburide, and the other combines metformin with glipizide. Combination medications are also available for metformin with thiazolidinediones, repaglinide and sitagliptin. The combination medications do not include the extended-release versions of metformin, and this may limit gastrointestinal tolerance for a few patients.
Thiazolidinediones
Thiazolidinediones (TZDs) are insulin-sensitizing agents that act as ligands of the nuclear transcription factor peroxisome proliferator-activated receptor γ (PPAR-γ) [36]. In so doing, they improve not only glycemia but may ameliorate dyslipidemia, inflammation and hypercoagulability associated with insulin resistance [37]. As a result, there is particular interest in their potential cardiovascular benefits in T2DM. The pleiotropic effects of TZDs beyond glycemic control lie in the role of their ligand, PPAR-γ, on lipid metabolism and inflammatory pathways. PPARs are members of a nuclear receptor superfamily that regulates gene expression in response to ligand binding. There are three known PPARs: α, δ (sometimes called β) and γ. PPAR-α is found in the liver, the heart, muscle and vascular walls, and binds fibrates, with subsequent free fatty acid oxidation, reduced triglycerides, improved high-density lipoprotein (HDL) cholesterol and a decrease in inflammation [37]. PPAR-γ is most abundant in adipose tissue, but also is in β-cells of pancreatic islets, vascular endothelium and macrophages. It regulates gene expression, influencing adipocyte differentiation, fatty acid uptake and storage, and glucose uptake [36,37].
Rosiglitazone and pioglitazone are both FDA approved for monotherapy and in combination with metformin and sulfonylureas [38–41]. Because of concerns about cardiovascular risk, rosiglitazone is no longer recommended with insulin or nitrate therapy. Pioglitazone is approved for use with insulin. A TZD predecessor, troglitazone, was FDA approved in 1997 but withdrawn in 2000 because of reports of fatal hepatotoxicity, which is not seen with current TZDs. In a meta-analysis of 13 randomized clinical trials, less than 0.3% of patients on pioglitazone or rosiglitazone had alanine aminotransferase (ALT) levels greater than three times the upper limit of normal, compared to nearly 2% of patients on troglitazone [42]. Nonetheless, it is recommended that liver function tests be performed prior to initiation of therapy and periodically thereafter. Of interest, recent reports suggest that TZD therapy may improve elevated transaminase values and even liver histology in patients with T2DM and non-alcoholic fatty liver disease or non-alcoholic steatohepatitis [43].
Both pioglitazone and rosiglitazone effectively lower HbA1c, up to about 1–1.5% (11–16 mmol/mol), with maximum doses in monotherapy [44] but less on average. Glycemic control is improved on average more than with nateglinide or α-glucosidase inhibitors, but less than with sulfonylureas or metformin in unselected patients, partly because of a heterogeneous response; some patients have a small response, but others have a robust response. When TZDs are added to sulfonylureas, metformin or insulin, an additional 0.8–1.5% (9–16 mmol/mol) HbA1c decline can generally be achieved [45]. A combination form of metformin and rosiglitazone is currently available. A combination pill of pioglitazone and metformin is now marketed. As with the sulfonylurea combination pills, the immediate-release form of metformin is used in these formulations. For very insulin-resistant patients (usually with high BMI, marked central obesity and hypertriglyceridemia) with preserved insulin secretion (shorter duration of diabetes), use of metformin with a TZD can be very effective. These same characteristics probably identify good TZD monotherapy responders. Combination pills of metformin and rosiglitazone or pioglitazone may be preferable for some patients because there are fewer pills to take or there is lower co-payment from insurance in some countries. TZD-secretagogue combinations are effective in insulin-resistant patients with moderate insulin secretory reserve. Combination pills are available, both for pioglitazone (with glimepiride) and rosiglitazone (with glimepiride).
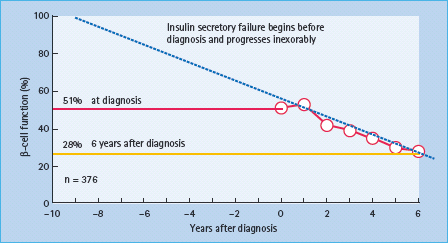
As described above, the ADOPT (A Diabetes Outcome Progression Trial) trial (Figure 31.3) [24] assessed the durability of glycemic efficacy of glyburide, metformin and rosiglitazone as a monotherapy. It used as a primary endpoint the ability to maintain fasting plasma glucose less than 180 mg/dL. Secondary outcomes included time from randomization to FPG >140 mg/ dL. Other prespecified outcomes included levels of FPG and glycated hemoglobin, weight and measures of insulin sensitivity and β-cell function as determined by homeostasis model assessment using the HOMA 2 method (Figure 31.5). The primary study endpoint, the proportion of those who failed to keep the FPG less than 180 mg/dL was achieved by 143 patients on rosiglitazone (2.9 per 100 patient-years), 207 on metformin (4.3 per 100 patient-years) and 311 on glyburide (7.5 per 100 patient-years). The percentages of patients HbA1c achieving <7.0% (<53 mmol/ mol) while on assigned therapy at study end were 26% for glyburide, 36% for metformin and 40% for rosiglitazone. Weight gain was greater with rosiglitazone than glyburide (2.5 kg) and greater still than metformin (6.9 kg) while hypoglycemia was more common with glyburide and gastrointestinal side effects with metformin as would be expected. Cardiovascular events were reduced with glyburide but not with the other two therapies. Overall, most editorialists have concluded that glitazones remain in the balance less attractive as initial therapy than metformin [46].
Figure 31.5 Based upon a homeostasis (HOMA) model, residual maximal insulin secretory reserve is depicted at the time of diagnosis and yearly for 6 years in subjects receiving diet only in the UK Prospective Diabetes Study. This figure illustrates that nearly half of insulin secretion is lost at diagnosis. It also shows the progressive loss of insulin secretory reserve, which predicts essentially complete insulin deficiency in about a dozen years if further loss were linear.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







