Fig. 12.1
Burkitt lymphoma. (a) In this example of a Burkitt lymphoma arising in the gastric wall of an HIV-positive patient, the tumor cells are slightly larger and more pleomorphic than in conventional BL (HE ×400). (b) EBER in situ hybridization discloses infection of the tumor cells with Epstein-Barr virus (EBER ×100)
Diffuse large B–cell lymphoma (DLBCL) accounts for roughly 25–30 % of malignant lymphomas in HIV-positive patients. In a certain contrast to DLBCL arising in immunocompetent individuals, HIV+ DLBCL frequently occurs at extranodal sites such as the CNS, the GI tract, and others. Histologically, DLBCL occurring in the setting of HIV resembles its counterparts in non-immunocompromised individuals ranging from centroblastic to immunoblastic types (Fig. 12.2a and b). IB lymphomas make up 20 % of HIV-associated DLBCL. There may be a higher frequency of cases with pleomorphic and HRS-like giant cells in this setting (Raphael et al. 2008). EBV infection of the tumor cells can be recognized in 30 % of cases, with 80 % of immunoblastic lymphomas harboring clonal EBV genomes (Subar et al. 1988; Ballerini et al. 1992; Shibata et al. 1993).
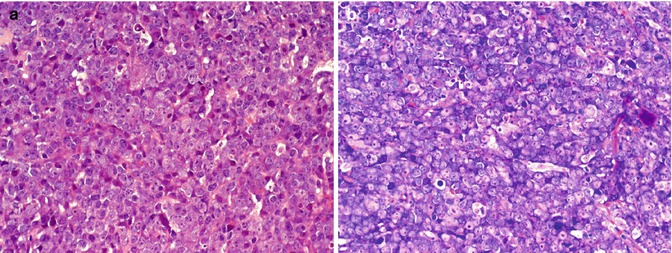

Fig. 12.2
(EBV-negative) DLBCL with immunoblastic features. (a and b) An EBV-negative DLBCL was diagnosed in a lymph node of a HIV+ patient. Giemsa staining discloses immunoblastic (-plasmablastic) differentiation of the tumor cells (b) (Giemsa ×400)
Classical Hodgkin lymphoma is the most common type of non-AIDS-defining lymphoma occurring in HIV-infected individuals (Spina et al. 2000). Generally speaking, the disease presents more aggressively, with frequently higher clinical stages. Bone marrow infiltration is observed in 50 % of cases and, of note, can be present as the sole site of disease manifestation. Histologically, mixed cellularity and lymphocyte-depleted subtypes prevail, often with higher HRS cell numbers. HRS cells in HIV+ HL, consistently, are EBV infected. In contrast to HL occurring in immunocompetent individuals, the reactive background of the tumor cells is depleted of CD4 cells and enriched for CD8+ T cells (Said 2011).
Plasmablastic lymphoma (PBL) has been initially described as a characteristic HIV-related tumor occurring in the oral cavity (Delecluse et al. 1997). In HIV-infected individuals, there is a tendency of these neoplasias to manifest in the oropharyngeal region, although they have been described in a large number of other (primarily extranodal) sites. Morphologically, they are indistinguishable from PBL occurring in immunocompetent individuals (Teruya-Feldstein et al. 2004). Plasmablastic lymphomas display a cellular spectrum from immunoblasts to plasmablasts and by definition, do not express pan B-cell antigens. In contrast, they are usually, but not always, positive for plasma cell markers such as CD138 and IRF4/MUM1, with clonal light-chain expression present in some cases. Most cases are EBV associated, but LMP-1 and EBNA-2 may not be expressed requiring EBER in situ hybridization for its unequivocal demonstration.
Other lymphoma types that have been associated with HIV infection, albeit occurring in smaller numbers, are extranodal marginal zone lymphoma of MALT type frequently occurring in the lungs of HIV+ children, plasma cell myeloma, and mature T/NK cell lymphoma. T/NK cell lymphomas occurring in the setting of HIV infection are uncommon but are increasingly encountered in HIV+-infected individuals. Most tumors are large-cell peripheral T cell lymphomas, NOS, but other lymphoma types such as Mycosis fungoides and anaplastic large-cell lymphomas (ALK+ and −) have been described (Said 2011).
In contrast to the aforementioned tumor types, primary effusion lymphoma, extracavitary HHV8+ lymphoma, and AIDS–related polymorphic lymphoproliferative disorders occur exclusively in the setting of HIV infection.
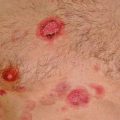
Primary effusion lymphoma (PEL) is a distinct tumor entity of the WHO classification invariably associated with immunodeficiency states, albeit of various nature (Said and Cesarman 2008). Most cases occur in HIV+ male homosexuals. The clinical hallmark of the disease is a malignant effusion of large blastic B cells in the pleura, the pericardium, or the peritoneal cavity (Fig. 12.3a). Extension into adjacent sites may occur, mostly with advanced disease. Human Herpes virus 8/Kaposi sarcoma virus (HHV8) infection is present in all cases and as such, a defining disease feature. HHV8 infection of the tumor cells can be demonstrated using immunohistochemistry (Fig. 12.3c). One third of patients will also have Kaposi sarcoma. Immunohistochemistry of PEL is characterized by the absence of B-cell antigen expression and the reactivity for plasma cell markers. Because of the occurrence of particular phenotypes such as CD30 and aberrant T cell marker expression, the clear-cut demonstration of HHV8 infection via immunohistochemistry is important. In the setting of HIV, in addition, most cases are EBV associated (Fig. 12.3d).
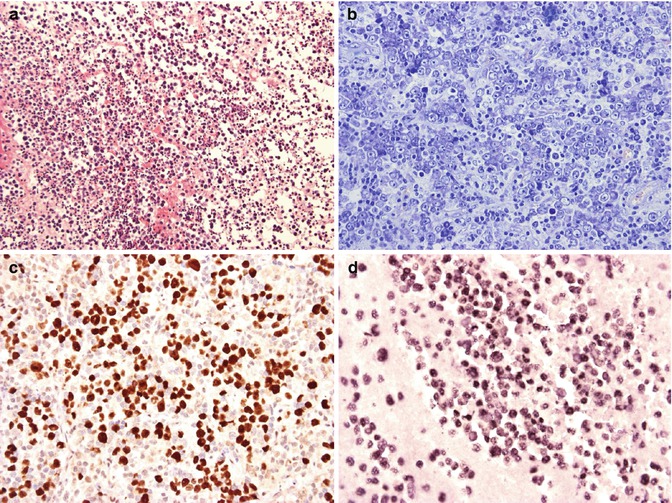

Fig. 12.3
HHV8-associated primary effusion lymphoma. (a and b) A cell block preparation from pleural fluid shows numerous large pleomorphic blasts (a, HE ×400). In this extracavitary HHV8+ solid variant, the immunoblastic/plasmablastic appearance of the tumor cells is well appreciated (b, Giemsa ×400). Latent HHV8 infection is demonstrated in this case using immunohistochemical detection of the LANA/HHV8 nuclear antigen (c, LANA ×400). In situ hybridization of PEL discloses infection of the tumor cells with EBV (d, EBER ×400)
Cytologically, most PEL demonstrate immunoblastic/plasmablastic or anaplastic morphology (Fig. 12.3b), with some cases harboring more pleomorphic and HRS-like cells. Extracavitary HHV8+ lymphoma is a solid variant of PEL presenting as a localized tumor in the GI tract, the lungs, the skin, or the lymph nodes, among others, in the absence of effusions(Chadburn et al. 2004). In contrast to PEL, up to 25 % of cases have been reported to express B-cell associated antigens but most are reflecting terminal B-cell differentiation, as do PEL. As with PEL, there is coinfection of the tumor cells with HHV8 and EBV (Fig. 12.3b and c).
12.4 Molecular Genetics
Immunoglobulin heavy-chain and/or light-chain rearrangement can be demonstrated in most cases of HIV+ lymphomas. Rearrangements for BCL6 and MYC are described in a minor fraction of DLBCL. In AIDS-associated Burkitt lymphoma, the structure of MYC rearrangements is similar to its non-HIV counterpart. In addition, TP53 mutations are relatively common (Ballerini et al. 1993). In contrast, PEL are negative for BCL2, BCL6, and MYC rearrangements, and no mutations in RAS or TP53 have been detected. Aberrant TCR rearrangements can be found in PEL (Raphael et al. 2008).
12.5 Differential Diagnosis
For the pathologist, the single most important information to be provided to is that the patient has HIV infection, because many morphological features in BL and DLBCL are shared by the tumor infiltrates in immunodeficient as well as in immunocompetent settings. BL in immunocompromised individuals may be more pleomorphic or demonstrate plasmacytoid differentiation. EBV infection in AIDS-associated DLBCL may be suspected by greater cellular pleomorphism, angioinvasive and angiodestructive growth, and – at times extensive – necrosis. In general, plasmablastic morphology in DLBCL should prompt examination of EBV examination, and DLBCL diagnosis in an effusion should prompt analysis for HHV8 LANA protein expression.
12.6 Evaluation
Patients should have a comprehensive medical history with attention paid to signs and symptoms of lymphoma and a detailed HIV history including prior opportunistic infections and history of HIV resistance, immune function, HIV viral control, and antiretroviral treatment. The physical examination should include a careful assessment of lymph node regions, the liver, and spleen. Relevant laboratory studies include a complete blood count, chemistry profile with lactate dehydrogenase (LDH) and uric acid levels, CD4 cell count, and HIV viral load. HIV and hepatitis B and C serologies should be assessed. A bone marrow aspirate and biopsy should be performed at initial diagnosis as involvement by lymphoma is found in up to 20 % of cases. Patients with aggressive B-cell lymphomas should have a lumbar puncture for analysis of cerebrospinal fluid by flow cytometry and cytology to check for leptomeningeal lymphoma (Hegde et al. 2005).
Imaging studies should include computed tomography (CT) scanning of the chest, abdomen, and pelvis. Radiographic evaluation of the head should also be performed preferably by magnetic resonance imaging (MRI). Fluorodeoxyglucose positron emission tomography (FDG-PET) is useful in HIV-negative aggressive lymphomas, but its role in HIV-associated lymphomas is very poorly studied at this point in time. One of the greatest limitations in using PET is that interpretation can be confounded by inflammation from HIV-associated nodal reactive hyperplasia, lipodystrophy, and infections (Dunleavy et al. 2010a, b). Prior experience evaluating FDG-PET in HIV-associated lymphoma is limited to small retrospective series where most scans were not predictive of remission.
12.7 Prognostic Factors
The International Prognostic Index (IPI) is the standard prognostic assessment tool in HIV-negative DLBCL. Its applicability to HIV-associated DLBCL, however, is controversial. While in some studies using CHOP or R-CHOP, the IPI score has divided groups prognostically, this has not been the case with DA-EPOCH and in a recent study of short-course EPOCH-R (infusional etoposide, vincristine, and doxorubicin with prednisone, cyclophosphamide, and rituximab) in newly diagnosed HIV-associated DLBCL, the IPI did not predict PFS or OS (Dunleavy et al. 2010a; Ribera et al. 2008; Kaplan et al. 2005). The prognostic importance of CD4 cell count and immune function in HIV-associated DLBCL, neither of which are part of the IPI, are the most likely confounding variables. Patients with CD4 counts less than 100 cells/μL are at increased risk of serious opportunistic infections and death. Furthermore, as noted earlier, patients with severe immune suppression have a higher incidence of immunoblastic subtypes, most of which are of ABC derivation, and a poor outcome compared to patients with preserved immunity, where the GCB subtype is more common (Dunleavy et al. 2010a). Although a recently reported study from the AIDS Malignancy Consortium (AMC) did not find an association between the cell of origin and outcome in HIV-associated DLBCL, their analysis was retrospective and included patients treated with a variety of different regimens (Sparano et al. 2010; Chadburn et al. 2009; Dunleavy and Wilson 2010). Involvement of the CNS, which is increased in HIV-associated aggressive B-cell lymphomas, also confers an adverse prognosis.
12.8 Treatment of HIV-Associated Lymphomas
The treatment of HIV-associated lymphoma has evolved over the last 30 years in line with improved control of HIV replication and preservation of immune function (Table 12.1).
Table 12.1
Pivotal trials in human immunodeficiency virus (HIV)-associated lymphomas
Study | Study type | Study design | Results |
|---|---|---|---|
Kaplan et al. (1997) | Prospective multicenter randomized phase III (n = 192) | Randomization to standard-dose m-BACOD with GM-CSF versus low-dose m-BACOD without GM-CSF. No cART | Similar efficacy of both regimens but less hematological toxicity with low-dose m-BACOD |
Ratner et al. (2001) | Prospective multicenter sequential phase II (n = 65) | First 40 patients received modified-dose (m) CHOP (50 % cyclophosphamide and doxorubicin) and the next 25 patients received standard-dose CHOP. cART was administered | CR higher with full dose CHOP compared to mCHOP (48 % vs. 30 %). Authors concluded that concomitant cART was safe but unable to conclude superiority of one regimen over another |
Sparano et al. (2004) | Prospective multicenter sequential phase II (n = 98) | First 43 patients received didanosine and the next 55 patients received cART with CDE | At 2 years, FFS and OS were 36 % and 43 %. Patients receiving concomitant cART had better survival and less toxicity |
Mounier et al. (2006) | Prospective multicenter phase III study | 485 patients were randomly assigned to different CHOP-based chemotherapy regimens according to an HIV score that was based on performance status, prior AIDS and CD4 count | Though HIV score, IPI score and cART affected survival, the intensity of CHOP-based chemotherapy had no effect on survival |
Little et al. (2003) | Prospective single center phase II (n = 39) | All patients received EPOCH and G-CSF with cART suspension | CR was 74 %. At 53 months, DFS and OS were 92 and 60 %. Patients in CR achieved CD4 recovery and HIV control following treatment. Conclusion that EPOCH with cART suspension is feasible and highly effective |
Kaplan et al. (2005) | Prospective multicenter randomized phase III (n = 150) | Randomization (2:1) to R-CHOP versus CHOP with concomitant cART. Some patients received maintenance rituximab | CR rate higher with R-CHOP compared to CHOP (57.6 % vs. 47 %). Increased infectious deaths with R-CHOP mostly in patients with low CD4 counts. Conclusion that rituximab does not improve clinical outcome |
Boue et al. (2006) | Prospective multicenter phase II (n = 61) | All patients received R-CHOP | CR in 77% of patients. Estimated 2-year OS was 75% |
Spina et al. (2005) | Retrospective analysis of 3 phase II trials | Pooled results from three trials of CDE with rituximab | CR rate was 70 %. At 2 years, FFS and OS were 59 % and 64 %. Conclusion that R-CDE is effective but rituximab may increase infections |
Sparano et al. (2010) | Prospective multicenter phase II study | 101 patients were randomized to receive either concurrent or sequential rituximab with DA-EPOCH | There was a superior outcome with concurrent rituximab and DA-EPOCH (CR rate 75 %) and this was considerably better when compared to the previous ANC results with CHOP +/− R |
Dunleavy et al. (2010a) | Prospective single center phase II (n = 33) | All patients received SC-EPOCH-RR with cART suspension | 79 % of patients needed only three cycles of treatment. At 5-year follow-up, PFS and OS were 84 % and 68 %. Outcome was better for GCB versus non-GCB DLBCL (5-year PFS of 95 % versus 44 %). |
In the pre-CART era, patients with HIV-associated lymphoma had poor outcomes with median survivals of 5–6 months. Because these outcomes were driven by both chemotherapy failure and infections, investigators have examined the effect of chemotherapy dose on survival. In one study, Kaplan and colleagues observed that higher doses of cyclophosphamide were associated with lower survival, suggesting that infections were a driving cause of death in these patients (Kaplan et al. 1989). In an attempt to reduce infectious deaths, the AIDS Malignancy Consortium (AMC) conducted a study of 192 untreated lymphoma patients randomly assigned to receive standard-dose m-BACOD (methotrexate, bleomycin, doxorubicin, cyclophosphamide, vincristine, and dexamethasone) with granulocyte macrophage colony-stimulating factor (GM-CSF) support or low-dose m-BACOD without GM-CSF in an effort to reduce the toxicity of chemotherapy (Kaplan et al. 1997). Compared to full-dose therapy, reduced-dose treatment had a similar response rate (52 % versus 41 %, respectively) and median survival (6.8 versus 7.7 months, respectively) but lower hematological toxicity. This led the authors to conclude that lower dose chemotherapy was preferable in HIV-associated lymphoma. One shortcoming of the study was that although the authors controlled for the absolute CD4 cell count in the survival analysis, they did not include enough patients with high CD4 counts and, ultimately, could not support a definitive recommendation for this group where the benefit of full-dose chemotherapy on cure of the lymphoma may outweigh the infectious risks (Little et al. 2003). Importantly, before completion of this trial, a randomized multicenter study in HIV-negative aggressive lymphoma showed CHOP to be equally effective as m-BACOD and less toxic (Fisher et al. 1993). The better therapeutic index of CHOP led to its acceptance as a standard for HIV-associated lymphoma (Lim and Levine 2005).
The introduction of CART some 15 years ago has had a dramatic effect on the outcome of HIV-associated lymphomas with increases in median survival. While the reasons are multifactorial, they can be ultimately attributed to salutary effects of CART on immune function. Patients with preserved immune function have a lower risk of infectious complications, thereby enabling optimal chemotherapy administration, and as noted earlier, a more favorable tumor biology (Little et al. 2003; Fisher et al. 1993; Lim and Levine 2005; Carbone and Gloghini 2005). Interestingly, in one study that looked at risk-adapted intensive chemotherapy in 485 patients with AIDS-related lymphoma (ARL), CART was significantly associated with survival while the dose-intensity of CHOP-based therapy was not (Mounier et al. 2006).
Although the benefit of rituximab is well established in HIV-negative DLBCL, its role in HIV-associated DLBCL has been controversial (Coiffier et al. 2002). This debate stems from an AMC randomized phase III study of CHOP ± rituximab in HIV-associated aggressive lymphomas that found rituximab was associated with significantly more infectious deaths but only a trend in improved tumor control; based on this, the authors concluded that rituximab does not improve the clinical outcome of HIV-associated DLBCL (Kaplan et al. 2005). A retrospective analysis of 3 phase II trials from Italy, where patients received infusional cyclophosphamide, doxorubicin, and etoposide (CDE) with rituximab, also concluded that rituximab might increase infections (Spina et al. 2001, 2005). On closer evaluation of the AMC trial, however, the increased infectious deaths occurred primarily in patients with very low CD4 counts, and many patients received “maintenance” rituximab after chemotherapy, which has not been shown to be useful in HIV-negative DLBCL (Dunleavy et al. 2006).
Subsequent to the AMC study, a French group performed a phase II study of CHOP plus rituximab in HIV-associated NHL and the CR rate of 77 % and 2-year survival rate of 75 % suggested that rituximab was beneficial and could be given safely to this group of patients (Boue et al. 2006). To further address the controversy of rituximab, the AMC performed another randomized phase II study. At the time that this study was designed, the results of the EPOCH regimen in this population were very promising, and they randomized patients to receive concurrent versus sequential rituximab with EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and hydroxydaunorubicin) (Sparano et al. 2010; Chadburn et al. 2009; Dunleavy and Wilson 2010; Kaplan et al. 1989, 1997; Little et al. 2003). Importantly they found that concurrent rituximab was not associated with increased infectious deaths (Sparano et al. 2010; Chadburn et al. 2009; Dunleavy and Wilson 2010; Kaplan et al. 1989, 1997; Little et al. 2003). The study also examined if the CR rate with EPOCH-R was superior to CHOP ± rituximab, employing a predetermined retrospective analysis, and if concurrent versus sequential rituximab was more toxic and/or more effective. There was no difference in toxicity between the arms, and the authors rejected the null hypothesis of 50 % (associated with CHOP ± rituximab) in favor of 75 % CR for EPOCH with concurrent rituximab (p = 0.005; power 0.89) (Sparano et al. 2010). Based on this study, we consider it very unwise to omit rituximab from upfront therapy in HIV-associated lymphoma.
While one group demonstrated good efficacy with R-CHOP in a multicenter setting, it is concerning that 15 % of enrolled patients were not evaluable for response due to early events or lacking clinical and radiological evaluations (Boue et al. 2006). Though the AMC’s conclusions regarding EPOCH-R’s superiority over R-CHOP are based on a historical comparison, the dramatic differential outcome with these two regimens in a similar patient population suggests that EPOCH-R may be a superior regimen in this population. Whether or not there are subgroups of patients with HIV-associated DLBCL who may do as well with R-CHOP is unknown at this time and has not been studied prospectively.
Following on from the initial promising results with EPOCH, a second-generation EPOCH regimen termed short-course EPOCH-RR was developed (Dunleavy et al. 2010a). This approach is designed to address the dual challenge of achieving excellent tumor control while preserving immune integrity. While it was previously demonstrated that 6 cycles of DA-EPOCH is highly effective (PFS and OS of 73 and 60 % at 53 months) in HIV-associated lymphoma, with 5 years follow-up, the PFS and OS of SC-EPOCH-RR are 84 % and 68 %, respectively, and 79 % of patients only required 3 treatment cycles (Dunleavy et al. 2010a, b; Ribera et al. 2008; Sparano et al. 2010; Chadburn et al. 2009; Dunleavy and Wilson 2010; Kaplan et al. 1989, 1997, 2005; Little et al. 2003). Interestingly, with this approach, the clinical prognostic characteristics that make up the IPI and the IPI itself do not predict PFS or OS. Only tumor histogenesis is associated with lymphoma-specific outcome with 95 % of GCB versus 44 % of non-GCB DLBCL progression free at 5 years. Although, both EBV positivity of the tumor and low CD4 count at diagnosis are significantly associated with an inferior overall survival, they are not associated with lymphoma-specific outcome.
12.8.1 Should CART Be Continued During Therapy?
The risks and benefits of continuing CART during curative chemotherapy of aggressive lymphomas have been variably interpreted. While many investigators rightly raise the concern that uncontrolled HIV replication during chemotherapy will worsen immune function, they often do not consider the potentially adverse effects of CART on lymphoma-specific outcomes because they are difficult to quantify. One of the first trials to assess concurrent CART was a nonrandomized AMC study of dose-reduced and standard-dose CHOP (Ratner et al. 2001). A potentially important finding of the study comes from the pharmacokinetic (PK) analysis which showed that cyclophosphamide clearance was reduced 1.5-fold, but doxorubicin clearance was unchanged compared to historical results. While it is reassuring that the doxorubicin PK was unaffected, the reduced clearance of cyclophosphamide – an inactive prodrug – could likely result in a reduction of active metabolites and potentially compromise efficacy. In this study, CD4 counts increased significantly during therapy, and the mechanism for increased CD4 cell counts raises the concern that CART protects T cells from chemotherapy-induced cytotoxic stress, an effect that might occur in the lymphoma cells (Johnson and Parkin 1998; Phenix et al. 2000). Other groups however have suggested that CART can be safely administered with chemotherapy, and it has not been well prospectively studied and controversies abound (Sparano et al. 2004; Vaccher et al. 2001). In that respect, it is important to note that many newer antiretrovirals with fewer drug interactions (than those studied in the past) are now available.
12.9 HIV-Associated Burkitt Lymphoma
Though, following the advent of CART, there was a significant improvement in the outcome of HIV-associated DLBCL, this was not the case initially with HIV-associated Burkitt lymphoma, as reported in a retrospective series by Lim et al. (2005). This lack of improvement is likely explained by the widespread use of CHOP-based regimens, which have poor efficacy in BL (Dave et al. 2006; Bishop et al. 2000). While dose-intense regimens such as hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) and CODOX-M-IVAC, with or without rituximab, have shown encouraging results in HIV-negative BL, they have not been studied too extensively in HIV-associated BL. One of the concerns with CODOX-M-IVAC is treatment-related toxicity in this population (Barnes et al. 2011; Cortes et al. 2002). In an attempt to reduce this, the AMC recently presented the results of a feasibility and toxicity study for HIV-associated BL and atypical BL and reported good overall survival rates with 65 % of patients completing treatment as per protocol (Noy et al. 2009).
Burkitt lymphoma highlights the necessity to balance treatment efficacy and toxicity by optimizing the therapeutic index, especially in patients who are immune suppressed and/or elderly. Based on its excellent activity in highly proliferative DLBCL and its favorable toxicity profile, EPOCH-R was studied in untreated BL and was highly effective (Dunleavy et al. 2011). The AMC also included several patients with BL or Burkitt-like lymphoma in their study of concurrent versus sequential EPOCH-R and reported high response rates in this group (Sparano et al. 2010).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree