George S. Deepe Jr.
Histoplasma capsulatum (Histoplasmosis)
Histoplasma capsulatum is one of the more common causes of infection in the U.S. Midwest and Southeast. Histoplasmosis, acquired by inhalation of mycelial fragments and microconidia, is most often self-limiting but can cause potentially lethal infection in patients with preexisting conditions. It remains a frequent cause of opportunistic infection in patients whose immune system is impaired by pharmaceutic agents or by the human immunodeficiency virus (HIV). This accelerating trend is unlikely to abate because the reservoir of H. capsulatum (soil) will never disappear.
History
The discovery of H. capsulatum was made in December 1905, when Samuel Darling, a pathologist stationed in Panama, examined visceral tissues and bone marrow from a young man from Martinique whose death was originally attributed to miliary tuberculosis.1 Peering through his microscope, Darling was struck by the presence of many small bodies, most of which were intracellular. Having been influenced by reports from Leishman and Donovan, he mistakenly thought that this organism was a protozoan. Because it lacked a kinetoplast, Darling assumed that it was a different Leishmania species. He termed this new species Histoplasma capsulatum because it seemingly exhibited a capsule. It was not until 1912, after reviewing tissue specimens, that da Rocha-Lima suggested that the organism resembled a yeast rather than a protozoan.2 More than 20 years later, the organism was finally isolated on artificial medium and observed to grow as a mold at room temperature and as a yeast at 37° C.3
For many years, the presence of pulmonary calcifications had become synonymous with healed tuberculosis by physicians. Amos Christie, a pediatrician at Vanderbilt University, dispelled that dictum.4,5 The presence of cutaneous reactivity to a skin test reagent, prepared from the mycelial phase of the organism, in an infant with disseminated histoplasmosis prompted large-scale testing during the 1930s. This endeavor unearthed the surprising finding that histoplasmosis was highly prevalent in the Ohio and Mississippi River Valleys.5 Moreover, many cases of presumed tuberculosis that were based on the presence of calcified nodules on chest roentgenograms were determined to be histoplasmosis instead.6 Eventually, many individuals residing in tuberculosis sanatoriums in the midwestern and southeastern United States were recognized to have been mistakenly admitted. They suffered from histoplasmosis, not tuberculosis. Some of these individuals contracted tuberculosis while housed in open wards with patients who had active pulmonary tuberculosis.
Ecology and Epidemiology
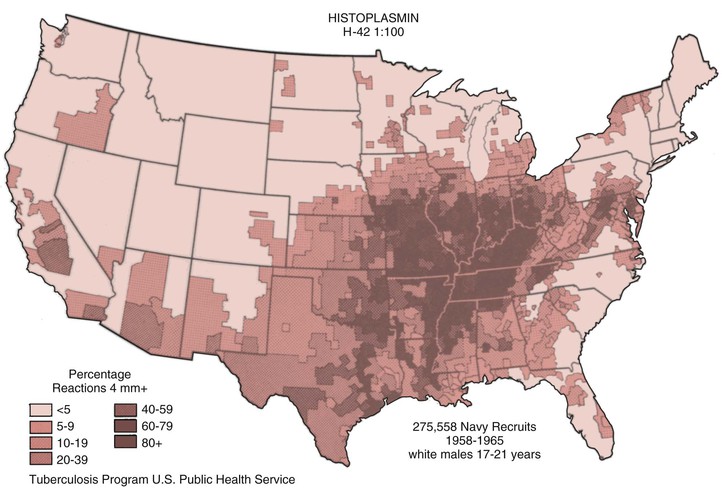
Cases of histoplasmosis have been reported from every continent except Antarctica. H. capsulatum is a soil-based fungus that has been isolated from many regions of the world and is most often associated with river valleys; as mentioned above, the most highly endemic region is the Ohio and Mississippi River Valleys (Fig. 265-1).6 The conditions that favor the growth of this fungus in soil are a mean temperature of 22° to 29° C, an annual precipitation of 35 to 50 inches, and a relative humidity of 67% to 87%. These conditions are typically found in the temperate zone between latitudes 45 degrees north to 30 degrees south.7 The organism is usually found within 20 cm of the surface, and it prefers soil that is acidic, has a high nitrogen content, and moist. In areas where avians roost, the fungus is found most often where the guano is decaying and mixed with soil.8 In such areas, infectious particles can exceed 105/g of soil. Fresh guano is less likely to contain any infectious particles. There is a strong association between the presence of bird and bat guano and the presence of H. capsulatum. In fact, the first isolation of the organism from an environmental source was from an area adjacent to a chicken house. Birds are not infected by the fungus, and attempts to isolate H. capsulatum from their cloaca have been unsuccessful. Bats, on the other hand, carry the fungus in their gastrointestinal tracts and shed it.9
Disruption of the soil by excavation or construction is one of the most common means of releasing infectious elements that are inhaled and eventually settle into the lungs. Those involved in recreational or work activities that expose them to disrupted soil are at highest risk for infection. Persons at risk include spelunkers who roam caves where bats reside and those who are engaged in agriculture, outdoor construction, or rehabilitation of buildings that have been inhabited by birds or bats. Human-to-human transmission via the pulmonary route has not been reported.
H. capsulatum contains between five to seven chromosomes. Differences in numbers of chromosomes are evident among strains. Originally, the organism could be distinguished by two chemotypes, but the advent of molecular biology has improved methods to distinguish strains of H. capsulatum. Eight clades of this fungus have been identified using molecular analysis10—two North American, two Latin American, and one each of Australian, Indonesian, Eurasian, and African clades. The spread of this fungus appears to have originated from Latin America between 3 and 13 million years ago. Interestingly, many of the isolates recovered from acquired immunodeficiency syndrome (AIDS) patients in St. Louis were found to be in clade 1, and these isolates are much less virulent in mice.11 Genetic differences can be associated with varied clinical manifestations. H. capsulatum from specific regions of South America often produce skin lesions, whereas isolates from North America do not. The findings suggest that H. capsulatum is highly diverse at the genetic level, perhaps based on the fact that the fungus undergoes sexual recombination in nature, thus allowing for exchange of genetic material.
The impact of histoplasmosis on health care costs is not precisely known because it is not a reportable disease. Estimates for the year 2002 indicate that the median hospitalization costs were $20,000 and $17,000 for children and adults, respectively. The in-hospital mortality rate was 5% for children and 8% for adults. Only 14% of adults who were hospitalized were immunosuppressed, whereas a third of children were.12 Another analysis of mortality spanning the years 1980 to 1997 demonstrated that the incidence of mortality in the United States from histoplasmosis ranged from 0.1 to 0.15 per 100,000 population.13 Earlier data indicated that disease develops in males more frequently than in females by a 4 : 1 ratio. This information was likely skewed because of the association of chronic pulmonary histoplasmosis with smoking that for many years was a male-dominated activity. But that gender bias has not been observed in recent years.
Mycology
H. capsulatum is classified as a member of the family of Ascomycetes and has a heterothallic form designated Ajellomyces capsulatum (see Chapter 257). Mating types (+) and (−) have been described and, when combined onto sporulating medium, they produce fruiting bodies containing asci. Isolates from patients carry the (−) mating type two to seven times more frequently than the (+) type, although the ratio of mating types in soil is 1 : 1.14
The organism has two morphotypes, the mycelial and yeast phases. The former is present at ambient temperature and the latter at 37° C or higher. The saprobic or mycelial phase can be divided into two colony types, brown (B) and albino (A). The A type grows more rapidly in culture and loses the capability to produce spores after prolonged subculturing. The B type generates a brown pigment. Yeast cells from the B type are more virulent in mice than those from the A type.
The basic elements of the nutritional needs of the organism are poorly defined because of the lack of a standardized medium. The organism requires vitamins, thiamine, biotin, and iron. Sulfhydryl groups in the form of cysteine or cystine are necessary for growth and maintenance of the yeast phase. The mycelial and yeast phases differ in their requirements for calcium. Chelation of this element from medium inhibits the growth of the mycelial but not the yeast phase.15
Microscopic evaluation of the mycelial phase reveals two types of conidia. Macroconidia are large ovoid bodies that span 8 to 15 µm in diameter. The surface is decorated with slender protrusions that are referred to as tuberculate. Microconidia are small, smooth oval bodies with a diameter ranging from 2 to 5 µm (Fig. 265-2). These forms are believed to be the infective phase because their size is small enough to lodge in the terminal bronchioles and alveoli.
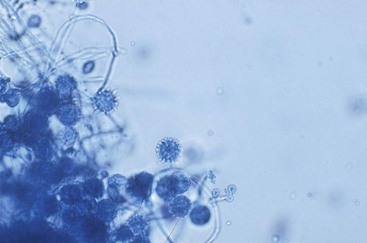
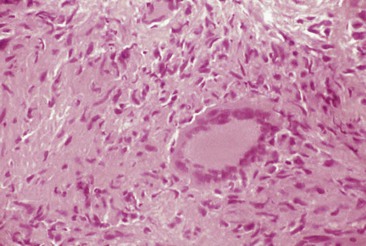
The transition from the saprobic to the yeast phase is a critical step in infectivity of the fungus. On exposure to 37° C, the organism undergoes genetic, biochemical, and physical alterations that result in the production of yeast cells that are uninucleate. These forms are small, typically 2 to 5 µm in diameter, and reproduce by multipolar budding (Fig. 265-3). The stimulus for the transition is heat, and the shift in temperature may be sensed by a change in the fluidity of the yeast membrane. Analysis of the conversion using microarrays has revealed numerous alterations in gene expression. The shift was associated with induction of genes contributing to conidiation, cell polarity, and melanin.16 Using insertional mutagenesis, a transcription factor termed Ryp1 has been found to be essential for growth of yeast cells at 37° C. In addition, two other genes, Ryp2 and Ryp3, that are crucial for the transition have been identified. Hence, there is a complex regulatory network that dictates the conversion to yeast cells upon an elevation in temperature.17 Three biochemical stages have been identified during the conversion following exposure to 37° C. Stage 1 is characterized by an uncoupling of oxidation-phosphorylation and a decrease in RNA and protein synthesis. In stage 2, no respiration is detectable, and in stage 3 there is a resumption of respiration. Chitin and α- and β-glucan content differ between the two phases.
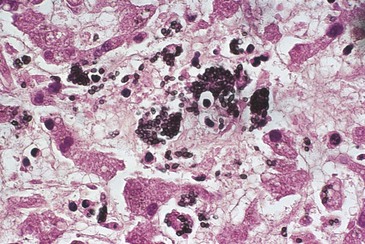
Within tissues, yeast cells may possess a morphology that differs from the usual ovoid shape. Misshapen or large yeasts have been observed in tissues and epithelial cells. These allomorphs may contain less α-1,3-glucan and appear to be less virulent in mice than oval-shaped yeasts.
Pathogenesis
The study of the pathogenesis of this fungus has accelerated as a result of technologic advances, including a transformation system to delete genes; silencing RNA; and insertional mutagenesis using Agrobacterium tumefaciens. These tools create the foundation for examining the influence of genes or gene regulators on the pathobiology of H. capsulatum.18,19
The transition from the mycelial to the yeast phase is the most critical determinant in the establishment of infection.20 This contention is supported by several findings. First, it is rare to find mycelial particles in tissues of humans or mammals with established infection. Rather, yeast cells are commonly detected. Second, exposure of H. capsulatum mycelia to p-chloromercuriphenylsulfonic acid (PCMS), a sulfhydryl inhibitor, irreversibly blocks the conversion to yeasts but does not alter growth of yeasts or mycelia. PCMS-treated mycelia fail to infect animals.
Iron and zinc are vital elements required for survival of H. capsulatum.21 The organism acquires iron from the intracellular environment by three means: release of iron scavenging siderophores, production of a ferric reductase, and modulating pH to remove iron from transferrin. α-1,3-Glucan has been found to be a key virulence factor in the pathogenesis of H. capsulatum.18 Synthesis of this carbohydrate, which is regulated by an amylase, blocks β-glucan binding to Dectin-1 and thereby suppresses generation of important proinflammatory cytokines.
After conidia settle into the alveoli, they bind to the CD11-CD18 family of integrins and are engulfed by neutrophils and macrophages.22 It is likely that the conversion of mycelia to the yeast phase transpires, at least partially if not entirely, intracellularly. The duration of the phase transition ranges from hours to days. Following transformation of conidia into yeasts in the lungs, yeasts migrate, presumably intracellularly, to local draining lymph nodes and subsequently to distant organs rich in mononuclear phagocytes (e.g., liver, spleen). The yeasts grow within resting macrophages. Activation of cellular immunity is necessary for restricting growth, and in primary infection this arm of immunity matures by 2 weeks.
Innate Immunity
In experimental pulmonary infection, neutrophils constitute one of the prominent cell populations that emigrate early into infected foci of lungs.23 These cells are capable of inhibiting the growth of yeast cells. Constituents from the azurophilic granules express fungistatic activity, and defensins also inhibit the growth of yeast cells.24 Neutrophils mount a respiratory burst in response to the fungus, but the oxygen intermediates are trapped intracellularly. Despite the burst, there is little evidence that toxic oxygen intermediates contribute to the anti-Histoplasma activity of these phagocytes. Resistance to these molecules is mediated in large part by superoxide dismutase 3, a copper/zinc-dependent enzyme.25
Macrophages and dendritic cells are the principal effector cells in host resistance to this fungus.22,26 The fate of yeast cells in each of these cell populations differs. Yeasts proliferate within resting mononuclear phagocytes, but this form is killed by dendritic cells. As noted, macrophages engulf yeast via CD11-CD18 receptors, whereas dendritic cells use the fibronectin receptor. Engagement of two disparate receptors may explain in part the different fates within these cell populations. The central importance of monocytes or macrophages, or both, in controlling H. capsulatum is highlighted by the discovery of individuals with congenital monocytopenia who present with disseminated histoplasmosis.27
In murine macrophages, a high percentage of yeast cells are located within phagolysosomes. Binding to the CD11-CD18 receptors and subsequent entry into human macrophages are mediated by heat shock protein 60 expressed on the surface of yeast.28 The fungus must contend with the adverse contents (e.g., acid proteinases) of this intensely hostile environment. A mechanism whereby yeasts survive is by alkalinization of the phagolysosome.29 Yeast cells raise the pH of the phagocytic compartment from 6 to 6.5. One reason for maintaining the pH within a narrow range is that yeast cells require iron to grow and, if the pH exceeds 6.5, they cannot acquire iron from the host.22
Nitric oxide produced by activated murine macrophages is a major mediator of anti-Histoplasma activity. The ability of this nitrogen intermediate to oxidize iron may explain its potent fungicidal activity.30 However, its influence in human infection remains unknown because human macrophages infected with H. capsulatum have not been reported to produce nitric oxide.
Macrophages from HIV-infected individuals manifest defective activity in their interaction with H. capsulatum. These cells bind fewer yeasts than cells from uninfected individuals, and a direct correlation exists between the CD4+ T-cell count and the capacity of macrophages to bind yeast cells. On entry into cells, yeasts grow more rapidly within macrophages from HIV-infected individuals or in macrophages that have been infected in vitro with a macrophage-tropic strain of HIV. The envelope glycoprotein 120 from the virus is responsible for the inhibition of binding yeasts to macrophages22 but not the altered growth characteristics of the yeasts in phagocytes.
Adaptive Immunity
Within the elements of the acquired immune response, T cells are pivotal in clearance of the fungus. Experimental studies indicate that neither B cells nor antibodies influence host resistance, although the data are limited. CD4+ cells are extremely important in controlling primary infection in mice.31 The central role of this T-cell subset in this species is supported by the finding that in HIV-infected individuals, most cases of histoplasmosis develop when the CD4+ cell count is lower than 200/µm.32 Mice deficient in CD8+ cells are impaired in their ability to reduce the fungal burden, but they can eventually eliminate the fungus.31 Vaccination of mice with yeast cells gives rise to a CD8+IL-17+ T-cell population that confers protective immunity. The outgrowth of this population provides an additional arm to adaptive immunity that can combat infection.33 In secondary infection, the absence of CD4+ or CD8+ cells diminishes the efficiency of yeast elimination, but mice survive. The loss of protective immunity only develops when both subsets are eliminated.
The primary contribution of T cells to host defense is the release of cytokines that eventually activate mononuclear phagocytes. Neutralization of endogenous interferon-γ (IFN-γ) or mice congenitally deficient in this lymphokine are exceptionally susceptible to infection.31 Other cytokines in mice that are necessary for host clearance are interleukin (IL)-12 and tumor necrosis factor-α (TNF-α). Blockade of endogenous production of either of these leads to the death of mice. The effect of IL-12 is mediated through the induction of IFN-γ.31 Interestingly, IL-12 is important in primary infection but not in reexposure histoplasmosis.31 TNF-α and IFN-γ are both necessary for controlling primary infection, and the former is required for secondary infection.31 Their importance in humans has been highlighted by the finding that TNF antagonists or individuals with defective IFN-γ signaling manifest an enhanced susceptibility to disseminated infection.34,35
In vitro, recombinant IFN-γ activates murine peritoneal macrophages to inhibit the growth of yeast cells. Macrophages from other tissue sources are either nonresponsive to this stimulus or require costimulation with lipopolysaccharide.21 The anti-Histoplasma action of IFN-γ is mediated by limiting iron acquisition, and this effect can be reversed by exposure to additional iron.29 Human macrophages, on the other hand, do not respond to human recombinant IFN-γ to inhibit yeast cell growth.22 The cytokines that activate these cells are macrophage colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, and IL-3.22 Granulocyte-macrophage colony stimulating factor appears to inhibit intracellular growth of yeast cells by limiting access to zinc in phagosomes.36 Although the infection is limited by cell-mediated immunity, tissues are not sterilized. Infected individuals contain yeasts, some of which remain viable for many years. The dormant organisms pose little risk unless the individual becomes immunosuppressed as a result of potent immunosuppressive agents used to combat various clinical conditions or immunosuppressive viruses such as HIV. The metabolic state of H. capsulatum in tissues is unknown. It is likely that some of the yeasts remain viable because individuals who have moved from endemic to nonendemic areas many years ago may have reactivated infection. The cascade of immunologic events that leads to activation of this form of the infection remains largely unknown. A murine model of reactivation histoplasmosis has been developed, and it should facilitate studies of the organism and the host in this form of infection. In mice, CD4+, CD8+, and a Thy-1.2, CD4−, CD8− cell must be eliminated to achieve progressive infection. B cells also appear to be important in the severity of reactivation disease.
Granulomas
The hallmark of the tissue response to this fungus is the development of caseating or noncaseating granulomas in which calcium may be deposited (Fig. 265-4). The granuloma consists of an admixture of mononuclear phagocytes and lymphocytes, principally T cells. The putative function of the granuloma is to contain fungal growth. Although IFN-γ and TNF-α are important in the generation of granulomas formed in response to other microbes, neutralization of these two cytokines does not prevent their formation in response to H. capsulatum. The organization of the Histoplasma granuloma has been characterized in mouse livers and lungs. CD4+ and CD8+ cells are present in the granulomas of mice. T-cell composition is polyclonal, and these cells are the source of IFN-γ and IL-17, whereas macrophages are the principal source of TNF-α.37
Organized granulomatous inflammation is typically observed in self-limited disease. Conversely, in progressive disseminated histoplasmosis, the more common histopathologic appearance of tissue is a massive influx of macrophages with scattered lymphocytes. Well-circumscribed granulomas are infrequently present, and the lack of an organized inflammatory response is indicative of a perturbed cellular immune response. Occasionally, the inflammatory response in mediastinal lymph nodes is exaggerated, resulting in excessive granuloma formation followed by fibrosis. The progressive scarring may affect the patency of the airways and major blood vessels.38
Delayed-type Hypersensitivity
In experimental infection, either cutaneous or in vitro delayed-type hypersensitivity responses to H. capsulatum antigens are detected approximately 2 weeks after exposure.31 In humans, delayed-type hypersensitivity responses are manifest within 3 to 6 weeks after exposure.39 These values are simply approximations because the precise time at which individuals are exposed in endemic areas is exceptionally difficult to determine. Reexposure to H. capsulatum in previously sensitized individuals is characterized by a more rapid tissue response. This finding is not surprising because H. capsulatum induces a memory response in which the immune system reacts in a much shorter time frame.
Infection with H. capsulatum produces a broad array of clinical and pathologic manifestations that must be recognized to diagnose and treat individuals afflicted with this fungus correctly. A summary of the clinicopathologic manifestations is depicted in Table 265-1.
TABLE 265-1
Spectrum of Histoplasma capsulatum-Induced Disease
| MANIFESTATIONS | ACUTE PULMONARY DISEASE | ACUTE CAVITARY PULMONARY DISEASE | DISSEMINATED DISEASE |
| Clinical | Often asymptomatic | Fever, productive cough, chest pain | Fever, weight loss, hepatosplenomegaly, hematologic disturbances* |
| Immunologic | |||
| Positive skin test | >90% | 70%-90% | 30%-55% |
| Lymphocyte transformation | +++ | + to +++ | ± |
| Antibody to Histoplasma capsulatum† | 25%-85%‡ | 75%-95% | 70%-90% |
| Antigenuria | 20%‡ | 40% | 60%-90% |
| Pathologic | |||
| Positive culture from lungs | <25% | 5%-70% | 50%-70% |
| Histology | Caseating and noncaseating granulomas, few yeasts, giant cells | Noncaseating granulomas, interstitial fibrosis, necrosis, yeasts, cavities, few to moderate yeasts | Diffuse macrophage proliferation, abundant few giant cells |
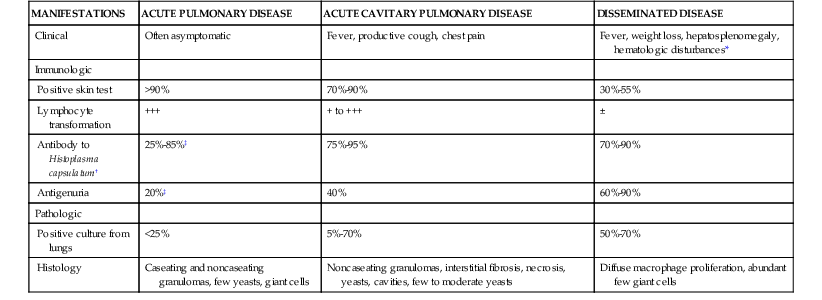
* Hematologic disturbances include anemia, leukopenia, and thrombocytopenia.
† Complement fixation titer of greater than or equal to 1 : 8.
‡ Higher incidence in those with symptomatic infection.
+, Indicates a proliferative response to antigen or mitogen that is 3 to 5-fold higher than background; ++, 5- to 10-fold higher than background; +++, more than 10-fold higher.
From Deepe GS Jr, Bullock WE. Histoplasmosis: a granulomatous inflammatory response. In: Gallin JI, Goldstein IM, Synderman R, eds. Inflammation: Basic Principles and Clinical Correlates. 2nd ed. New York: Raven Press; 1992:943.
Pulmonary Histoplasmosis
Acute Infections
Acute Primary Infection
Symptoms
The vast majority of primary infections (>90%) go unrecognized medically. Usually, they are asymptomatic or result in mild influenza-like illness for which individuals do not seek medical attention. However, there is a small proportion of patients who become overtly ill. The major determinant for the development of symptoms is likely to be the inoculum size, although differences in strain virulence cannot be excluded.40,41 Other contributing factors include age and underlying diseases. Thus, older adults, those younger than 2 years of age, and individuals whose immune systems are compromised are more likely to develop progressive disseminated disease symptoms.
In those who become ill, the typical incubation time is 7 to 21 days, and most individuals manifest symptoms by day 14.40–42 Fever that may reach 42° C, headache, nonproductive cough, chills, and chest pain are the most common symptoms noted. The latter is described usually as a substernal discomfort, although in an outbreak in children, it was more often located in the anterior chest.42 Pleuritic chest pain is uncommon. The chest pain is believed to be caused by enlargement of the mediastinal or hilar lymph nodes, or both. Malaise, weakness, fatigue, and myalgias are observed in a distinctly smaller percentage of patients. Most symptoms resolve within 10 days but can persist for several weeks if there is an exposure to a heavy inoculum. Acute pulmonary infection can be accompanied by a number of rheumatologic manifestations. Arthralgias, erythema nodosum, and erythema multiforme are present in approximately 6% of patients, most of whom are women.43 In some, these manifestations of histoplasmosis may be the presenting complaint. Frank arthritis is distinctly uncommon.
Clinical Findings
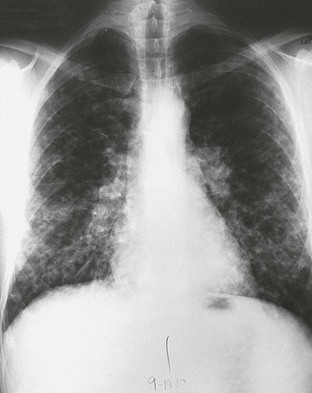
Physical findings in acute pulmonary histoplasmosis are minimal. Crackles may be detected on auscultation of the lungs and, rarely, hepatosplenomegaly. The common roentgenographic features are characterized by a patchy pneumonitis that eventually calcifies and hilar lymphadenopathy (Fig. 265-5). If a heavy exposure has transpired, numerous patches of pneumonitis that calcify may develop, and these produce the so-called buckshot appearance on the chest roentgenogram.35 Pleural effusions are distinctly uncommon. The white blood cell count is usually within the normal range, but approximately 30% of patients will have leukocytosis or leukopenia during the course of this infection. Another laboratory abnormality is a transient increase in the serum alkaline phosphatase level. Pulmonary function studies, performed in only a few patients, have demonstrated reversible restrictive defects, impaired diffusing capacity of lung for carbon monoxide (DLCO), and obstructive defects.44
Pericarditis
At least 6% of patients who acquire histoplasmosis suffer from acute pericarditis.45 This figure may underestimate the true incidence because only those most seriously affected seek medical attention. Precordial chest pain and fever are frequent. A high proportion of patients report a respiratory illness approximately 6 weeks before the onset of the pericarditis. A pericardial friction rub is auscultated in more than 75% of patients with pericarditis, and pulsus paradoxus is also present in more than 75%. An enlarged cardiac silhouette is usually seen on chest roentgenogram. Electrocardiographic abnormalities indicative of pericarditis—for example, ST segment elevation—are often observed. Only a small percentage of individuals develop cardiac tamponade. The likely cause of the pericarditis is not direct invasion of the organism because it is rarely found in tissue specimens or in pericardial fluid, but rather the granulomatous inflammatory response that is mounted in mediastinal lymph nodes adjacent to the pericardium.
Differential Diagnosis
Acute pulmonary histoplasmosis must be distinguished from influenza and other forms of community-acquired pneumonia.46 This task is difficult unless a thorough exposure history is obtained. Of greater concern, however, are patients who present with mediastinal lymphadenopathy. This finding is often considered to be caused by a hematologic malignancy rather than histoplasmosis. In such cases, patients may undergo unnecessary surgical procedures in an attempt to establish a diagnosis. Sarcoidosis also should be considered and distinguishing between them can be difficult. Both may have similar histopathologic features, and serum angiotensin-converting enzyme levels are elevated in each disease.47 Thus, in all patients who present with mediastinal or hilar lymphadenopathy, it is critically important that histoplasmosis be considered in the differential diagnosis in patients who reside or have recently been in an endemic region.
A Ghon complex and pulmonary calcifications are common in healed pulmonary histoplasmosis. Another characteristic feature of resolved primary infection is the presence of splenic or liver calcifications. In fact, the presence of these on a routine roentgenogram should be considered evidence of resolved histoplasmosis if the patient has been in an endemic area. Although splenic and liver calcifications are also noted in healed tuberculosis, the most likely cause of these findings remains histoplasmosis because the incidence of tuberculosis in the United States is much lower than that of histoplasmosis. Positron emission tomography studies performed to diagnose malignant pulmonary nodules are reported to be falsely positive in healed histoplasmosis lesions, especially if there is active inflammation.48
Acute Reinfection
It is not uncommon for those who reside in endemic areas to be exposed more than once to H. capsulatum. Those who are reexposed to a large inoculum in heavily endemic areas present with a milder influenza-like illness. The onset can begin within 3 days, which is shorter than in primary infection. The characteristic chest roentgenogram is one of numerous small nodules that are diffusely scattered throughout both lung fields. This feature has been referred to as miliary granulomatosis. Hilar or mediastinal lymphadenopathy is absent. The duration of illness is often briefer than in primary infection.40,41,49
Complications of Primary Histoplasmosis
Histoplasmoma
An infrequent complication of primary histoplasmosis is the development of a mass lesion that resembles a fibroma.50 When it arises, it is found most often in the lung. Instead of resolving, a nidus of infection gradually enlarges over years to form a concentric mass. Presumably, the growth is caused by persistent antigenic stimulus from the yeasts. It is composed of active inflammation at the periphery and fibrous tissue within the inner sphere, and eventually the central portion calcifies. Roentgenographically, the histoplasmoma may have a central core of calcium or rings of calcium, and these findings are useful in distinguishing it from a neoplastic growth.
Mediastinal Granuloma and Fibrosis
Another complication of primary infection is a massive enlargement of the mediastinal lymph nodes that is caused by the granulomatous inflammation mounted in response to the fungus.51 The diameter of these nodes can reach 8 to 10 cm. The nodes are caseous and contain a fibrotic shell that may be up to 5 mm thick. Often, this process is asymptomatic. Occasionally, however, the nodes may impinge on major airways and impair gas exchange. During the healing process, the fibrotic tissue can cause retraction of the airways, leading to postobstructive pneumonias, hypoxemia, and bronchiectasis. The fibrosis also may constrict the esophagus or the superior vena cava, resulting in dysphagia, superior vena cava syndrome, or both.50,51
Calcific deposits that originate within the lungs occasionally produce lithoptysis. More common, however, is the penetration of enlarged, calcified nodes into the airways and the generation of particles of calcium that can be expectorated. If the calcified mass is large, airway obstruction may ensue. Another consequence of enlarged nodes is the creation of sinuses or fistulas between the airways and pericardium or esophagus.50
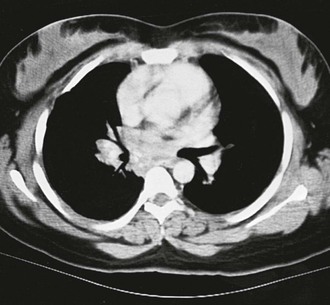
A rare but dire consequence of mediastinal involvement is mediastinal fibrosis.52 This syndrome is similar to that observed with tuberculosis, in which the infection leads to a massive deposition of fibrotic tissue within the mediastinum (Fig. 265-6). The mechanism underlying this exuberant immune response is unknown. However, it appears not to be triggered by massive numbers of yeast because they are observed infrequently in lesions. The reaction to the antigen or antigens from H. capsulatum must be host specific, based on its infrequent development. Genetic susceptibility loci for this entity have not been identified. The fibrosis encroaches on all the structures of the mediastinum, including the major airways, superior vena cava, and esophagus. The symptoms that arise from the fibrotic process are attributable to the narrowing of the patency of these structures. Hypoxemia, shortness of breath, superior vena cava syndrome, and dysphagia may ensue as the fibrotic process progresses.