HERPES SIMPLEX VIRUS
RICHARD J. WHITLEY
Eight herpesviruses routinely cause human disease. The current classification of herpesviruses into subfamilies serves the purposes of identifying evolutionary relatedness and summarizing unique properties of each member. The three subfamilies are alpha that include herpes simplex virus 1 (HSV-1), HSV-2, and varicella-zoster virus (VZV); beta consisting of cytomegalovirus (CMV), human herpesvirus-6 (HHV-6) and HHV-7; and the gamma herpesviruses, Epstein-Barr virus (EBV), and Kaposi sarcoma herpesvirus (i.e., HHV-8). One simian herpesvirus, B virus (Cryptotetia crypta), also an alpha herpesvirus, can inadvertently infect humans, resulting in devastating central nervous system (CNS) disease. These viruses share similar molecular and biologic characteristics, including the unique ability to establish latency and reactivate. These agents, among the most common encountered by humans, are common causes of CNS infections (1). Members of the alpha herpesvirus subfamily are characterized by a very short reproductive cycle, prompt destruction of the host cell, and ability to establish latency, usually in sensory ganglia. Both HSV-1 and HSV-2 are routine causes of CNS disease and the subject of this chapter.
GENERAL CHARACTERISTICS OF HERPESVIRUSES
All members of the family Herpesviridae have a similar molecular structure. These viruses contain double-stranded DNA, which is located at the central core. The DNA is surrounded by a capsid, consisting of 262 capsomers and providing icosapentahedral symmetry to the virus. Tightly adherent to the capsid is an amorphous tegument. Loosely surrounding the capsid is a bilayered lipid envelope. The overall size of herpes virions varies from 120 to approximately 300 nm, depending on the virus. The envelope consists of polyamines, lipids, and glycoproteins. The glycoproteins confer distinctive properties to each virus, providing unique antigens to which the host is capable of responding.
Herpesvirus DNA varies in molecular weight from approximately 80 to 150 million and consists of 120,000 to 230,000 base pairs. Base composition of herpesvirus DNA varies between 31% and 75% of guanine plus cytosine. Of all the herpesviruses, HSV-1 and HSV-2 are the most closely related, with approximately 50% genomic homology. With the exception of HSV-1 and HSV-2, the structural and nonstructural proteins coded by the DNA of the HHVs are not immunologically related. However, HSV-1 and HSV-2 share common types of proteins; therefore, cross-antigenic reactions do occur.
HISTORY
Infections caused by HSV have been recognized since the time of ancient Greece. Greek physicians used the word herpes to mean creeping or crawling in reference to observable skin lesions. Likely, this word was used to describe various skin conditions ranging from cancer to shingles and probably even fever blisters. The Roman scholar Herodotus associated mouth ulcers and lip vesicles with fever (2). He called this event herpes febrilis. Genital herpetic infections were described first by a physician to the French royalty, Astruc (3).
The transmissibility of these viruses was established unequivocally by passage of virus from human lip and genital lesions to either the cornea or the scarified skin of the rabbit (4). Goodpasture (5) further demonstrated that material derived from the lesions of herpes labialis consistently produced encephalitis when inoculated onto the scarified cornea of rabbits.
Since the first suggestions of herpes simplex encephalitis (HSE) by the Mathewson Commission in 1926 (6) and subsequent description of the histopathologic changes (7), HSV is reported as the most common cause of sporadic fatal encephalitis in the United States. Intranuclear inclusion bodies consistent with HSV infection were first demonstrated in the brain of a neonate with encephalitis (7) in 1941, as is described later in this chapter. Virus was subsequently isolated from this brain tissue (7). The first adult case of HSE providing similar proof of viral disease (i.e., intranuclear inclusions in brain tissue and virus isolation) was described in 1944 (8). The most striking pathologic findings in this patient’s brain were apparent in the left temporal lobe where perivascular cuffs of lymphocytes and numerous small hemorrhages were found. This temporal lobe localization subsequently has been determined to be characteristic of adult HSE, and it differs from the patchy diffuse encephalitis of neonates with HSV brain infection.
In the mid-1960s, Nahmias and Dowdle (9) demonstrated two antigenic types of HSV. Viral typing allowed the demonstration that HSV-1 was historically primarily responsible for infections “above the belt” (including brain disease in adults), whereas HSV-2 was primarily responsible for infections “below the belt” (brain disease in neonates). However, more recent studies (10,11) indicate that either virus can infect the mouth, genital tract, or brain.
INFECTIOUS AGENT
Recent detailed reviews highlight the importance of these organisms as models of viral replication and as pathogens for human infection (11). Our current understanding of the structure of HSV indicates that the genome has a molecular weight of approximately 100 million. The DNA encodes about 80 polypeptides. The DNA strands of HSV-1 and HSV-2 are colinear with reasonable but not identical matching of base pairs. Of note, there is considerable overlap in the cross reactivity between the HSV-1 and HSV-2 glycoproteins, although uniqueness can be demonstrated, as discussed later in this chapter. Distinction between the two viral types can be demonstrated by restriction enzyme analysis of viral DNA patterns. This technique has been applied to epidemiologic investigations of human HSV infections, as well.
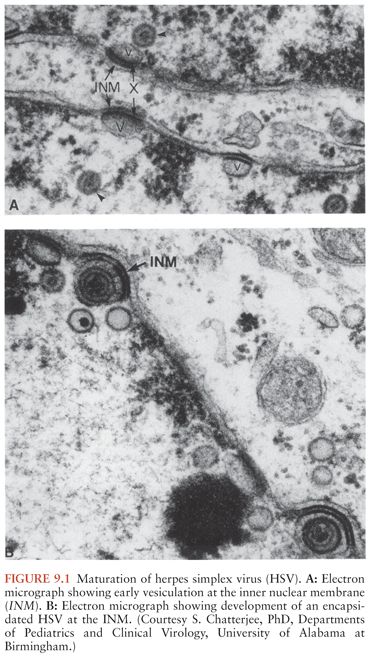
Replication of HSV is characterized by the expression of three gene classes: immediate early (alpha), early (beta), and late (gamma), which are expressed temporally and in a rolling-circle sequence (11). A few relevant events will be noted. There are five “immediate early” genes, one of which is necessary for initiating viral replication. The “early” gene products include those enzymes necessary for viral replication (such as HSV thymidine kinase), as well as the regulatory proteins. Current antiviral drugs with selective mechanisms of action are activated at the level of early gene expression. Acyclovir is an example of such a drug, being converted to its active monophosphate derivative by HSV thymidine kinase. Early gene expression coincides with an irreversible shutoff of host cellular macromolecular protein synthesis, which results in cell death. Structural proteins are usually of the late gene class. Assembly of the virus begins in the nucleus, and the envelope is acquired as the capsid buds through the inner lamella of the nuclear membrane, as shown in Figure 9.1. Virus is transported through the cytoskeleton to the plasma membrane, where lysis of the cell and release of progeny virions occur. The replicative efficiency of HSV is poor, as indicated by the ratio of infectious to noninfectious virions. With the completion of replication, 11 glycoproteins are expressed on the plasma membrane (12).
PATHOLOGY AND PATHOGENESIS
General Observations of Pathology
The pathologic changes induced by replicating HSV are similar for both primary and recurrent infection and for both adults and newborns but vary in the quantitative extent of cytopathology. The histopathologic characteristics of a skin lesion induced by HSV represent a combination of virus-mediated cellular death and associated inflammatory responses. Changes induced by viral infection include ballooning of infected cells and the appearance of chromatin within the nuclei of cells; this is followed by degeneration of the cellular nuclei, generally within parabasal and intermediate cells of the epithelium. Cells lose intact plasma membranes and form multinucleated giant cells. As host defenses are mounted, an influx of mononuclear cells can be detected in infected tissue.
Pathology of Central Nervous System Disease
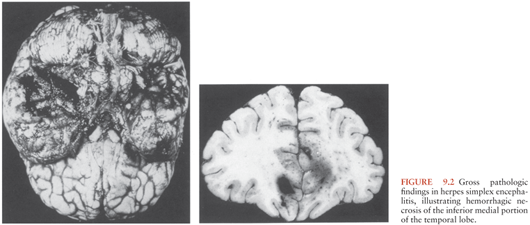
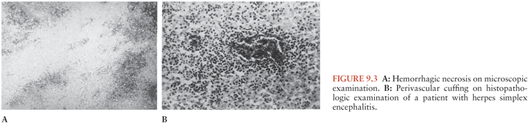
HSE results in acute inflammation, congestion, and/or hemorrhage, most prominently in the temporal lobes and usually asymmetrically in adults (13) and more diffusely in the newborn. Adjacent limbic areas show involvement as well. The meninges overlying the temporal lobes may appear clouded or congested. After approximately 2 weeks, these changes proceed to frank necrosis and liquefaction, as shown in Figure 9.2.
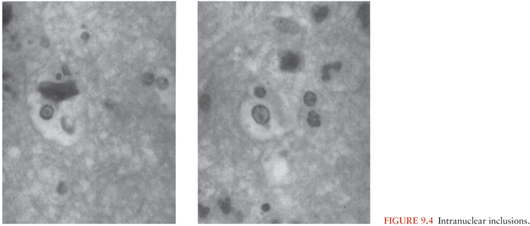
Microscopically, involvement extends beyond areas that appear grossly abnormal. At the earliest stage, the histologic changes are not dramatic and may be nonspecific. Congestion of capillaries and other small vessels in the cortex and subcortical white matter is evident; other changes are also evident, including petechiae. Vascular changes that have been reported in the area of infection include areas of hemorrhagic necrosis and perivascular cuffing (Fig. 9.3). The perivascular cuffing becomes prominent in the second and third weeks of infection. Glial nodules are common after the second week (14,15). The microscopic appearance becomes dominated by evidence of necrosis and eventually inflammation; the latter is characterized by a diffuse perivascular subarachnoid mononuclear cell infiltrate, gliosis, and satellitosis neuronophagia (13,16). In such cases, widespread areas of hemorrhagic necrosis, mirroring the area of infection, become most prominent. Oligodendrocytic involvement and gliosis (as well as astrocytosis) are common, but these changes develop very late in the disease. Although found in only approximately 50% of patients, the presence of intranuclear inclusions supports the diagnosis of viral infection, and these inclusions are most often visible in the first week of infection. Intranuclear inclusions (Cowdry type A inclusions) are characterized by an eosinophilic homogeneous appearance and are often surrounded by a clear, unstained zone beyond which lies a rim of marginated chromatin, as shown in Figure 9.4.
General Observations on the Pathogenesis of Human Disease
The pathogenesis of human disease depends on intimate, personal contact of a susceptible individual (namely, one who is seronegative) with someone excreting HSV. Virus must come in contact with mucosal surfaces or abraded skin for infection to occur. With viral replication at the site of infection, the capsid is transported by neurons to the dorsal root ganglia, where after another round of viral replication, latency is established. These events have been demonstrated in a variety of animal models (17). Transport of the virion is by retrograde axonal flow (18). In some instances, replication can lead to severe CNS infection; however, more often, the host–virus interaction results in latency. After latency is established, reactivation can occur, with virus shedding at mucocutaneous sites appearing as skin vesicles or mucosal ulcers or being totally asymptomatic. Occasionally, primary infection can become systemic, affecting other organ systems besides the CNS and the peripheral nervous system. Such circumstances include disseminated neonatal HSV infection with multiorgan involvement, multiorgan disease of pregnancy, and infrequently dissemination in patients undergoing immunosuppressive therapy. Multiorgan disease is likely the consequence of viremia in a host not capable of limiting replication to mucosal surfaces.
Infection with HSV-1 is transmitted either by respiratory droplets or through direct contact (to a susceptible individual) with infectious secretions (such as virus contained in orolabial vesicular fluid). Acquisition of HSV-2 infection is usually the consequence of transmission via genital routes. Under these circumstances, virus replicates in the vaginal tract or on penile skin sites, with seeding of the sacral ganglia.
Pathogenesis of Latency
All of the herpesviruses have the ability to become latent, persist in an apparent inactive state for varying durations, and be reactivated by a provocative stimulus, as yet unidentified (11,19), as recently reviewed. As a biologic phenomenon, latency has been recognized since the beginning of the twentieth century (20–26). In 1905, Cushing (27) noted that patients treated for trigeminal neuralgia (by sectioning a branch of the trigeminal nerve) developed HSV lesions along the innervated areas of the sectioned branch, as suggested previously by Goodpasture (28,48). Several investigators have demonstrated that microvascular surgery of the trigeminal nerve tract for tic douloureux resulted in recurrent herpetic lesions in more than 90% of seropositive individuals (29–32). Axonal injury and attempts at excision of lesions have been associated with recurrences (33). Reactivation of latent virus appears to depend on an intact anterior nerve route and peripheral nerve pathways.
Recurrences occur despite both cell-mediated and humoral immune responses and can be either symptomatic or asymptomatic. Recurrences are spontaneous, but there have been associations with physical or emotional stress, fever, exposure to ultraviolet light, tissue damage, and immune suppression (25,34,35). Viral DNA can be detected in neuronal tissue in the absence of cutaneous lesions (17,36–39). Latent virus has been retrieved from the trigeminal, sacral, and vagal ganglia of humans (20–22,36,39).
Pathogenesis of Encephalitis
The pathogenesis of HSE in children (older than 3 months) and adults is only partly understood. Both primary and recurrent HSV infections can cause disease of the CNS. From studies performed by the National Institute of Allergy and Infectious Diseases (NIAID) Collaborative Antiviral Study Group (CASG), approximately one third of the cases of HSE are the consequence of primary infection. For the most part, the patients with primary infection are younger than 18 years. The remaining two thirds of cases occur in the presence of preexisting antibodies, but only approximately 10% of patients have a history of recurrent herpes labialis. Patients with preexisting antibodies are considered to have HSE as a consequence of reactivation of HSV (40). A limited number of patients, approximately 15%, have active herpes labialis and HSE, allowing comparison of the DNA by restriction enzyme analyses. The isolates are usually identical; however, this is not always the case. The virus isolated from the peripheral site can be different from that retrieved from the CNS (41). Thus, the issues of reactivation of virus directly within the CNS, the potential for enhanced neurotropism of certain viruses, and the selective reactivation and access of one virus by the trigeminal route or other routes to the CNS remain for further elucidation.
The route of access of virus to the CNS in primary infection is a subject of debate, especially in humans. Studies performed more than five decades ago defined pathways for HSV access to the brain in animals, including both the olfactory and trigeminal nerves among others (42). However, which of these nerve tracts uniformly leads to HSV infection in the CNS of humans is not clear. The anatomic distribution of nerves from the olfactory tract into the limbic system, along with the recovery of virus from the temporal lobe (the site of apparent onset of HSE in the human brain), suggests that viral access to the CNS via this route is a tenable hypothesis. Reports in the literature have found electron microscopic evidence that suggests this has been the case in some individuals with HSE (43–45). Animal model data support the contention that the olfactory tract provides one neurologic avenue for viral access to the CNS and causes localization of the infection in brain regions analogous to medial temporal structures in humans (46,47). Definitive proof of such progression in humans is lacking.
Reactivation of HSV, leading to focal HSE, is a similarly confusing problem from the standpoint of pathogenesis. Evidence of latent virus within infected brain tissue exists (48); however, virus reactivation at that site remains purely hypothetical. Reactivation of virus peripherally (namely, in the olfactory bulb or the trigeminal ganglion) with subsequent neuronal transmission to the CNS has been suggested (42,49,50). Nevertheless, a relevant observation is that with recurrent herpes labialis, whereby reactivation of virus from the trigeminal ganglia occurs, HSE is a very uncommon event. Furthermore, HSE does not occur more frequently in immunocompromised patients.
Host immunity plays an important, but undefined, role in the pathogenesis of HSE. Possibly, the CNS is particularly prone to HSV infection because intraneuronal spread may shelter virus from host defense mechanisms. HSE is no more common in the immunosuppressed host than in the normal host; however, when it does occur, the presentation is atypical, with a subacute but progressively deteriorating course.
More recently, a host genetic deficiency has been found to play a role in recurrent HSE but certainly does not exist in all patients (51).
EPIDEMIOLOGY
Herpes Simplex Virus, Type 1
The epidemiology of HSV infections is multifaceted. Because the focus of this book is CNS infections, only a brief review of non-CNS HSV infections follows. The reader is referred to more complete reviews (11). HSV infections are distributed worldwide and have been reported in both developed and developing countries, including remote Brazilian tribes (52). Animal vectors for human HSV infections have not been described; therefore, humans remain the sole reservoir for transmission of these viruses to other humans during close personal contact. There is no seasonal variation in the incidence of infection. Because infection is rarely fatal, and because these viruses become latent, more than two thirds of the world’s population can have recurrent HSV infections and, therefore, can transmit infection during episodes of reactivation. HSV disease ranges from mild (even indiscernible) in most patients to sporadic, severe, and life-threatening disease in a few infants, children, and adults. Children, particularly those younger than 5 years, are most often infected; however, primary infections can also occur in older individuals. With clinical illness, oropharyngeal disease, namely gingivostomatitis, usually is the manifestation. Primary infection in young adults has been associated with pharyngitis and often a mononucleosis-like syndrome (53). Seroprevalence studies have demonstrated that acquisition of HSV-1 infection is related to socioeconomic factors, namely lower socioeconomic populations acquire infection earlier in life than more affluent individuals. The identification of primary gingivostomatitis that was proven to be caused by HSV infection (54,55) led to the definition of the natural history of infection, including the appearance of neutralizing antibodies (56), absence of virus shedding in children younger than 6 months (57), and a higher rate of occurrence among individuals of lower socioeconomic status. Contemporary surveys document the viral shedding data, ranging from 2% to 5% (58–63).
Antibody surveys have helped clarify the epidemiology of HSV infection. Geographic location, socioeconomic status, and age all influence the acquisition of HSV infection (54,64–66). In developing countries, seroconversion occurs early in life. In Brazilian Indians, HSV antibodies are detectable in more than 95% of children by the age of 15 years (67). Similarly, serologic studies performed in New Orleans demonstrated acquisition of antibodies in more than 90% of children by the age of 15 years (68). In developing countries, such as Uruguay, or in lower socioeconomic populations in the central United States, the appearance of antibodies occurred at similar but lower frequencies (68–71). By 5 years of age, approximately one third of patients had seroconverted; this frequency increased to 70% to 80% by early adolescence.
Middle-class individuals of industrialized societies acquired infection later in life. Seroconversion occurred during the first 5 years of life in 20% of children; there was no significant increase until the second and third decades of life, at which time the prevalence of antibodies increased to 40% and 60%, respectively (72,73). One study of university students demonstrated that seroconversion of susceptible individuals occurred at an annual frequency of approximately 5% to 10% (53,74,75). In summary, primary infection occurs very early in children of underdeveloped countries and in those of lower socioeconomic classes; however, in developed countries and more affluent classes, primary infection is delayed until adolescence or, perhaps, even adulthood. The frequency of direct person-to-person contact is the major mediator of acquisition of infection.
Over the past decade, HSV-1 has been increasingly associated with primary genital herpes, reflecting a change in the epidemiology of infection (11).
Herpes Simplex Virus, Type 2
Because HSV-2 infections are usually acquired through sexual contact, antibodies to this virus are rarely found before the age at onset of sexual activity. Although most genital HSV infections are caused by HSV-2, an ever-increasing proportion is attributable to HSV-1, as noted earlier, now as high as 50% of all new primary infections (10,76–78). Approximately 1.5 million new cases of HSV-2 occur annually in the United States (79). Genital HSV infections are not reportable in the United States (80). Current estimates of infected individuals with genital herpes in the United States range from 40 to 60 million (76–78,81).
Women have the highest rates of infection, particularly prostitutes and others with multiple sex partners, including those with HIV infection. The incidence of genital HSV infections in both indigent women and those of middle and upper socioeconomic classes is significantly lower than the incidence found among women attending sexually transmitted disease clinics (82). As with HSV-1 infections of the mouth, HSV-2 can be excreted in the absence of symptoms at the time of primary, initial, or recurrent infection (83,84). The actual frequency of asymptomatic excretion of HSV-2 in women by culture is approximately 3% to 5% of all days, and by polymerase chain reaction (PCR) 15% to 20%. Furthermore, some individuals can start and stop shedding multiple times during the same day (85). Its occurrence creates a silent reservoir for transmission of infection (86,87). The appearance of HSV-2 antibodies reflects the time of exposure or more simply the acquisition of infection and is positively correlated with the onset of sexual activity (70,71,88). However, crowded living conditions may indirectly contribute to antibody prevalence (89). If HSV-2 type-specific antibodies are sought in healthy women, there is a wide discrepancy in prevalence, ranging from averages of 10% in England and Italy to 25% in the United States and 77% in Uganda (90,91). Up to 50% to 60% of lower socioeconomic populations in the United States and elsewhere develop antibodies to HSV-2 by adulthood. The reader is referred to a review for worldwide seroprevalence of HSV-2 (90). Seroprevalence is a function of age, number of sexual partners, race, and marital status (92–94).
Latent Genital Herpes Simplex Virus Infections
Latent genital infection with subsequent reactivation is the largest reservoir for transmission of HSV-2. As with HSV-1 infection, recurrent HSV-2 infection can be either symptomatic or asymptomatic; however, recurrence is usually associated with a shorter duration of viral shedding and fewer lesions. Several studies have implicated a frequency of recurrence as high as 60% (88,95). The type of genital infection, HSV-1 versus HSV-2, is predictive of the frequency of recurrence (95–97), with HSV-1 infection recurring less frequently than HSV-2 (98,99).
HERPES SIMPLEX ENCEPHALITIS
Background
HSV infections of the CNS are among the most severe of all viral infections of the human brain. Currently, HSE is estimated to occur in approximately 1 per 250,000 to 500,000 individuals per year. At the University of Alabama at Birmingham, the diagnosis of HSE was proven by brain biopsy in an average of ten patients per year, for an incidence of approximately 1 in 300,000 individuals, an incidence similar to those in Sweden and England (100,101). With the advent of PCR, brain biopsy is no longer used. In the United States, HSE accounts for 10% to 20% of viral infections of the CNS (102).
The economic cost of HSE is considerable, as estimated in 1983 for hospitalization alone of adults to be more than $25 million (103). The total medical cost is considerably higher because of the long-term care and support services required for many of the survivors.
HSE occurs throughout the year and in patients of all ages, with approximately one third of cases occurring in patients younger than 20 years but older than 6 months and approximately one half in patients older than 50 years (102). Caucasians account for 95% of patients with biopsy-proven disease. Both sexes are affected equally.
The severity of disease is best determined by the outcome of patients who have received either no therapy or an ineffective antiviral medication, such as idoxuridine or cytosine arabinoside. In such situations, mortality is in excess of 70%; only approximately 2.5% of all patients with confirmed disease (9.1% of survivors) returned to normal function after recovery from their illness (104–108). Because brain biopsy with isolation of HSV from brain tissue was the method of diagnosis in these early studies, a far broader spectrum of HSV infections of the CNS actually was thought to exist. However, with the more recent use of PCR for diagnosis of HSE, virtually all patients have a focal neurologic disease, suggesting a limited spectrum of disease (109).
Diagnosis
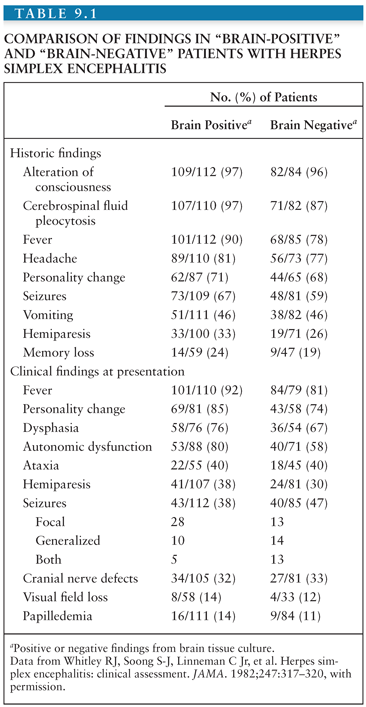
Several aspects relating to the diagnosis of HSE merit discussion: (a) the clinical presentation in regard to the sensitivity and specificity of various clinical characteristics, (b) the historical use of brain biopsy to establish the diagnosis, (c) conditions that mimic HSE, and (d) noninvasive means of diagnosis. Data from the NIAID CASG compare presentation and outcome for brain biopsy–positive and brain biopsy–negative patients (137). Of 202 patients who were evaluated for HSE because of focal neurologic findings, HSV was isolated from brain tissue of only 113. Only three of the remaining patients had combinations of serologic and clinical findings suggestive of HSE. These patients were subsequently shown to have HSV DNA in their cerebrospinal fluid (CSF) by PCR.
As shown in Table 9.1, most patients with biopsy-proven HSE presented with a focal encephalopathic process, including (a) altered mentation and decreasing levels of consciousness with focal neurologic findings; (b) CSF pleocytosis and proteinosis; (c) the absence of bacterial and fungal pathogens in the CSF; and (d) focal electroencephalographic (EEG), computed tomographic (CT), and/or magnetic resonance image (MRI) findings (102,110–120). The frequency of headache and CSF pleocytosis is higher in patients with proven HSE than in patients with diseases that mimic HSE. Nearly uniformly, patients with HSE present with fever and personality change. Seizures, whether focal or generalized, occur in only approximately two thirds of all patients with proven disease. Thus, the clinical findings of HSE are nonspecific and do not allow for empirical diagnosis of disease predicated solely on clinical presentation. Although clinical evidence of a localized temporal lobe lesion is often thought to indicate HSE, various other diseases can mimic this condition.
Examination of the CSF is indicated in patients with altered mentation, provided it is not contraindicated because of increased intracranial pressure. In patients with HSE, CSF findings are nondiagnostic, being similar in patients with confirmed disease or diseases that mimic HSE. Both the CSF white blood cell (WBC) count (lymphocytes predominance) and the CSF protein level are elevated. The average CSF WBC count is 100 cells/µL; the protein averages approximately 100 mg/dL. Sequential evaluation of CSF specimens from patients with HSE indicates increasing cell counts and levels of protein. The presence of CSF red blood cells is not diagnostic for HSE. Approximately 5% to 10% of patients have a normal CSF formula on first evaluation.
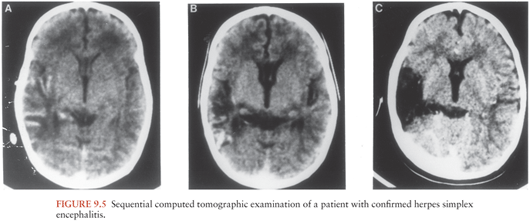
Noninvasive neurodiagnostic studies support a presumptive diagnosis of HSE. These studies have included EEG, CT, and MRI. Focal changes of the EEG are characterized by spike and slow-wave activity and periodic lateralized epileptiform discharges, which arise from the temporal lobe (114–118). Early in the disease, the abnormal electric activity usually involves one temporal lobe and then spreads to the contralateral temporal lobe as the disease evolves, usually over 7 to 10 days. The sensitivity of the EEG is approximately 84%, but the specificity is only 32.5%. CT scans initially show low-density areas with mass effect localized to the temporal lobe, which can progress to radiolucent and/or hemorrhagic lesions (119,120). Bitemporal disease is common in the absence of therapy, particularly late in the disease course (Fig. 9.5). When these neurodiagnostic tests are used in combination, the sensitivity is enhanced; however, the specificity remains inadequate. None of these neurodiagnostic tests is uniformly satisfactory for diagnosing HSE. MRI detects evidence of HSE earlier than CT scan (111).
A sensitive and specific means of diagnosis is the isolation of HSV from tissue obtained at brain biopsy (121). However, PCR detection of HSV DNA in the CSF has become the diagnostic procedure of choice. Brain biopsy is of value in confusing clinical presentations. Complications, either acute or chronic, occur in approximately 3% of patients. Fears of potentiating acute illness (by incising the brain in a diseased area) or of causing chronic seizure disorders have not been substantiated by follow-up studies of patients in the NIAID CASG.
Serologic Evaluation
Several strategies using antibody production as a means of diagnosing HSE have been utilized (58). Because most encephalitic patients are HSV seropositive at presentation, seroconversion per se is usually not helpful because fever alone can reactivate labial herpes, resulting in antibody elevations. A fourfold rise in serum antibody was neither sensitive nor specific enough to be useful. A fourfold or greater rise in CSF antibody occurred significantly more often within a month after onset of disease in patients with biopsy-proven HSE: 85% versus 29%. By 10 days after clinical presentation, however, only 50% of brain biopsy–positive patients had a fourfold rise in CSF antibody. This test is useful only for retrospective diagnosis. The use of a ratio of serum to CSF antibody of 20 or less did not improve sensitivity during the first 10 days of disease.
Polymerase Chain Reaction Detection of Viral DNA
PCR detection of HSV DNA in the CSF is the diagnostic method of choice (121–127). Data from the NIAID CASG defined the sensitivity and specificity as 94% and 98%, respectively. These CSF specimens were obtained from patients with biopsy-proven or biopsy-negative disease. Notably, the specificity would have been higher except that some tissue specimens were fixed in formalin, which killed infectious virus, prior to an attempt to isolate virus. HSV DNA persisted in 80% of tested CSF specimens for 1 week or more.
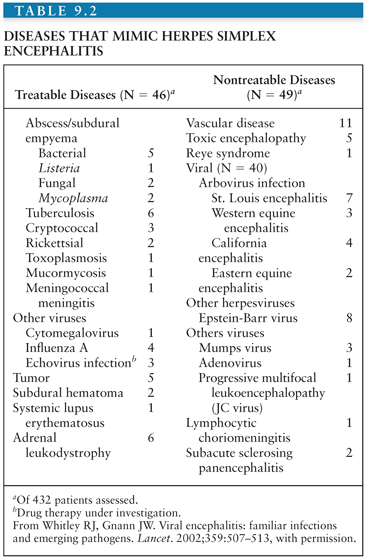
Diseases That Mimic Herpes Simplex Encephalitis
In a compilation of the NIAID CASG data, 193 (45%) of 432 patients undergoing brain biopsy for a focal encephalopathic process had HSE (128). As shown in Table 9.2, the remaining patients were evaluated for diseases that mimic HSE (128). Thirty-eight had disease amenable to other forms of therapy, including brain abscess, tuberculosis, cryptococcal infection, and brain tumor. An additional 19 patients had diseases that were indirectly treatable, and another 38 patients had an alternative diagnosis established for which there was no current therapy, usually other viral infections. Thus, those diseases that mimic HSV infection of the CNS and that require immediate medical intervention should be considered if the PCR is negative for HSV DNA.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree