HELMINTHIC INFECTIONS
JOSE A. SERPA, WON K. CHUNG, AND A. CLINTON WHITE, JR.
The helminths include a broad range of organisms that are common human parasites. There are three major groups of helminths: the nematodes (or roundworms, phylum Nematoda), the trematodes (or flatworms or flukes, phylum Platyhelminthes, subphylum Trematoda), and the cestodes (tapeworms, phylum Platyhelminthes, subphylum Cestoda). Numerous members of each group cause human central nervous system (CNS) infection (Table 46.1). Helminths are part of the animal kingdom and are eukaryotic multicellular organisms with well-developed organs including reproductive and digestive tracts (nematodes and trematodes) or digestive surface (cestodes). Helminths are often large enough to be visible to the human eye. They are extracellular parasites. The host response to helminths is dominated by mechanisms aimed at extracellular organisms, resulting in the development of eosinophilia.

Most helminths have complex life cycles, with different morphologic forms and often involving multiple hosts. Most reproduce primarily by sexual reproduction. The host in which sexual reproduction occurs is termed the definitive host. The host in the life cycle in which no or only asexual reproduction occurs is termed the intermediate host. In many cases, humans are a dead-end host and not part of the parasite life cycle. In that case, humans are termed an incidental host.
CENTRAL NERVOUS SYSTEM DISEASE CAUSED BY CESTODES
Neurocysticercosis
Taenia solium is a cestode parasite with a wide distribution globally (1–3). The parasite has two forms: an intestinal tapeworm, found only in human hosts, and the cystic larval form, termed the cysticercus, which is typically found in pigs. Humans can also be host to the cysticercus. Cysticercus infection of the CNS is termed neurocysticercosis (NCC), which is now recognized as a common cause of neurologic disease worldwide (1–3).
Epidemiology
The endemic regions for T. solium infection include most areas of the world where pigs are raised including Latin America, sub-Saharan Africa, India, Southeast Asia, China, Indonesia, and other regions less well characterized such as New Guinea and Eastern Europe (1–3). However, the burden of disease was not recognized until the availability of neuroimaging studies in the early 1980s resulted in identification of NCC as a common neurologic problem among immigrants to the United States and other developed countries and later among residents of endemic areas. Review of studies from endemic areas around the world document NCC in approximately 30% of patients with seizures (3). Several studies have documented a high prevalence of infection throughout Latin America (4,5) with estimates ranging between 15% and 38%. Similar studies have also demonstrated NCC in a high proportion of African patients with seizures, with the disease prevalent throughout the region (6,7). The prevalence of seizures due to NCC in sub-Saharan Africa has been estimated at 1.7 to 24.8 per 1,000 persons.
When computed tomographic (CT) scans were initially used in adults with new-onset seizures in India, most patients were noted to have abnormalities, including single calcifications or single enhancing lesions. In a series of 991 patients with symptomatic localization-related seizures in south India, 40% of patients were found to have either active NCC, calcifications on CT scan consistent with prior cysticercosis, or single enhancing CT lesions, consistent with cysticercal granulomas (8). These findings were confirmed in a study of children with seizures regardless of features of localization and in a study from northern India (9,10). The single enhancing CT lesions were initially attributed to tuberculosis or to the effects of seizures. However, when excisional biopsies were performed on these patients, nearly all showed histopathologic evidence of cysticercosis (11). More than half of 401 patients presenting with a single enhancing lesion were subsequently proven to have NCC (12). As mentioned earlier, NCC is also prevalent in other parts of Asia including Indonesia, Southeast Asia, China, and Korea (13). Recent data suggest an average prevalence of T. solium infection in China of 0.11% (range, 0.05% to 15%); which translates to about 1.26 million cases of taeniasis and 3 to 6 million cases of cysticercosis. In the United States, estimates range from 0.2 to 0.6 cases of NCC per 100,000 general population and 1.5 to 5.8 cases per 100,000 Hispanics (14).
Parasitology
The T. solium life cycle requires two hosts and two major forms of the parasite (Fig. 46.1). The cysticerci are found primarily in muscle of the pig, the intermediate host. Humans are the host for the intestinal tapeworm form. Humans become infected with the tapeworm form (taeniasis) by ingesting undercooked pork infested with T. solium cysticerci. After ingestion, the scolex (or head) evaginates and attaches to the small intestines by means of hooks and suckers (Fig. 46.2). Proglottids (segments) develop from the base of the scolex. As new segments arise at the base of the scolex, the older proglottids form a chain that can reach a length of up to 30 feet, with the larger more mature proglottids at the distal end. The mature proglottids are off-white, opaque, and approximately 1 cm wide, 1 to 2 cm long, and 1 to 3 mm thick. The proglottids are hermaphroditic and mate with themselves to produce ova. The ova or proglottids are shed intermittently in the stool. Most tapeworm carriers note few or no symptoms other than possibly noting proglottids in the stool. Excretion is intermittent, so stool examinations for the ova or parasites are usually negative.
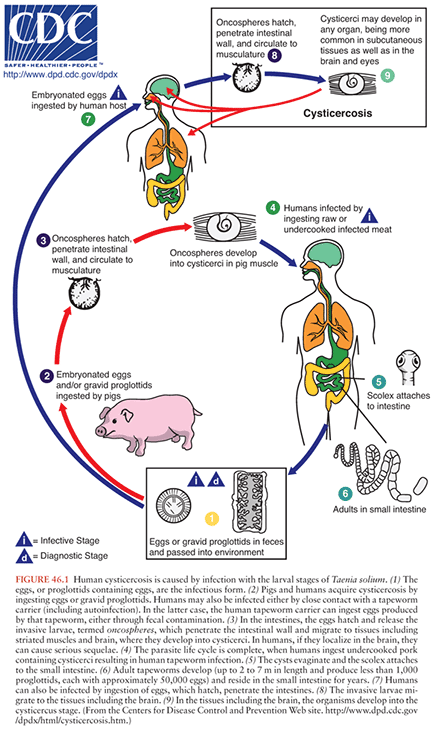

Porcine cysticercosis is endemic in regions where pigs consume human fecal material. Pigs, when raised under these conditions, ingest the ova or proglottids from the tapeworm carrier. In the intestines, the eggs hatch, release the invasive larvae (also termed oncospheres), which penetrate the intestinal mucosa using hooklets and excretory proteases, enter the bloodstream, migrate to the tissues, and mature into cysticerci. In the muscle, the cysticerci appear as thin-walled, translucent, oval cysts, approximately 1 cm in diameter. The invaginated scolex is found as a 1- to 2-mm opaque nodule attached to one side of the wall.
Human cysticercosis occurs when people ingest ova shed by a tapeworm carrier. As in pigs, the eggs hatch in the intestine, enter the bloodstream, migrate to the tissues, including muscle, brain, and eye, and mature into cysticerci. Most epidemiologic studies suggest that close personal contact with or perhaps food preparation by a tapeworm carrier is associated with NCC. The tapeworm carriers can also be infected themselves, probably by the fecal-oral route, but cysticercosis is not acquired directly from pork. This is illustrated by an outbreak that occurred in an Orthodox Jewish community in New York City (15,16). In all the exposed households, there was a history of employment of live-in housekeepers who had recently emigrated from Latin American countries. Examination of six housekeepers revealed an active taeniasis in one and a positive serologic test result in another. There have been a total of 78 autochthonous cases of cysticercosis reported in the United States between 1954 and 2005 (17). A confirmed or presumptive source of infection was identified among household members or close personal contacts of 16 (21%) case patients.
Pathogenesis and Pathology
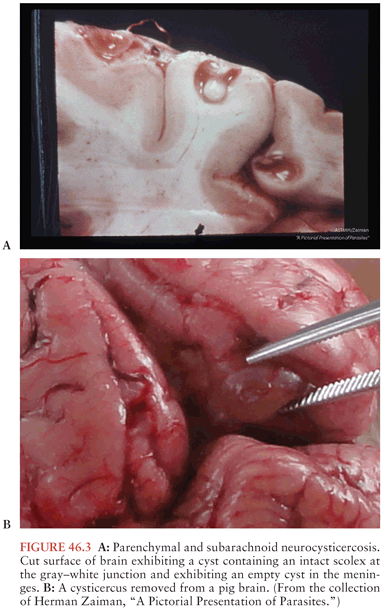
Despite the fact cysticerci reach their final size in a few weeks, there is a period of several years between infection and onset of symptoms (18,19). Thus, the presence of the parasites in the CNS does not explain symptoms. Autopsy studies of individuals in whom cysticerci were an incidental finding demonstrate viable cysticerci in brain that appear similar to the viable cysticerci from pig muscle (20) (Fig. 46.3A and B). By contrast, autopsies of patients with cysticercosis with seizures demonstrate a prominent inflammatory infiltrate. Studies have elaborated a number of molecular mechanisms used by the parasites to suppress the host inflammatory and immune responses (21). Similarly, our group has demonstrated that parasite granulomas—not the parasite itself—are the cause of seizures in an animal model. Thus, seizures likely result from the host response rather than from the parasitic infection per se (22). After months to years, the cysticerci lose their ability to control the host response. The cysticerci become infiltrated and surrounded by host inflammatory cells composed primarily of mononuclear cells, with variable numbers of eosinophils and neutrophils, and pass through a series of stages of inflammation (23,24). This response is associated with elaboration of substance P and type 1 cytokines such as interleukin-12 (IL-12), interferon-γ (IFN-γ), and IL-2 (25,26). Recent findings indicate that substance P (SP) is a critical mediator of seizures in NCC, and this may be prevented with SP-receptor antagonists (27). The walls of the cysticercus degrade. The cyst cavity is invaded by inflammatory cells (colloid stage). As the host response progresses, fibrosis encompasses the cysticercus with collapse of the cyst cavity (granular nodular stage). Eventually, the parasite is replaced by progressive fibrosis, which may calcify (calcific stage). This stage may have a minimal lymphocytic infiltrate but may also become more inflamed if the granulomas break down.
Findings similar to these pathologic stages have been noted on neuroimaging studies (28,29). Invasion of the CNS and early development of the cysticercus may present as an area of focal edema or enhancement. The viable cysticercus appears as a cystic area that is isodense with cerebrospinal fluid (CSF) (Fig. 46.4A). The cyst wall has the same density as brain parenchyma and lacks surrounding edema or contrast enhancement. As the cysticercus becomes inflamed, the density of the cyst wall increases and may enhance with contrast (Fig. 46.4B and C). Associated edema or enhancement is noted in the brain parenchyma. The cyst fluid increases in density. As the cysticercus becomes fibrotic or collapses, neuroimaging studies reveal an area of focal enhancement, suggestive of a granuloma. Finally, the calcific stage is defined by the appearance of a focal area of calcification, typically several millimeters in diameter (Fig. 46.4C). Recent studies have demonstrated edema and contrast enhancement associated with symptomatic calcified lesions (30,31). This likely results from breakdown of the calcified granulomas, triggering host inflammation.
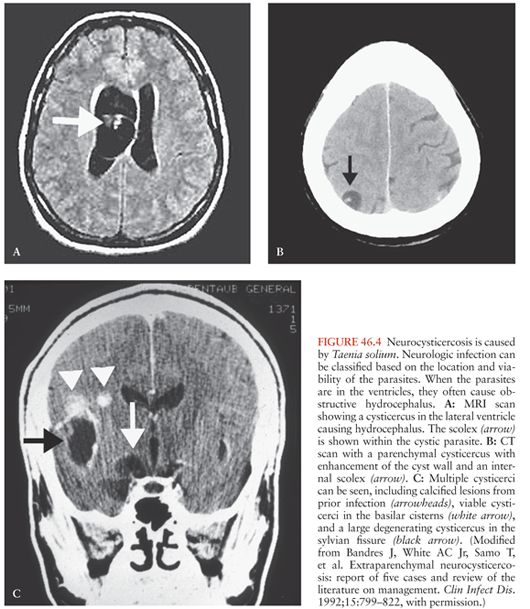
Cysticerci can also be found in the cerebral ventricles and typically float within the CSF (Fig. 46.4A). However, cysticerci in the ventricles can cause hydrocephalus, primarily by mechanical obstruction of CSF flow (32). In most cases, the obstruction is caused by viable cysticerci (33). When the cysticerci become inflamed, the ependymitis and accompanying astrocytosis cause the cysticercus to adhere to the walls of the ventricles or block CSF flow directly (especially in the aqueduct of Sylvius).
Cysticerci in the basilar cisterns are associated with a form of NCC termed subarachnoid cysticercosis. Some of the cysticerci within the subarachnoid space may enlarge and occasionally lose the scolex (Figs. 46.3 and 46.4C). The cysticerci without a scolex were termed racemose. The term racemose cysticercosis was used to describe subarachnoid disease. Because the clinical presentation, pathogenesis, and treatment are the same, whether the scolex is present or not, the term racemose may not be ideal. Furthermore, many of the so-called racemose cysticerci actually contain hooks, evidence that they previously contained a scolex.
Subarachnoid NCC is characterized by a prominent basilar arachnoiditis (34). Patients may have meningeal signs, communicating hydrocephalus, or vasculitis. The communicating hydrocephalus is presumed to be caused by CSF outflow obstruction or blocked ventricular outflow (32). The accompanying vasculitis may present as lacunar infarctions, or occasionally, erosion into larger vessels may cause large vessel strokes (35,36).
Cysticerci within the subarachnoid space can grow to sizes larger than the 1 to 2 cm typical of parenchymal cysticerci (Fig. 46.4C). These giant cysticerci may expand to sizes up to 10 cm in diameter and can cause mass effects (37). The mass effects are especially prominent when the cysticerci are inflamed either from spontaneous degeneration or after drug treatment.
Classification and Clinical Manifestations
The clinical manifestations of NCC depend on the number and location of the cysticerci, as well as the associated host response. Clinical classifications are usually based on neuroimaging (38–40). The most common scheme separates NCC into parenchymal and extraparenchymal. Extraparenchymal NCC is subdivided into ventricular and subarachnoid (including spinal). Experts agree that the term inactive NCC be used to describe cases in which there is no longer evidence of either a viable or a degenerating parasite. CT scans may reveal one or more calcifications, typically 2 to 10 mm in diameter. These correspond to the calcific stage described pathologically. Often, these lesions are symptomatic causing recurrent seizures associated with contrast enhancement (perilesional edema) (30,41–44). Some patients may also present with hydrocephalus without evidence of cysts. These patients have had prior arachnoiditis or granular ependymitis resulting in obstruction of CSF flow (e.g., aqueductal stenosis) or CSF outflow obstruction.
Symptomatic parenchymal infection typically results from the host inflammatory response and carries a favorable prognosis. Extraparenchymal disease tends to have a worse prognosis, with associated hydrocephalus (usually requiring surgical therapy), and can be fatal if not properly managed (45,46).
Parenchymal Neurocysticercosis. Parenchymal infection is the most common form of disease, and in most series, the majority of cases are inflamed cystic lesions. By contrast, noninflamed cysts rarely cause symptoms. For example, Garcia and Del Brutto (47) have described a group of patients with numerous, noninflamed cysticerci but few, if any, symptoms. Thus, symptomatic infection likely occurs when the cysticercus can no longer control the host inflammatory and immune responses. Neuroimaging studies reveal round cysts, typically 4 to 20 mm in diameter. The cyst fluid is often isodense with CSF. In viable cysticerci, the cyst wall is thin (<1 mm thick) and is usually not visible. If seen, the scolex appears as a round or tubular mass 1 to 3 mm long on the side of the cyst wall. The inflamed cyst wall becomes denser and may display contrast enhancement of the wall (ring enhancement), cyst fluid, or surrounding tissues. There is often edema surrounding the cysticercus, especially on T2-weighted magnetic resonance imaging (MRI) scans. Later stage cysticerci appear as focal areas of enhancement or granulomas. The number of cysticerci varies from one to several thousand. However, in areas where most patients with new-onset seizures undergo CT scanning (e.g., India and the United States), most cases of parenchymal NCC have only a single cyst (48–50).
A subgroup of patients with active parenchyma disease present with diffuse cerebral edema and a clinical presentation resembling encephalitis (51). This form, termed cysticercal encephalitis, is more common in children than adults. Cysticercal encephalitis results from an inflammatory reaction to large numbers of cysticerci in the brain parenchyma. The accompanying inflammatory response causes diffuse cerebral edema.
Ventricular Neurocysticercosis. In some series, 5% to 30% of patients with NCC had cysticerci in the ventricles (1,40,50). These cysticerci can obstruct CSF flow causing hydrocephalus. The cysticerci are found in any of the ventricles. Older series noted most of the ventricular cysticerci in the fourth ventricle, but this may have reflected more severe disease when the cysticerci are in that location. In most cases, the cysticerci cause symptoms while still viable (33). Because viable cysticerci have thin walls with cyst fluid isodense with CSF, they may be difficult to detect by CT scanning, which usually only shows evidence of obstructive hydrocephalus or distortion of the shapes of the involved ventricle. The cysticerci are frequently visible on MRI (52,53). However, the findings are subtle and can be missed unless an expert familiar with cysticercosis reviews the scan. These patients may also have parenchymal cysticerci.
Subarachnoid Neurocysticercosis. When patients have cysticerci in the gyri of the cerebral convexities, the radiologic appearance, clinical presentation, and pathogenesis overlap with active parenchymal NCC. However, the cysts may be slightly larger and are more likely to persist after antiparasitic chemotherapy.
With cysticerci in the fissures (especially the sylvian fissure), the cysticerci can enlarge to several centimeters in diameter, termed giant cysticerci (37). Isolated cysticerci in the fissures carry a similar prognosis to parenchymal cysts with symptoms resulting from parenchymal inflammation. In some patients, however, the cysts may enlarge to the point of causing mass effects or midline shift. Most of the time, giant cysticerci are accompanied by cysticerci in the parenchyma or basilar cisterns. The giant cysticerci are readily visualized by CT or MRI, but the accompanying cysticerci in the basilar cisterns may not be seen on CT or MRI. Cysticercosis of the basilar cisterns carries a grave prognosis. Most patients are infected with numerous cysticerci that fill the basilar cisterns. Cisternal cysticercosis is characterized by arachnoiditis, which may be seen as focal or diffuse meningeal enhancement or vasculitis with strokes (34,36). Patients often develop communicating hydrocephalus. The spinal subarachnoid space is commonly involved in patients with basal subarachnoid NCC (54).
Spinal Neurocysticercosis. Only a small percentage of patients with NCC have recognized spinal involvement (1,40). Most cases of spinal NCC result from cysticerci in the subarachnoid space (45). Initially, the cysticerci are free floating, and they may move between levels. When the cysticerci degenerate, they eventually become fixed at one level. The accompanying inflammation may cause cord compression from mass effect with obstruction of flow on myelogram. Cysticerci are rarely intramedullary and may cause symptoms or signs from mass effect or accompanying inflammation.
Other Forms of Cysticercosis. Cysticercosis can involve the eye, with either intravitreal or subretinal cysticerci (55). Cysticerci may involve the muscles. Only rarely do they cause more than minor symptoms. Cysticerci can involve the subcutaneous tissues, where they present as a palpable cystic lesion that is often confused with a sebaceous cyst.
Mixed Forms. Clinical cases, especially those involving large numbers of cysticerci, often include more than one of the aforementioned forms. The pathogenesis and presentation may reflect each location. For example, patients who present with seizures will usually have parenchymal cysticerci. However, some also have cysticerci in the basilar cisterns that can progress to hydrocephalus later if not properly managed.
Clinical Manifestations
The most common clinical manifestation of NCC is seizures (56–58) (Table 46.2). The seizures may be either focal, focal with secondary generalization, or generalized (56,57,59). Most patients with seizures have evidence of parenchymal cysticerci with associated edema or enhancement, corresponding to the colloid or granular nodular stages. Electroencephalographic (EEG) studies for patients with active disease may reveal focal abnormalities. The seizures are usually easily controlled and often resolve as the inflammation subsides (57).

Some patients have seizures from calcified parenchymal NCC (60). The calcified lesions are thought to represent scarring from prior active infection (calcific stage). In spite of the presentation with seizures, few will have focal abnormalities on EEG studies. Patients who develop calcifications are more likely to have recurrence of seizures if anticonvulsants are withdrawn (61,62). Thus, patients with calcifications are classified as having remote symptomatic seizures (i.e., epilepsy) (56,57). They usually require continuous treatment with antiepileptic therapy. Recent MRI studies have documented that some patients with NCC and seizures have calcified lesions with associated enhancement (30,41,43,60,63). There is no evidence that these lesions are associated with viable parasites. Instead, the enhancement may result from breakdown of the calcified granulomas, with antigen release resulting in restimulation of host inflammation (63).
Headaches are common among patients with NCC and may be seen with parenchymal, ventricular, or cisternal NCC. Headaches may be hemicranial or bilateral (64,65). The headaches can be confused with uncomplicated migraines or tension headaches. The pathogenesis is also variable. In some cases, headache is the initial symptom of raised intracranial pressure (ICP). The strong association of parenchymal NCC with migraine-like headaches suggests vascular involvement (64). Headaches may also reflect vasculitis associated with cisternal cysticercosis.
Patients may also present with symptoms or signs of raised ICP. Symptoms may include nausea or vomiting, altered mental status, visual changes, or dizziness. In adults, raised ICP usually results from obstructive hydrocephalus from cysticerci obstructing CSF flow in the ventricles. Patients with numerous cysticerci in the basilar cisterns can also present with communicating hydrocephalus. Some pediatric patients present with large numbers of inflamed cysticerci, causing cerebral edema (51,66). These patients with cysticercal encephalitis may have altered mental status, raised ICP, or seizures.
Neurocognitive defects and decreases in quality of life have been described with cysticercosis (67,68). Infected children are thought to suffer from learning disabilities (69). There also appears to be an association of cysticercosis with depression and perhaps psychosis (70). By contrast, acute alterations of mental status usually reflect ongoing seizures or hydrocephalus. In our experience, altered mental status that does not resolve after a brief postictal period usually results from hydrocephalus.
Less common manifestations include spinal cysticerci, which can present with radicular symptoms or myelopathy, ocular disease with visual changes, subcutaneous nodules, which may be confused with sebaceous cysts, and muscular disease presenting as a myopathy.
Diagnosis
Because the symptoms of NCC are similar to those of several other diseases and the parasite material is not readily accessible, diagnosis has proven problematic (71). A consensus panel proposed diagnostic criteria based on neuroimaging studies, serologic tests, clinical history, and exposure (71). Patients with either an absolute criterion or two major criteria alone with two minor or epidemiologic criteria were considered to have a definite diagnosis. One major criterion plus two other criteria or two minor criteria plus exposure were considered to establish a probable diagnosis (Table 46.3).

Demonstration of T. solium infection in biopsy or autopsy material makes a conclusive diagnosis but is rarely available. Parasites may be visualized directly when there is ocular involvement (55). Neuroimaging studies may reveal a cystic lesion with an associated scolex (demonstrated as a 1- to 3-mm mural nodule) (Fig. 46.4A and B). This is thought to be pathognomonic for cysticercosis, although this has never been rigorously tested (53,72). All of the aforementioned criteria were considered absolute and diagnostic of NCC by Del Brutto et al. (71).
Neuroimaging studies that do not reveal a scolex, although suggestive of NCC, are usually not diagnostic. Neuroimaging studies highly suggestive of NCC were considered major diagnostic criteria. These included cystic lesions, single or multiple ringlike or nodular enhancing lesions, and typical parenchymal brain calcifications. Parenchymal cysts are typically round and 5 to 20 mm in diameter (Fig. 46.4B). They are usually found in the cerebral cortex or the basal ganglia. Cysts in the subarachnoid space are often larger, attaining a diameter of up to 60 mm. They may be either round or lobulated. Cysticerci may also be located in the cerebral ventricles. Ring-enhancing or nodular enhancing lesions are also highly suggestive of NCC. However, a number of other diseases including tuberculomas, brain abscesses, and tumors can cause similar lesions. Cysticercal lesions are usually smaller than 20 mm in diameter and rarely cause midline shift (12). Parenchymal brain calcifications are a common CT finding in NCC. In cysticercosis, the calcifications tend to be solid, dense, supratentorial, and 2 to 10 mm in diameter, and in the absence of evidence of other illnesses should be considered as highly suggestive of NCC. Thus, characteristic calcifications are highly suggestive of NCC (71).
Which imaging modality to use depends on a number of factors. CT scanning is more sensitive than MRI at detecting intracerebral calcifications (53,72). By contrast, MRI is much better at detecting cysticerci in the CSF (e.g., ventricular or cisternal cysticercosis) (52,53,73,74). MRI may also reveal the scolex, which is usually not visible on CT scans (75). Either method is adequate for imaging intraparenchymal cysticerci or hydrocephalus.
Clinical criteria have not generally proved helpful in making a diagnosis. Rajshekhar and Chandy (12) identified combined clinical and radiologic criteria for cysticercosis for patients who presented with seizures and single enhancing lesions. The constellation of a single, round enhancing lesion less than 20 mm in diameter with no midline shift in patients without increased ICP, focal neurologic deficits, or evidence of systemic disease was highly suggestive of NCC. In a cohort of more than 400 patients with a single enhancing lesion, the criteria were about 99% sensitive and specific for NCC in an endemic population. The diagnosis is further supported by resolution of the lesion spontaneously or after anticysticercal therapy or by precipitation of symptoms by antiparasitic drugs (76). Thus, the consensus panel felt that satisfying these criteria was a major diagnostic criterion (71).
Serologic tests have been examined as additional diagnostic criteria. Antibody-detection assays that employ crude antigen have proven problematic in cysticercosis because of cross reactions to other common parasites and nonspecific binding of antibody by the parasite. Tsang et al. (77) at the Centers for Disease Control and Prevention (CDC) developed an immunoblot assay employing semipurified membrane antigens (termed the enzyme-linked immunotransfer blot [EITB]). Binding to any one of seven bands is considered positive. This EITB assay was initially reported to be 98% sensitive and 100% specific for cysticercosis (77). Subsequent studies have confirmed nearly 100% specificity, but the sensitivity is limited in patients with either a single lesion or only calcified lesions (5,78–80). Thus, detection of antibody by the EITB assay is another major diagnostic criterion (71). Other serologic tests are less accurate and are generally not considered major diagnostic criteria. However, studies mostly published since the consensus conference suggest that assays to detect parasite antigens may prove to be important diagnostic studies (81–87) in serum, CSF, and even urine. A number of antigen-detection tests are being developed.
Minor criteria include lesions compatible with NCC, but less suggestive. These include isolated basilar meningitis, hydrocephalus, or filling defects in the spinal subarachnoid space without discrete cysticerci. Each of these may be the only neuroradiologic finding in NCC. However, the radiographic picture is not distinct, with similar findings observed in other diseases. Clinical symptoms of seizures or hydrocephalus, though present in most symptomatic cases, can also be caused by other diseases. Thus, the clinical pattern is also a minor criterion. Finally, evidence of cysticercosis outside the CNS (subcutaneous nodules demonstrated to be due to T. solium, cigar-shaped muscle calcifications) is not diagnostic of NCC and hence is only a minor criterion. Epidemiologic criteria include residence or prolonged visits to endemic areas or contact with a tapeworm carrier.
Management
Symptomatic therapy, antiparasitic drugs, antiinflammatory drugs, and surgical therapy are all important in the management of different forms of NCC. Their use should be guided by knowledge of the pathogenesis of different forms of disease. Thus, treatment of NCC should be individualized based on the clinical presentation, the number, location, and viability of cysticerci, and the host response.
Symptomatic Therapy. Symptomatic therapy plays a critical role in the management of NCC. Seizures should be treated with anticonvulsant drugs and are generally easily controlled (57,59,88). Most published experience is with phenytoin and carbamazepine, but other agents such as levetiracetam and oxcarbazepine are likely effective. Among patients in whom neuroimaging studies normalize and seizures are controlled, antiepileptic treatment can often be tapered off (56,57,59). However, the presence of residual calcifications is a marker for a high risk of recurrent seizures and an indication for continued treatment (61).
Hydrocephalus should generally be treated surgically. If obstruction is due to parasites in the lateral or third ventricles, primary endoscopic removal may be both symptomatic and definitive therapy. Obstructive hydrocephalus can be treated acutely by CSF diversion procedures such as ventriculoperitoneal shunting. In the past, there was a high rate of shunt failure, partly because of aspiration of the cysticerci into the shunt. Recent studies have demonstrated that antiparasitic drugs or treatment with corticosteroids decreases the rate of shunt failure (1,33,89).
Antiinflammatory Drugs. Corticosteroids are frequently used in NCC to control inflammation. The main indications for corticosteroid use are for forms of the disease in which the host response causes life-threatening reactions. These include cysticercal encephalitis, subarachnoid NCC, and spinal intramedullary NCC. Meningitis, vasculitis with stroke, and communicating hydrocephalus can result from the host inflammatory response and accompanying arachnoiditis in subarachnoid NCC. Chronic antiinflammatory therapy is a key component of treatment in these cases. Steroids are also used along with antiparasitic drugs to treat parenchymal NCC to prevent the inflammatory reaction when the parasites die. Methotrexate is increasingly being used as a steroid-sparing agent when antiinflammatory therapy needs to be prolonged (90).
Antiparasitic Drugs. Considerable controversy has existed on the role of antiparasitic drugs in the treatment of NCC. Prior to the use of CT and MRI scanning, diagnosis of NCC was difficult. Thus, the spectrum of cases diagnosed tended to be weighted toward more severe disease. Two developments led to a dramatic change in the prognosis. First, the development and dissemination of CT and subsequently MRI scanning led to more widespread diagnosis of milder cases. Secondly, praziquantel and later albendazole were recognized as antiparasitic agents that could kill the parasites. In Latin America, initial uncontrolled trials of antiparasitic drugs were accompanied by resolution of neuroimaging abnormalities and improved prognosis (91). It was generally assumed that the improvements were a consequence of drug effects. By contrast, in the United States, neuroimaging studies were readily available, but because of regulatory hurdles, antiparasitic drugs were available only later. Thus, U.S. clinicians recognized that many cases of NCC resolved spontaneously without antiparasitic therapy (88). By contrast, when antiparasitic drugs became available, there were reports of worsening symptoms with treatment (92). The first randomized controlled trials were published in the 1990s (93). A metaanalysis of randomized clinical trials evaluating the use of antiparasitics in cystic parenchymal or subarachnoid convexity lesions demonstrated a slightly higher likelihood of radiologic resolution with antiparasitics. Antiparasitics also seemed to be associated with higher resolution of enhancing lesions, although this difference did not reach statistical significance. In addition, antiparasitics were associated with a reduction in seizure recurrence in patients with enhancing lesions (94–96).
Praziquantel was the first antiparasitic drug reported to be effective in NCC. It has been widely available since the early 1980s. Praziquantel is absorbed well after oral administration, but with extensive first-pass metabolism. Metabolism is induced by antiepileptic drugs (carbamazepine, phenytoin, and probably phenobarbital) and corticosteroids (97,98). This induction can be inhibited by cimetidine (e.g., 400 mg orally three times a day), which may increase efficacy when praziquantel is coadministered with anticonvulsants or corticosteroids (99–102). Dose-ranging studies have shown greater effects at doses of 50 mg/kg day in three daily doses for 14 days. Subsequent studies demonstrated that doses as high as 100 mg/kg per day could be given safely (103). Studies with praziquantel in parenchymal NCC given in three doses of 25 mg/kg separated by only 2 hours suggest similar efficacy as with longer courses of therapy (102,104,105).
Adverse reactions to praziquantel in NCC mainly include worsening neurologic function (e.g., headaches, dizziness, seizures, and increased ICP) thought to be caused by the host inflammatory response to the dying parasite. Based on these observations, some observers recommend use of corticosteroids along with antiparasitic drugs (92), but routine use of corticosteroids may decrease efficacy.
Albendazole is a benzimidazole anthelminthic agent with broad-spectrum activity versus most nematodes and cestodes. Albendazole is given is a dose of 15 mg/kg per day in two daily doses. Side effects include minor gastrointestinal (GI) side effects and neurologic effects similar to those described for praziquantel. Imaging studies demonstrated resolution of parenchymal cysticerci as good or better than those noted with praziquantel (106,107). Controlled trials in parenchymal NCC showed no difference in neuroradiologic resolution with treatment for 7 days versus longer courses (107–109). Albendazole was subsequently studied in cases of extraparenchymal disease and was associated with improvement (37,110–114). However, none of these trials was controlled. The only randomized controlled trial that included evaluation of extraparenchymal lesions showed higher resolution with albendazole, but this was not statistically significant (115).
Recent studies have employed albendazole in combination with praziquantel. Animal studies suggest that the combination is synergistic (116). Pharmacologic studies have demonstrated higher levels of albendazole sulfoxide (117). A small, randomized trial of combination therapy in single enhancing lesions noted slightly more rapid resolution, but this was not statistically significant. Two trials of combination therapy suggest enhanced parasiticidal activity, but these trials have not yet been published.
Antiparasitic drugs are not recommended in patients with calcified parenchymal lesions, because there are no signs of viable parasites. These patients typically present with seizures. Seizures are clustered near the time of diagnosis, but they may recur years later (56,57,61). Thus, these patients may require chronic antiepileptic therapy. If patients maintain therapeutic levels of the drugs, the risk of recurrent seizures is very low (59). Some patients will have contrast enhancement or edema on imaging studies (30,41,63). Whether they would benefit from antiinflammatory treatment is unclear. Some patients have hydrocephalus related to scarring from prior infection (e.g., aqueductal stenosis) (118). In this case, hydrocephalus can be corrected by ventriculoperitoneal shunting without the need for antiparasitic drugs.
Parenchymal Neurocysticercosis. Patients with cystic parenchymal lesions are usually asymptomatic. In most symptomatic cases, neuroimaging studies demonstrate evidence of edema or contrast enhancement. Thus, in most cases, symptoms are associated with transitional parasites that are in early stages of degeneration. Neuroimaging studies will typically normalize within 1 to 2 years regardless of whether the patients receive antiparasitic drugs (88,119–123). Thus, seizures tend to cluster near the time of presentation (57). On the other hand, seizures are more common while there is residual parasite material present (57) and most studies demonstrate more rapid radiographic normalization with antiparasitic therapy (120). Accordingly, antiparasitics have shown to decrease the rate of seizure recurrence in patients with parenchymal NCC. Although there is no consensus, most experts also agree that the prognosis for patients with a single inflamed lesion is generally favorable with only symptomatic therapy (88,93,119,124). Many experts think that the prognosis is worse when there are multiple lesions (93,119). Such patients are more likely to have noninflamed viable parasites, and resolution may not be synchronized. Thus, experts are more likely to recommend antiparasitic drugs (47,93,119). In cases with numerous inflamed lesions with associated diffuse cerebral edema (cysticercal encephalitis), antiparasitic drugs are contraindicated (93). Instead, the management of cerebral edema with high-dose corticosteroids (e.g., dexamethasone) or in some cases, mannitol is of utmost importance.
Ventricular Neurocysticercosis. Ventricular NCC usually presents with hydrocephalus. Management should focus on reversing hydrocephalus, reducing the risk for recurrence, and minimizing treatment-associated morbidity (106). The traditional treatment is with emergent surgical removal of the cysticercus from the ventricle. Because open brain surgery is associated with significant morbidity, alternative approaches have been tried. Recent studies have demonstrated that cysticerci can usually be removed by endoscopic surgery (125–131). Cysticerci in the lateral and third ventricles can be removed with rigid endoscopes and the fourth ventricle can be approached with flexible endoscopes. When possible, this is considered the preferred treatment for ventricular cysticerci (93).
Some patients require emergent CSF diversion by placement of a ventriculoperitoneal shunt. In older series, up to 75% of patients with shunts developed shunt failure requiring revision or replacement. Recent studies have demonstrated that shunt revision is infrequently required among patients who are also treated with antiparasitic drugs (33,48,113, 114,132). Similarly, corticosteroids may decrease the risk of shunt obstruction (89).
Subarachnoid Neurocysticercosis and Giant Cysticerci. NCC with involvement of the basilar cisterns is a rare form of NCC associated with a poor prognosis. In one series of patients with this form of NCC, those treated with only CSF diversion had a 90% fatality rate at 10 years (133). Complications include mass effect, communicating hydrocephalus, vasculitis with stroke, and basilar meningitis (34,118).
No controlled trials have been performed on the management of subarachnoid NCC. However, recent case series employing antiparasitic drugs, corticosteroids, and shunting for hydrocephalus have demonstrated a markedly improved prognosis compared to earlier studies (37,45,111,112). A large observational study that investigated medical treatment of patients with giant cysts in the sylvian fissure documented good responses to repeated courses of antiparasitics (37). Thus, most experts consider subarachnoid NCC a clear indication for more intensive antiparasitic therapy. This may take the form of prolonged treatment or repeated courses of albendazole, higher doses of albendazole, or combinations of praziquantel with albendazole.
Other Forms of Neurocysticercosis. NCC in the spine can be located in the subarachnoid space or can be intramedullary. Because of the risks of paralysis from cord swelling, intramedullary infection is usually approached surgically (93). Spinal subarachnoid cysticerci may respond to antiparasitic drugs (45). Ocular disease may also respond to antiparasitic treatment, but the standard therapy is still surgical removal (93).
Prevention
Transmission of T. solium in developed countries has been largely eliminated by meat inspection, improved sanitation, and better animal husbandry. Human infection with adult tapeworms can also be prevented by destruction, freezing, or adequate cooking of infected (measly) pork. Treatment of the tapeworm carrier might result in elimination of the disease. However, detection of tapeworm carriers has been problematic. Mass chemotherapy of humans has been tried with limited short-term success as a field intervention. Development of an effective animal vaccine against cysticercosis may provide the best potential tool toward the eradication of the disease. A recombinant vaccine has been developed for Taenia ovis that can provide nearly complete protection. This vaccine is commercially available in several countries. T. solium antigens have been cloned, and initial study results suggest vaccines based on these proteins may be protective (134). The optimal control measure for cysticercosis may require mass chemotherapy of tapeworm carriers with praziquantel or niclosamide, treatment of pigs with oxfendazole, and vaccination (135).
Echinococcus
Human infection with the different echinococcal species is termed hydatid disease (136–139). The word hydatid refers to the fluid-filled larval forms found in the intermediate hosts (hydatid is derived from a Latin hydatis, or drop of water). Echinococcus granulosus and related species cause cystic hydatid disease, which is characterized by cystic lesions found mainly in the liver or lungs. Isolated brain lesions or second lesions in the brain occur in less than 5% of patients. Echinococcus multilocularis causes alveolar hydatid disease, characterized by tumor-like collections of vesicular parasites in the liver. Isolated lesions in the brain are very rare, but metastatic lesions from the liver to the brain may occur. Echinococcus vogeli and Echinococcus oligarthrus cause polycystic hydatid disease, a rare cause of visceral organ infection noted only in Latin America. CNS involvement has not been described.
The Parasites, Life Cycles, and Epidemiology
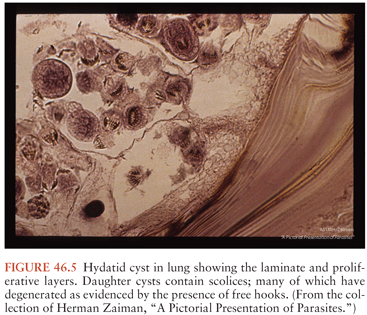
E. granulosus has two obligate mammalian hosts (136–139). The dog and other canines are the main definitive host containing the tapeworm form. The tapeworms are 2 to 5 mm in length and contain only three to four proglottids. The normal intermediate hosts are ruminants, which are infected when eating material contaminated with ova passed in the feces of the canines. In the intermediate host, the eggs hatch and release the invasive larva (termed the oncosphere), which then penetrates the intestine and migrates to the tissues, primarily the liver. The larva develops into a large cystic lesion containing an external laminar membrane, a germinal layer (the brood capsule), and a central fluid layer. Within the cyst fluid are numerous protoscolexes, which form from the brood capsule (Fig. 46.5). When ingested by the definitive host, the protoscolexes develop into tapeworms. If the cyst ruptures, however, the protoscolexes can develop into additional hydatid cysts. A number of separate life cycles have been noted for what was previously assumed to be a single species (140). However, recent molecular evidence suggests that there may be separate species infecting sheep, cattle, horses, and perhaps camels. The sheep strains appear to be most pathogenic for humans, hence the association between human disease and sheep raising. E. granulosus has a wide geographic distribution. Highly endemic areas include countries surrounding the Mediterranean Sea, the Middle East, East Africa, parts of Russia, and many countries in South America. Locally acquired cases are occasionally noted in Australia, New Zealand, North America, China, and South Asia.
E. multilocularis is mainly found in alpine and arctic zones. Foxes and wolves are the main definitive hosts. However, domestic dogs can also be infected. The normal intermediate hosts are rodents. Cases are increasing in Central Europe. Endemic areas also include Russia, parts of China, Canada, and Alaska. In contrast to E. granulosus, the parasite develops as multiple adjacent vesicles, usually without internal protoscolexes. However, the vesicles may bud and may spread as metastatic lesions.
Pathology and Pathogenesis
Humans are infected by ingesting the ova shed by dogs or other canines. After hatching in the intestine, the invasive larva (or oncosphere) is released, attaches to and penetrates the intestine, and spreads hematogenously (136–140). In the case of E. granulosus, approximately 70% of the parasites develop in the liver. Another one third develops in the lung. Less common sites include bone, pelvis, spleen, and the CNS. The echinococcal cysts slowly expand and generally remain asymptomatic until symptoms result from their expanding size or their space-occupying effect. Because years may elapse before cysts enlarge sufficiently to cause symptoms, cysts may be discovered incidentally on routine x-ray or ultrasound studies.
E. multilocularis characteristically presents as a slowly growing hepatic tumor, with progressive destruction of the liver and extension into vital structures (138,141). Patients commonly complain of right upper quadrant and epigastric pain, and obstructive jaundice may be apparent. The liver lesions metastasize to brain or lung.
Clinical Manifestations
Patients with hepatic echinococcosis who are symptomatic most often present with abdominal pain or a palpable mass in the right upper quadrant. Rupture or leakage from a hydatid cyst may produce fever, pruritus, urticaria, eosinophilia, or anaphylaxis. CNS disease typically presents as a mass lesion. In this case, the slowly enlarging lesion may cause headaches, seizures, or focal neurologic abnormalities (137,142–145).
Diagnosis
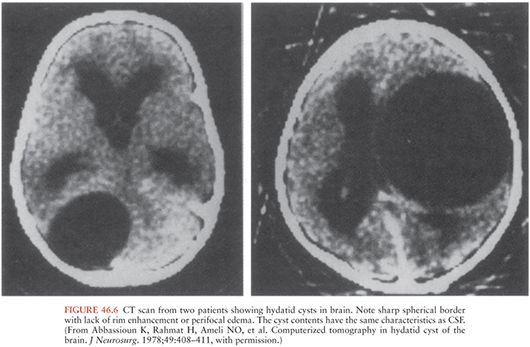
Diagnosis is primarily made by imaging studies. In the case of E. granulosus, CT or MRI scans of brain typically demonstrate a spherical cystic lesion with smooth borders (144,146) (Fig. 46.6). The cyst wall may or may not be visible. Daughter cysts within the main cyst and degenerative forms are occasionally visualized by MRI. When the internal images from the protoscolexes (or “hydatid sand”) are seen, the image can be diagnostic. Midline shift and distortion of the ventricles are common. Lesions are usually not inflamed and thus do not have surrounding edema or enhancement. Cysts may be found outside of the CNS, which suggests that the CNS lesion is also due to hydatid disease. The CT appearance of E. multilocularis is less distinct. It may present as an ill-defined mass lesion. Most cases will also have evidence of liver involvement.
In doubtful cases, serologic assays can be diagnostic. Enzyme-linked immunosorbent assay (ELISA) or indirect hemagglutination assays are readily available and can be confirmed by immunoblot assays. However, the sensitivity is not optimal for extrahepatic disease. For E. multilocularis, the EM2 ELISA with purified antigen is the confirmation test.
Treatment
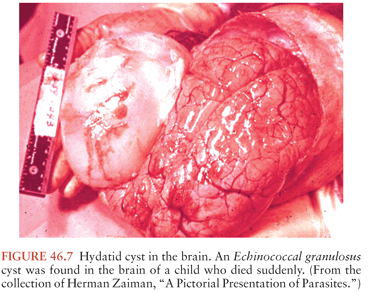
The treatment of choice for CNS hydatid disease is surgical removal. In the case of E. granulosus, care must be taken not to rupture the cyst with resultant spread of the protoscolexes (Fig. 46.7). Complications can include recurrent disease and death (147,148). This is more readily accomplished if the diagnosis is made before surgery. Antiparasitic drugs, particularly albendazole, are routinely recommended before surgery in hepatic cases (138,149). Although the role of chemotherapy in CNS disease is not clear, data suggest that adjuvant chemotherapy for CNS disease is associated with an improved outcome (143). The practice of aspirating the cyst and injecting scolicidal agents is no longer recommended because of the increased risk of spillage of the protoscolexes. Drapes and potentially contaminated surfaces should be covered with hypertonic saline.
In the case of E. multilocularis, CNS involvement is often a manifestation of advanced disease (141). Few cases of alveolar hydatid disease can be cured with surgery alone, so chemotherapy is now routinely recommended postoperatively (149). In patients in whom the lesion is completely resected, albendazole is recommended for 2 years after surgery. In other patients, albendazole should be continued indefinitely. There are also reports of treatment of cerebral disease with radiosurgery plus albendazole (150).
Prevention
Peridomestic transmission of E. granulosus is linked to canine infection, which can be prevented by not feeding dogs on viscera of ruminants. People should also limit exposure to material potentially contaminated by dog feces. The vaccine has already been developed and licensed in New Zealand. E. multilocularis may be acquired from foxes or other animals. Care should be taken to avoid exposure to material potentially contaminated by feces (e.g., wearing gloves when gardening).
Sparganosis
Sparganosis, caused by Spirometra mansoni, Spirometra ranarum, and Spirometra erinaei, is a parasitic infection caused by the migratory larvae of these tapeworms. The adult tapeworm parasitizes the intestines of domestic and wild carnivores, such as dogs and cats. The intermediate hosts are usually frogs or snakes. Humans are an incidental host.
Epidemiology
Sparganosis infections occur worldwide but are most commonly reported in China, Korea, Japan, and Southeast Asia (24,151–157). Most cases have a history of eating frogs or snakes (152,153,155). Topical application of frog or snake poultices is also an important risk factor. Less than 70 cases have been reported from the United States. The most common species causing human infections in the Western Hemisphere is Spirometra mansonoides. In the Far East, the most common species is S. ranarum followed by S. mansoni and S. erinaei (24).
Parasitology
Adult tapeworms (measuring 3 to 40 cm in length) are found in the small intestines of domestic and wild carnivores (24). Eggs are shed in the animal’s feces into freshwater. Two intermediate hosts are involved in the sparganosis infection cycle. The first includes an aquatic copepod crustacean, Cyclops, where the procercoid larva develops. A second host, such as frogs, snakes, mammals, and birds, can ingest these infected copepods. The procercoid larva develops into a motile form, called the sparganum, in the second intermediate host’s GI tract. From the GI tract, the sparganum is able to attach to the mucosa, penetrate the intestinal wall, and migrate into various tissues. The life cycle of Spirometra species is completed when domestic or wild carnivores eat the infected tissues of the second intermediate host. Humans serve as accidental secondary intermediate hosts when ingesting polluted freshwater containing Cyclops; eating raw or inadequately cooked infected tissues of secondary intermediate hosts, such as frogs, fish, and snakes; or by the direct application of infected tissues to the eye or an open wound as in traditional medicines (24).
Pathogenesis and Pathology
The spargana are off-white, motile, ribbon-shaped larvae with a broad evaginated anterior end (future scolex) that can protrude and retract (24,158) (Fig. 46.8). This protruding and retracting motion helps it traverse the host’s tissues. When the larvae reach the host tissues, they will either remain coiled or can become elongated. Nodules (2 to 3 cm) are then formed in the subcutaneous tissues and superficial muscles and less commonly the abdominal cavity, perirenal fat, breast, scrotum, ureter, lymphatics, orbit, and the CNS. These nodules can be tender, inflamed, painful, or pruritic. Larva can be dissected out of fibroadipose tissue in extracted nodules. When the parasites die, a strong inflammatory reaction is elicited, leading to an abscess containing the Spirometra larvae.

This organism rarely enters the brain parenchyma, ventricular system, or spinal canal. Cerebral sparganosis usually involves the cerebral hemispheres, with occasional extension to the external and internal capsules and basal ganglia (158–162). White matter degeneration occurs, probably caused by migration of the surviving larvae along the fiber tracts. The cerebellum, brainstem, and spinal cord are less commonly involved. In the CNS, the larva is surrounded by a collagenous capsule, granulomatous layer of inflammatory cells, and the formation of new capillaries. Degenerated worms develop into punctate calcifications.
A rare and lethal form of sparganosis, termed proliferative sparganosis, has been described in Japan in which spargana proliferate in tissues and throughout the body, ultimately leading to death (24,163).
Clinical Manifestations of Central Nervous System Sparganosis
CNS involvement with sparganosis occurs in variable proportions of cases (152,153,155,159). CNS involvement may be subacute or chronic. The most common neurologic manifestations included seizures, hemiparesis, and headache (152,159). When the migrating worms penetrate the ventricles, they can lead to intraventricular hematomas and obstructive hydrocephalus. Cerebral sparganosis can result in vasculitis leading to cerebral hemorrhage or infarction.
Diagnosis
Sparganosis should be suspected in patients from endemic areas with a history of eating frogs, fish, or snakes, or characteristic findings on imaging studies (159–162). Serodiagnostic tests for sparganum-specific immunoglobulin G (IgG) in either serum or CSF can help support the diagnosis but are not widely available. An ELISA developed in Korea was reported to have a sensitivity of 80% to 85% (151). Definitive diagnosis is made by the finding of a live worm or a lesion consisting of the remains of a sparganum. Stereotactic biopsy has also been used in making the diagnosis (164).
CNS imaging by CT or MRI may show characteristic slightly hypointensity on T1 images and hyperintense on T2 (151,159–161,165). The CT triad of white matter hypodensity with adjacent ventricular dilation, irregular or nodular enhancing lesion, and small punctate calcifications is specific for cerebral sparganosis, occurring in most patients (151,160). Movement of nodules or serpiginous tubular tracts, as shown by repeated CT or MRI of the head, represents larval migration in the brain (159,165). This may occur in one third of patients. MRI is less sensitive than CT for detecting small punctate calcifications. However, MRI is more sensitive in detecting inflammatory changes, degenerated white matter, thickening of the meninges, and nodular and subcortical lesions. Intracerebral petechial hemorrhage or hematoma may also be seen. These lesions represent capillary or venous injury from the migrating worm. MRI may show linear or curvilinear contrast-enhancing lesions, conforming to the shape of the worm. Cerebral sparganosis can be confused with primary or metastatic malignancies and granulomatous diseases.
Treatment
Treatment consists of surgical removal of nodules for patients with migrating nodules or lesions or worsening of CT findings (Fig. 46.8) (151,159,164). Whether patients with nonmigrating nodules, lesions, or calcifications should undergo surgical removal of lesions is not clear. Medical therapy with praziquantel (50 mg/kg per day for 14 days) can kill the parasites and might provide benefit for patients with surgically inaccessible CNS sparganosis (141). When patients have seizures, antiepileptic drugs should be given. Whether there are any benefits to administering antiepileptic drugs to asymptomatic patients is not known.
Prevention
Individuals who live in or are traveling to endemic areas should avoid ingestion of frogs, fish, snakes, and contaminated water.
CENTRAL NERVOUS SYSTEM DISEASES CAUSED BY NEMATODES
Angiostrongyliasis
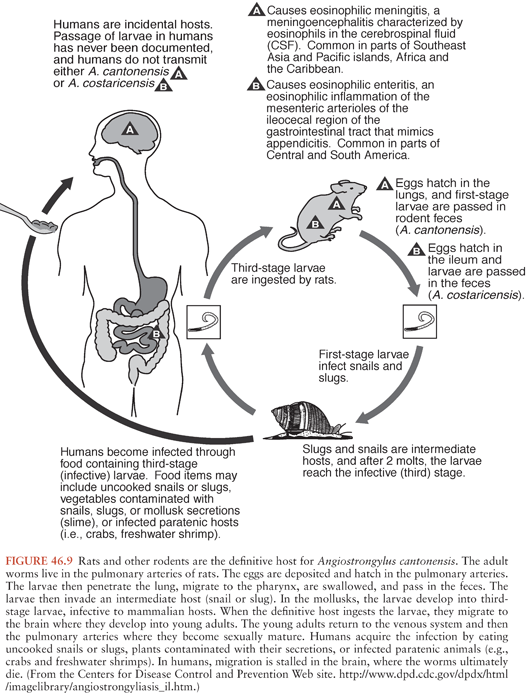
Angiostrongylus cantonensis, the rat lungworm, is responsible for most human cases of eosinophilic meningitis and meningoencephalitis. This parasite is found in many Pacific islands and in Southeast Asia, but has recently spread to Hawaii, the Caribbean, and the Gulf of Mexico (157,166–172). The mortality rate of A. cantonensis in human eosinophilic meningitis is low, but fatalities do occur. Human CNS infections with this nematode are characterized by headache, paresthesias, and CSF leukocytosis with striking eosinophilia. The related species Angiostrongylus costaricensis causes eosinophilic gastroenteritis.
Epidemiology
Angiostrongyliasis occurs worldwide. The disease was first described in rats in 1933, and the first human case was described in 1944 (166,167). Initial reports came from Southeast Asia (Thailand, Malaysia, Vietnam, Indonesia, and southern China) (166,167,173,174). Subsequent cases have been noted from the Pacific Islands, Egypt, the Caribbean, and Brazil (166,167,170,175). In the United States, angiostrongyliasis is endemic in Hawaii and has been reported along the Gulf coast (171,172). The wide geographic spread of infection is attributed to rats that are carried on ships. Increasing transcontinental travel, influx of refugees, and importation of food from Southeast Asian countries are thought to be factors. The endemic focus in the Caribbean likely represents spread from Asia (175).
Parasitology
The definitive hosts for A. cantonensis are rats (Fig. 46.9). The adult worm (2 to 3 cm long) lives and deposits eggs in the pulmonary arteries of rats and other rodents (176). The eggs reach the lung and hatch into first-stage larvae in the terminal branches of the pulmonary arteries. The larvae then migrate up the rat’s trachea to reach the pharynx. The rat swallows the larvae, allowing access to the GI tract and subsequent passage in the feces.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree