Primary central nervous system tumors constitute a relatively rare, but significant, fatal health problem in the United States and the rest of the world. Every year in the United States approximately 44,000 new primary brain tumors are diagnosed, or 14 in every 100,000 citizens. Of these, approximately 60% are malignant and 45% are gliomas. Malignant gliomas are the second leading cause of cancer mortality in people under the age of 35, the fourth leading cause in those under the age of 54, and kill approximately 13,000 people per year. Epidemiologic evidence suggests children younger than age 14 years and patients older than age 70 years have a higher incidence of primary brain tumors compared with other age groups Median age at diagnosis for primary brain tumors is between 54 and 58 years old Metastatic brain tumors arising from other primary cancer types are substantially more common, affecting more than 150,000 patients each year in the United States.
Glioblastoma is a particular type of infiltrative malignant glioma with a very poor patient prognosis and few effective treatment options. Despite advances in surgical procedures, radiation, and chemotherapy, median survival is only around 14 months for glioblastoma patients when treated with radiotherapy and concurrent temozolomide, followed by adjuvant temozolomide (“Stupp protocol”). Tumor cell invasion into normal parenchyma, otherwise undetected by standard neuroimaging techniques, along with resistance to treatments remains the primary reason for poor prognosis in glioblastoma. Low-grade gliomas have a substantially longer median survival, ranging from 6 to 10 years when including both astrocytomas and oligodendrogliomas. Except for medulloblastoma and embryonal tumors, younger patients generally have a better survival rate than older patients, and patients with any type of glioma who survive more than 2 years from diagnosis have more than a 75% chance of surviving more than 5 years.
Primary brain tumor classification
Central nervous system tumors are currently classified based on histologic features and the particular tumor cells of origin. Examination of histologic features includes assessing the general degree of tumor cellularity (i.e., cell density), architecture of the tumor, organization of cellular arrangements (e.g., rosette formations, whirling patterns, angiogenesis), cytologic features of the tumor cells themselves (e.g., identification of cellular processes, cytoplasm characteristics), and cytologic atypia suggestive of malignancy. The use of specialized histochemical or immunohistochemical stains, including glial fibrillary acidic protein (GFAP), further help differentiate the particular type of brain tumor. Table 23-1 summarizes the main classifications of primary intracranial central nervous system tumors.
|
Neuroimaging in brain tumors
Modern neuroimaging techniques and biomarkers in brain tumors are intimately tied to clinical understanding of in vivo human brain tumor behavior. Almost all brain tumor treatment decisions are based on neuroimaging findings and interpretations ( Figure 23-1 ).
Magnetic resonance imaging (MRI) constitutes the most widely used modality for both structural and physiologic imaging, followed by computed tomography (CT) and molecular imaging techniques such as positron emission tomography (PET). In fact, the first medical use of MRI was in describing the characteristics of a patient with a primary brain tumor. Novel contrast agents, MR pulse sequences, and molecular tracers, combined with postprocessing techniques using advanced quantification, simulation, and computer vision algorithms can provide the clinician with a host of information on which to base treatment decisions.
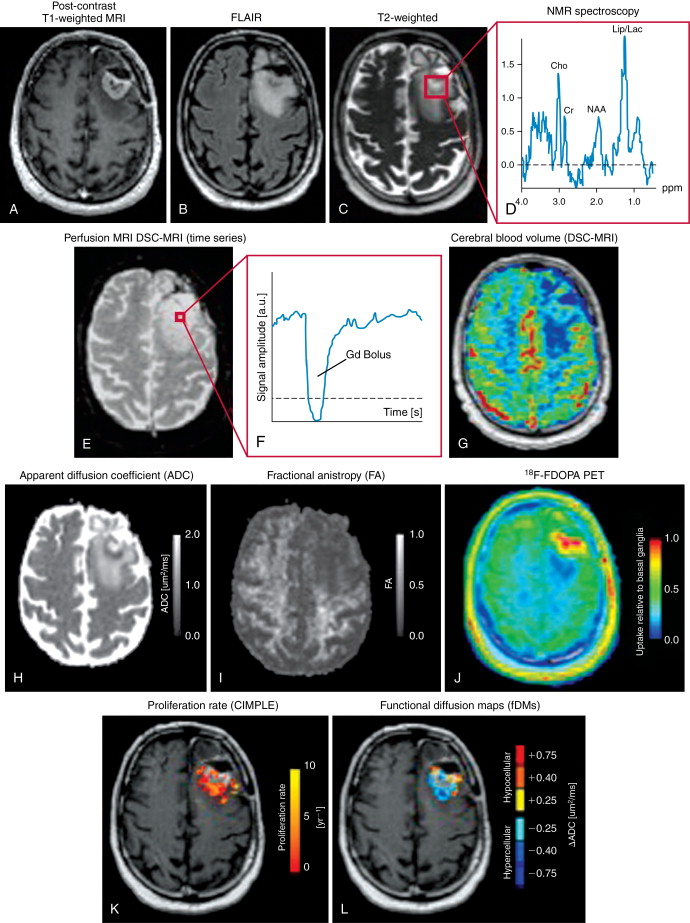
Standard MRI. The minimal MR examination of a patient with a primary brain tumor consists of a T2-weighted, fluid-attenuated inversion recovery (FLAIR), and both a pre- and postcontrast T1-weighted image series. Gadolinium contrast agents are the most widely used in MR brain examinations because of hyperintense (bright) contrast on T1-weighted images due to the significant increase in longitudinal relaxivity (an MR property), which accounts for the increased signal seen.
Diffusion MRI. Diffusion MRI is a physiologic imaging technique that can be used to quantify the apparent diffusion coefficient (ADC), a quantity representing the magnitude of microscopic motion of water molecules. ADC is inversely proportional to cell density, likely reflecting the degree of extracellular space, but ADC also is sensitive to edema and necrosis. Thus, ADC is potentially clinically useful for assessing both cytotoxic and antiangiogenic treatment response. , Diffusion tensor MRI is a modification of the standard diffusion MR pulse sequence where direction as well as magnitude is quantified. Diffusion of water molecules is anisotropic in the central nervous system, meaning water molecules diffuse faster in one particular direction compared with other directions. Myelination and cylindrical symmetry in cerebral white matter regions creates high diffusion anisotropy in the normal brain. This anisotropy can be exploited to create pseudo-axonal tracts for visualization and quantification of white matter integrity and connectivity, a technique termed diffusion tensor tractography . Registration of ADC maps obtained from patients over time allow for voxelwise changes in ADC to be quantified, a technique termed functional diffusion mapping (fDM ). , , FDMs allow for the visual assessment and quantification of tumor response to treatment, and are particularly useful when tumors are highly heterogeneous.
Perfusion MRI. Perfusion MRI techniques are useful for detection and quantification of neovascularization, which is characteristic of malignant transformation in brain tumors. Perfusion MRI can be performed using the susceptibility (T2*) contrast changes observed during injection of an exogenous (typically gadolinium-based) contrast agent in a first-pass bolus tracking experiment termed dynamic susceptibility contrast (DSC)-MRI . DSC-MRI is a quick (typically less than 2 minutes) scan that can be used to easily estimate relative cerebral blood volume (rCBV), relative cerebral blood flow (rCBF), and mean transit time (MTT) and allows for quantification of vessel permeability using a global leakage correction algorithm (K2). Perfusion MRI can also be performed using assessment of longer-lasting longitudinal relaxivity changes caused by injection of exogenous MR contrast agents. By obtaining dynamic T1-weighted images over 8–10 minutes and applying a simple two-compartment pharmacokinetic model to the resulting signal-vs-time curves, dynamic contrast enhanced (DCE)-MRI estimates of blood volume fraction ( v e ) and vascular permeability ( K trans ) can be obtained. Arterial spin labeling (ASL) is a perfusion MRI technique that magnetically tags arterial blood water and images the resulting water once it enters the cerebral vasculature, effectively using blood water as an endogenous contrast agent. Absolute quantification of cerebral blood flow (CBF) can be obtained using ASL techniques.
Functional MRI. Functional MRI (fMRI), or more specifically blood oxygenation level dependent (BOLD) fMRI, is commonly used during surgical planning in gliomas to avoid important functional regions of the brain. During a functional paradigm such as finger tapping, auditory cues, visual stimuli, or language-specific tasks, regions of the cortex responsible for these tasks consume a higher level of oxygen, resulting in different concentrations of oxyhemoglobin and deoxyhemoglobin within these regions. The difference in magnetic susceptibility between oxyhemoglobin (diamagnetic) and deoxyhemoglobin (paramagnetic) causes signal differences when scanned using MRI, which can be used to create maps of functional activation.
NMR Spectroscopy. Single-voxel nuclear magnetic resonance spectroscopy (MRS) or multivoxel chemical shift imaging (CSI) can be used to quantify brain tumor metabolites having NMR signatures. Choline (Cho), N -acetylaspartylglutamate (NAA), along with lipids and lactate signatures are among those commonly altered in malignant brain tumors. Increased Cho is suggestive of an increase in cell turnover due to rapidly dividing cells. NAA, an intracellular neuronal marker, is commonly decreased in growing primary neoplasms. The ratio of Cho/NAA is commonly used as an NMR spectroscopic measure of malignant potential, with values higher than one indicative of a rapidly growing neoplasm. Lipid and lactate peaks, when present, are largely thought to reflect the degree of microscopic necrotic tissue composition, which can indicate an aggressive tumor. Other metabolites, including creatine and myo-inositol, , are also implicated in different malignant features or metabolic characteristics of different brain tumor subtypes.
Positron emission tomography. Molecular imaging using PET and radiolabeled tracers is rapidly becoming popular for serial assessment and diagnostic questions in patients with primary brain tumors. 2-[ F]-fluoro-2-deoxyglucose ( F-FDG) PET is the most common radiotracer used to detect malignant lesions in the body, and increased uptake of F-FDG is present in malignant gliomas (WHO grades III-IV) compared with low-grade gliomas (grade II). , Studies have shown elevated uptake of F-FDG in glioblastoma compared to contralateral normal brain, which has shown prognostic value with respect to time-to-progression (TTP). This change in F-FDG uptake can be detected within days of initiating radiochemotherapy ; however, no improvement in overall survival (OS) or progression-free survival (PFS) has been demonstrated by targeting FDG-avid areas with supplemental radiation treatment, which may speak to the complexity in delineating treated tumor from normal tissue that also exhibits elevated glucose consumption. This high basal F-FDG uptake in normal brain is one of the primary disadvantages of using F-FDG PET clinically, as it has significantly lower specificity compared with other PET tracers. Amino acid and amino acid analog PET tracers comprise a fundamentally different category of molecular imaging agents that show elevated uptake in tumor tissue with low uptake in normal brain tissue, resulting in higher specificity for delineating tumor. , The most common and most widely studied amino acid tracer is C-methionine ( C-MET) ; however, the short half-life of C has resulted in increased use of F-labeled amino acid analogs, including O -(2-[ F]-fluoro-ethyl)-l-tyrosine ( F-FET) and 3,4-dihydroxy-6-[ F]-fluoro-l-phenylalanine ( F-FDOPA). The uptake of F-FET and F-FDOPA has been reported to be similar to C-MET, , suggesting results obtained using C-MET will be similar to those obtained with newer F-labeled amino acid analogs. Although F-FDG and amino acid tracers constitute the majority of PET tracers used in routine clinical practice, the use of less routine PET tracers also show promise, including 3′-deoxy-3′-[ F]-fluorothymidine ( F-FLT) to visualize tumor regions undergoing proliferation or [ F]-fluoro-misonidazole ( F-FMISO) for visualizing hypoxic tumor regions.
Gliomas
The World Health Organization (WHO) classifies primary nervous system tumors into grades I to IV in order of increasing malignancy and worse prognosis. According to the WHO classification, gliomas can be divided into either diffuse or localized subtypes, for which diffuse gliomas can be further subdivided into astrocytomas, oligodendrogliomas, and mixed oligoastrocytomas, depending on the particular histologic features of the tumor. Glioblastoma, or grade IV astrocytoma, can be subdivided into primary and secondary glioblastoma. Secondary glioblastomas arise from malignant transformation of grade II/III gliomas, as opposed to primary glioblastomas, which arise de novo. Ependymomas and choroid plexus tumors, which are also considered of glial origin, constitute most of the remaining proportion of primary brain tumors in adults.
Astrocytoma
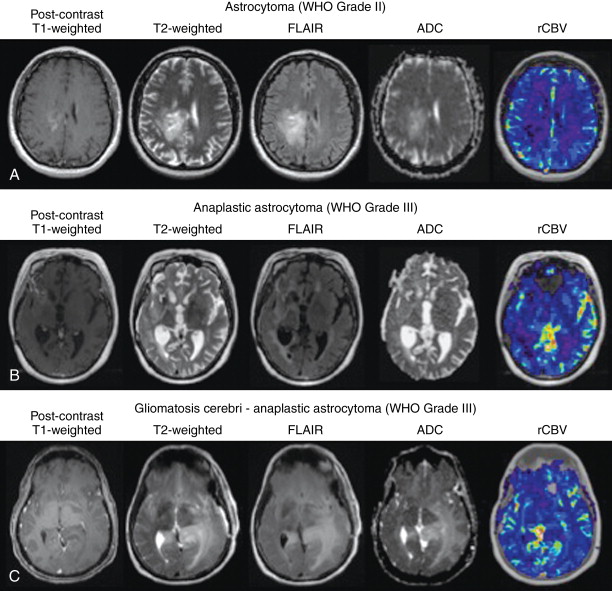
Astrocytomas (WHO grade II) are diffusely infiltrative gliomas composed of neoplastic astrocytes. Astrocytomas typically have ill-defined boundaries, low cellularity, and few nuclear atypia. , Lacking malignant features such as endothelial microvascular proliferation, high mitotic activity and proliferation rate, and microscopic necrosis, astrocytomas are typically of low aggressivity compared to higher-grade tumors. Postcontrast T1-weighted MR images of low-grade astrocytomas typically lack contrast enhancement, as contrast uptake is indicative of blood–brain barrier compromise commonly caused by microvascular proliferation, characteristic of malignant transformation. Diffusion MR estimates of apparent diffusion coefficient (ADC), a measure of water mobility inversely proportional to cell density and proportional to extracellular space, is often low in distinct regions thought to contain viable tumor, while elevated in regions of infiltration and/or edema.
Anaplastic astrocytoma
Anaplastic astrocytomas (WHO grade III) are malignant astrocytomas with many of the histologic features of grade II astrocytomas, but lacking the endothelial microvascular proliferation and necrosis found in glioblastoma (WHO grade IV) tumors. Anaplastic astrocytomas are typically more highly cellular compared to low-grade astrocytomas and exhibit mitotic activity (Ki-67), typically of 12%–14%. , Increased cytoplasmic pleomorphism and elevated cellularity in anaplastic astrocytomas manifest as lower mean ADC values compared with low-grade astrocytomas, whereas slightly higher cerebral blood volume is also observed in high- compared to low-grade astrocytomas. Anaplastic astrocytomas typically demonstrate a higher degree of infiltrative features on standard and advanced magnetic resonance imaging (MRI) compared to low-grade astrocytomas, including blurring of the gray–white matter interface, and have higher rates of enhancement ( Figure 23-2 ).
Oligodendroglioma
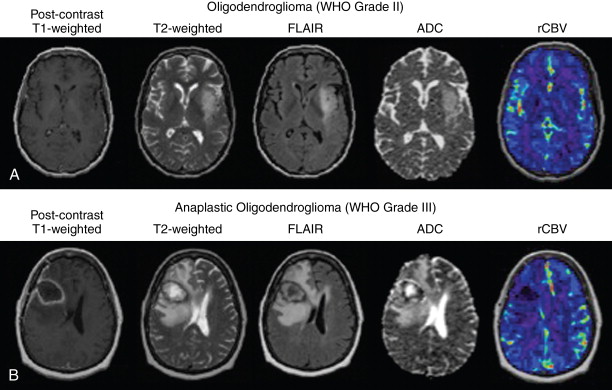
Oligodendrogliomas (WHO grade II) comprise 2.1% of all primary brain and CNS tumors and 6.6% of all primary brain and CNS gliomas. Oligodendrogliomas occur approximately three times more often in white compared with black patients, and occur most often in the fourth and fifth decades of life. Almost two-thirds of patients with oligodendrogliomas present with seizures. Oligodendrogliomas are often gelatinous masses that tend to blur the white–gray matter interface on anatomic MRI. Oligodendrogliomas also often exhibit cystic degeneration and necrosis on MRI ( Figure 23-3 ). Calcifications within oligodendrogliomas are often detectable as hypointensities on gradient recall echo (GRE) or susceptibility-weighted MRI sequences, while presenting as hyperdensity on computed tomography (CT). Similar observations on MRI are also common because of hemorrhages within oligodendrogliomas. Histologically, oligodendroglioma tumor cells have indistinct cytoplasm and round nuclei, giving them a “fried egg” appearance, and are often distributed along regions of neovascularization with distinctive vascular patterns or “chicken wire” patterns. These geometrically distinct vascular patterns manifest as elevated blood volume on perfusion imaging compared with the same-grade astrocytomas, and the tightly packed oligodendroglioma tumor cells often result in a lower ADC compared with astrocytomas. Grade II oligodendrogliomas do not present with histopathologic features of anaplasia or malignant transformation; therefore, these tumors may have low a mitotic index (Ki-67) between 2% and 5%.
Anaplastic oligodendroglioma
Anaplastic oligodendrogliomas (WHO grade III) often occur as a result of anaplastic transformation of oligodendroglioma WHO grade II tumors, but can also occur de novo. Although they maintain many of the histologic features of low-grade oligodendrogliomas, anaplastic oligodendrogliomas have additional malignant features including increased mitotic activity, endothelial cell proliferation, microscopic necrosis, increased nuclear and cytoplasm pleomorphism, and tumor cell infiltration. Anaplastic oligodendrogliomas have both better clinical and prognostic outcomes compared to anaplastic astrocytomas, primarily because of the increased chemosensitivity in oligodendrogliomas. ,
Mixed and anaplastic oligoastrocytoma
Mixed oligoastrocytomas (WHO grade II) and anaplastic oligoastrocytomas (WHO grade III) are primary brain tumors consisting of both neoplastic oligodendroglial and astrocytes. Although no definitive histologic criteria exist for diagnosis of oligoastrocytomas, identification of neoplastic cells from both populations appears to be necessary, either diffusely distributed or focally isolated. As with oligodendrogliomas, mixed oligoastrocytomas or anaplastic oligoastrocytomas have a more favorable prognosis compared with pure astrocytomas of the same grade or with glioblastoma. The extent of the oligodendroglial component of these tumors correlates with improved overall survival.
Glioblastoma
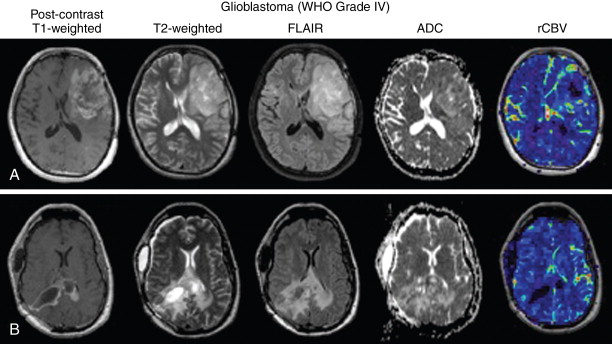
Glioblastoma, previously referred to as glioblastoma multiforme (GBM) , are the most malignant and most common primary brain tumors, constituting nearly 54% of all primary gliomas. Glioblastomas occur most frequently in older patients, and patterns in glioblastoma location suggest that the most frequent region of tumor occurrence moves from anterior to posterior with increasing age. Glioblastomas are highly infiltrative, proliferative, and aggressive brain tumors with a very poor patient prognosis. Despite advances in surgical resection, radiotherapy, and chemotherapy, median survival for patients with glioblastoma is around 14.6 months (radiotherapy and temozolomide) and 12.1 months for radiotherapy alone. Microscopically, glioblastomas exhibit many of the malignant features of anaplastic gliomas, with the addition of endothelial microvascular proliferation and pseudopalisading necrosis. Anatomic MRI exhibits contrast enhancement on postcontrast T1-weighted images in the majority of cases, and this contrast enhancement often occurs in a “ring-shape” enhancement with an area of central T1 hypointensity indicative of macroscopic necrosis. Perfusion imaging demonstrates elevated blood volume and blood flow in regions of contrast enhancement, corresponding to regions with elevated angiogenesis. T2-weighted or fluid-attenuated inversion recovery (FLAIR) images often show extensive tumor infiltration, along with “fingers of edema” presented as hyperintense regions on T2-weighted images respecting the gray–white matter interface at the cortical ribbon. T2-isointense or T2-hypointense regions within T2-hyperintense regions may signify dense bulk tumor regions in glioblastoma ( Figure 23-4 ).
Surgical planning and guidance
Imaging plays a large role in current surgical planning and intraoperative guidance for the treatment of gliomas. A number of imaging techniques are useful in surgical management of patients with gliomas. fMRI, MRI with diffusion tensor imaging (DTI), intraoperative monitoring, and image-guided needle biopsies are all useful in planning and execution. The overall goal of imaging is to assist the surgeon in obtaining a maximal resection while preventing neurologic complications . Extent of resection has been shown to correlate with overall survival.
Preoperative fMRI is used to localize critical areas involved in learning, memory, speech, motor, and sensory function. , fMRI testing maps areas of basic functions with regard to the tumor location. Mass effect from tumor or edema can distort the normal architecture of the brain, making localization by traditional landmarks difficult. fMRI facilitates surgical planning by localizing areas of eloquent cortex prior to surgery.
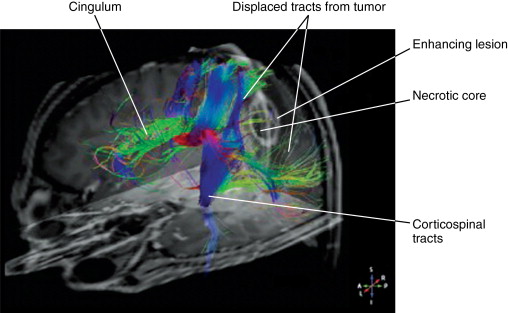
MRI with DTI provides information about major white-matter tracks associated with eloquent cortex ( Figure 23-5 ). It can determine if tumor is displacing critical white-matter tracks, enabling the surgeon to resect the tumor without causing significant neurologic deficits. Whereas fMRI helps the surgeon avoid critical areas of the cerebral cortex, MRI with DTI identifies the major white matter pathways and connections, assisting the surgeon with resection in the white matter and avoiding major fiber tracts. Interoperative MRI can account for brain shift that occurs during surgery, either from changes in pressure or resection of tumor. Image-guided needle biopsy provides real-time feedback on the location of the biopsy needle, increasing the accuracy of a blind needle biopsy to a few millimeters. These techniques minimize the potential for neurologic complications from surgery while helping the surgeon safely resect as much tumor as possible.
Treatment
Grade II gliomas
Treatment of grade II astrocytomas, oligoastrocytomas, and oligodendrogliomas are similar and are largely customized to the clinical situation and tumor location. Although guidelines exist for treatment, there is no level 1 evidence to define a standard treatment course. If maximal resection is not feasible, subtotal resection or biopsy should be considered for debulking and tissue diagnosis. Radiation has shown a clear benefit in grade II gliomas. However, there is some debate about when to start radiation therapy. When upfront radiation was compared to radiation at tumor progression, there was no difference in overall median survival (7.4 vs 7.2 years), but there was an increase in progression-free survival in the upfront setting and better control of symptoms such as seizures. The decision to treat is left to the clinician and often depends on the remaining tumor bulk, extent of resection, and involvement in or near eloquent cortex. Temozolomide chemotherapy alone, though less effective than radiation, is sometimes used up front in patients wishing to delay radiation in order to postpone long-term cognitive side effects of radiation therapy. Patients with low-grade gliomas are followed with serial MRI imaging, initially every 3–6 months for the first 5 years, and then at least yearly thereafter.
Grade III gliomas
Treatment of anaplastic astrocytomas and anaplastic oligodendrogliomas is also similar. Again, much of the clinical management is dictated by expert consensus, and data from phase III studies is largely lacking. The initial treatment for grade III gliomas is gross total resection as assessed by routine MRI. When gross total resection is not possible, subtotal resection and biopsy are used for tissue diagnosis.
Following resection, treatment is most often 6 weeks of radiation therapy followed by chemotherapy. , Radiation therapy is fractionated into 30–33 treatments over 6 weeks. Chemotherapy is usually temozolomide, 5 days of treatment followed by 23 days off, for one 28-day cycle. Temozolomide was initially approved by the FDA for recurrent anaplastic astrocytomas. The PCV regimen of procarbazine, lomustine (CCNU), and vincristine have also been used. This regimen has been largely replaced by temozolomide alone, given the improved tolerability of temozolomide in comparison to PCV.
Patients are followed with serial MRI imaging, starting with the first postsurgical scan 2–6 weeks following radiation therapy, prior to the start of adjuvant chemotherapy ( Figure 23-6 ). Patients are monitored by MRI for response or progression every 2–4 months for 2–3 years, then less frequently afterwards. If recurrence is local, a second surgery can be considered, with or without the addition of carmustine (BCNU) wafers to the surgical cavity, followed by a change in the chemotherapy regimen. Reirradiation is also considered, especially if recurrent disease is outside the original field of radiation, but this is controversial.
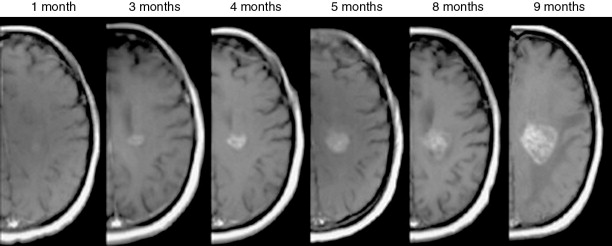
Glioblastoma (who grade IV)
The standard of care treatment for glioblastoma and gliosarcoma has become the Stupp regimen. This consists of maximal resection if possible, followed by 6 weeks of daily chemotherapy with temozolomide plus radiation therapy, which is fractionated into 30–33 treatments over that initial 6-week period. Postsurgical imaging is recommended within 72 hours in order to assess the extent of resection and possible ischemia before postsurgical blood products cause significant T1 shortening (hyperintensity on precontrast T1-weighted images), as this can often mask residual contrast enhancement on postcontrast T1-weighted images. A second postoperative imaging session is usually acquired 2–6 weeks after 6 weeks of concurrent radiation and temozolomide in order to assess the response to the initial treatment regimen, prior to initiating adjuvant cycles of temozolomide. The goal of this imaging regimen is to first assess the initial extent of resection. Near gross-total resection is associated with a good prognosis. Next, imaging is obtained a few weeks after initial radiation and chemotherapy to establish a baseline tumor burden once postsurgical and initial postradiation changes have stabilized. This baseline is used to assess for tumor progression ( Figure 23-7 ). Adjuvant temozolomide is typically given 5 consecutive days every 28-day cycle as described above. Bevacizumab is a monoclonal antibody to vascular endothelial growth factor, which is FDA approved for recurrent glioblastoma, and is used alone or with other chemotherapy agents, such as irinotecan. , Patients are encouraged to participate in clinical trials at disease recurrence. Participation in clinical trials at the time of initial diagnosis is becoming more common as well.
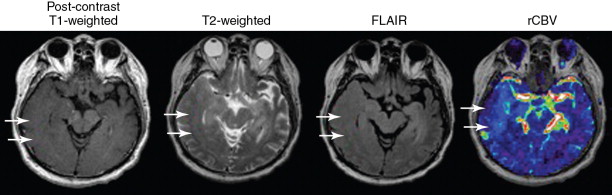
Schwannomas
Epidemiology and presentation
Schwannomas, which represent 8% of all intracranial tumors, are the second most common intracranial extraaxial tumor of the central nervous system after meningiomas. These tumors arise from the cells of the nerve sheath, including Schwann cells, fibroblasts, and perineural cells. The most common of schwannomas are vestibular schwannomas (also called acoustic neuromas ), which arise from the inferior division of the vestibular branch of the eighth cranial nerve. The two variants of vestibular schwannomas are the sporadic form, which presents in the fourth to eighth decade, and the autosomal dominant form, which presents frequently bilaterally in younger patients with neurofibromatosis type II. Nonvestibular schwannomas affect the cranial nerve V next in frequency (40%), followed by cranial nerve VII (23%), with the rest distributed among the lower cranial nerves IX, X, XI, and XII (20%).
Affected patients typically present with symptoms referred to the cranial nerve involved. For lesions involving cranial nerve VIII, the most common presenting symptoms are sudden or progressive high-frequency unilateral or bilateral sensorineural hearing loss, tinnitus, and vertigo. In lesions affecting cranial nerve V, trigeminal neuralgia or paresthesias, sensory loss, and wasting of the muscles of mastication, or an absent corneal reflex may be the presenting symptoms and signs. For lesions affecting cranial nerve VII, facial nerve palsy is the predominant symptom. For lesions affecting cranial nerve IX, swallowing difficulty and nonspecific symptoms referred to a parapharyngeal mass are the main findings. Interestingly, neurofibromatosis type 1 is associated with an increased incidence of schwannomas of the spinal nerve roots.
Imaging characteristics
Diagnosis is most effectively achieved with MR including T1- and T2-weighted MR imaging. Lesions are typically hypo- to isointense to gray matter on T1-weighted images, hyperintense relative to the pons on T2-weighted spin-echo imaging, and hypointense to cerebrospinal fluid (CSF) on T2-weighted 3D imaging. These lesions enhance homogenously with contrast administration, although tumors larger than 2.5 cm may demonstrate intratumoral areas of cystic change or necrosis. Tumoral enhancement may vary by Antoni histologic subtypes of tumor and tumor size, with subtype A tumors being small and more uniformly enhancing, whereas subtype B tumors are larger, more cystic, and more heterogenously enhancing. Tumoral enhancement can be homogeneous (50%–60%), heterogeneous (30%–40%), and cystic (5%–15%). Other characteristics that may suggest the diagnosis of schwannoma include enlarged cranial nerve foramina; micro-hemorrhage on T2-weighted MR, and associated communicating hydrocephalus, that may be related to elevated protein in the CSF. The extrameatal component of tumors may appear characteristically spherical with a funnel-shaped cone associated with the internal acoustic meatus, which can be expanded. Although meningiomas can occasionally extend into the internal acoustic meatus, they typically do not expand it. Enhancement of the adjacent meninges is possible in vestibular schwannoma and is not specific to meningioma. , CT may assist in characterizing schwannomas in patients with MR contraindications. On CT, these lesions are typically hypo to isodense, may be associated with cystic degeneration or hemorrhagic conversion, and show marked enhancement with contrast administration.
Advanced imaging techniques including diffusion-weighted imaging (DWI), ADC map, DSC perfusion MR, and proton NMR spectroscopy can be helpful for further distinguishing schwannomas from other pathology. Fast spin-echo, heavily T2-weighted MR may demonstrate high T2 signal in the dorsal brainstem ipsilateral to the tumor that reflects a degenerating vestibular nucleus. DWI may demonstrate tumor isointensity to normal parenchyma, with ADC values ranging from 1.1 to 1.7 × 10 −3 mm /s, elevated above ADC values for both normal brain values (1.4 × 10 −3 mm /s) and for meningiomas (1 × 10 −3 mm /s). Perfusion MR may demonstrate a lower mean relative tumor blood volume in vestibular schwannomas compared to meningiomas, and mean rCBV ratios that are lower than that reported for meningiomas (3 vs. 8, respectively). , Spectroscopy demonstrates a myo-inositol peak at 3.55 ppm in schwannomas, compared to alanine that is found in meningiomas. Additional notable features of schwannomas include size of tumor (best assessed on axial sections along the extracanicular portion), the distance between the intracanalicular portion and fundus (which is correlated with hearing prognosis) ; and localization and identification of the facial nerve and its position on contrast enhanced T2-weighted MR.
The differential for vestibular schwannomas includes other lesions of the cerebellopontine angle including meningioma, metastases, aneurysm, cavernoma, sarcoidosis, tuberculosis, and Erdheim–Chester disease (a rare form of systemic non-Langerhans histiocytosis). The nonvestibular schwannomas share imaging characteristics described above but arise in different locations (with the exception of facial schwannomas).
Trigeminal schwannomas, representing 1%–8% of all intracranial schwannomas, may arise anywhere along the course of the nerve from the cerebellopontine cistern to Meckel cave to the cavernous sinus, or superior inferior orbital fissure to the foramen ovale. The differential for lesions of Meckel cave or of the proximal cranial nerve V include meningioma, epidermoid, dermoid, lipoma, and metastases and inflammatory lesions. Facial schwannomas may arise anywhere along its course from the internal auditory canal (IAC) to the distal branches in the parotid gland, and may erode into the middle ear. Enlargement of the facial nerve canal is often suggestive. Because facial schwannomas of the IAC/cerebellopontine angle (CPA) may arise in the same spaces as vestibular schwannomas in the IAC or glomus tumors, features such as the dumb-bell shape with extension into the temporal bone, and suggestive round mass projecting into middle cranial fossa can provide clues to its diagnosis.
Finally, vagal, glossopharyngeal, spinal accessory, and hypoglossal schwannomas are collectively called jugular foramen schwannomas. They may typically arise more posteriorly, but extend cranially and may extend up through the CPA. The differential for jugular foramen schwannomas includes meningioma, glomus jugulotympanicum, and vestibular schwannomas (and less commonly, lymphoma, metastasis, and giant cell tumor). On CT, schwannomas demonstrate jugular foramen enlargement with sclerosis with preserving borders between tumor and bone, whereas glomus tumors and meningiomas are associated with erosive destruction and decalcification type changes. MR can be used to demonstrate intraluminal invasion of adjacent vascular structures, which is more typical of glomus tumors and meningiomas, and external compression, which is more suggestive of schwannomas. Isointensity on T1-weighted MR and hyperintensity on T2-weighted MR of schwannomas also distinguish schwannomas from paragangliomas (which are hypointense on T1-weighted MR and isointense on T2-weighted MR). In comparison, metastases or lymphoma of the jugular foramen are most often associated with osteolytic or destructive bony changes with less sharply marked bone borders. A recent study demonstrated that multidetector CT performed in arterial phase can more effectively differentiate neuromas from glomus tumors of the jugular foramen based on contrast enhancement difference than MR, and may additionally identify the lesions that can benefit from preoperative embolization.
Treatment
The goals of treatment are to relieve mass compression and retard tumor growth in order to slow the progression of cranial neuropathies and related neurologic dysfunction.
For trigeminal schwannomas, the tumor can be resected at the cost of anesthesia of the branch affected. Resection is complete in more than 95% for tumors greater in size than 3 cm, unless there is involvement of the cavernous sinus or adherence to the brainstem. Other than deterioration of preoperative sensation in 20%–70% of cases and improvement in postoperative sensation in only 19%–44% of cases, the rate of improvement in facial pain postoperatively is excellent (73%–100%). Recurrence rates range from 2% to 13% with follow-up between 2 and 13 years, with time to recurrence in the range of 13 months to 8 years. Gamma knife radiosurgery (GKS) for these tumors gives comparable outcomes, with a recurrence and progression rate of 0%–14 %.
The trend in facial nerve schwannoma management has shifted from gross total resection with nerve grafting, to more conservative nerve-sparing approaches (House Brackman grades I and II). Surgical excision with nerve grafting typically leads to paresis and postoperative recovery can achieve no better than House-Brackman grades III and IV. Observation with serial MR follow-up is recommended in the setting of normal facial nerve function (House Brackman grade <III). Surgical decompression in the setting of bone-confined tumor with increasing size is offered to offset worsening facial nerve function, and may be combined with nerve grafting for enlarging tumors associated with brainstem compression or hydrocephalus or in patients with House Brackman grade >IV facial palsy. Meanwhile, stereotactic radiation may be offered to patients with an enlarging tumor in the cerebellopontine angle or middle fossa to arrest tumor growth and decrease risk of local compression. Although case series managed with stereotactic radiation report promising facial nerve function preservation rates, larger studies are needed to define a control rate.
Jugular foramen schwannomas are ideally resected, but the optimal surgical approach and acceptable postoperative morbidity have not yet been defined. In patients with stable tumor causing tolerable neuropathies, an argument can be made for serial imaging and observation as well. Stereotactic radiosurgery can be applied to tumors associated with minimal deficits, or tumors that exhibit intimate involvement with neurovascular structures, making surgery high risk. In elderly patients in whom postoperative lower cranial nerve deficits would be less well tolerated, expectant management with serial imaging and monitoring of neuropathies of the lower cranial nerves IX, X, and XI should be elected.
Acoustic schwannomas are typically observed until enlargement significantly compromises function or limits future treatment options for hearing preservation. Given a growth rate of approximately 1–2 mm per year, patients with minimally symptomatic acoustic neuromas and elderly patients who are poor surgical candidates are often observed. In these cases, the average-sized lesion measures ∼11.8 mm, follow-up is typically for 3.2 years, and 57% of patients remain stable without tumor growth, with 51% developing hearing loss and 20% electing to have further treatment. During expectant management, annual MRIs are recommended to follow tumor growth. For others with symptomatic neuromas who are surgical candidates, the historical mainstay is microsurgical resection, with radiation being a more recent alternative.
The gold standard treatment for acoustic neuroma is complete microsurgical resection, which can be achieved in up to 74% of patients at the cost of vestibulocochlear and facial nerve dysfunction. Postoperative facial nerve dysfunction ranges between 3% and 22% and hearing loss from baseline ranges as widely as 40%–80%. , Because of a low postoperative recurrence rate of 8.8% with a latency to recurrence of ∼100 months, baseline postoperative imaging obtained between 6 and 12 months postoperatively can be used to evaluate for stable postoperative changes, and then may be followed by serial annual imaging until at least 5 years postoperatively.
Stereotactic radiotherapy (SRT) and radiosurgery (SRS) are noninvasive therapeutic alternatives for patients with tumors up to 3–4 cm in size, who are poor surgical candidates, or who have recurrent tumor. These modalities may achieve excellent tumor control rates >90%, and excellent rates of preserved facial nerve and trigeminal nerve function (>95%) in association with factors such as tumor volume <15 mm in diameter, patient age younger than 60 years, and radiation dose <13 Gy. In more heterogenous populations, mixed hearing preservation rates may range from 13% to 71%, with follow-up ranging from 3 to 11 years. Two single-institution nonrandomized prospective trials of SRT and SRS for acoustic neuromas reported comparable tumor control and preservation of cranial nerve VII function. , A form of SRS, gamma knife radiosurgery has expanded its application as an outpatient procedure for tumors up to 3–4 cm in size, beyond its originally targeted patient populations (elderly patients, patients who cannot tolerate surgery, patients with bilateral tumors, and patients with inoperable tumors). Outcomes are reported to be >90% progression-free survival at 5 years, in a representative case series. Efforts to determine whether reduction in radiation doses to 12–13 Gy can be made without sacrificing efficacy are ongoing. So far, the use of lower doses in SRS has been associated both with a wide range of hearing preservation rates and risk of undertreatment with local failure. With the use of GKS, median interval to salvage surgery ranges between 27 and 37 months for patients with recurrence. , Tumors have been shown to swell between 12 and 18 months post-GKS, so surveillance imaging has been recommended at 2 and 5 years postirradiation.
Lastly, proton beam therapy applied in fractionated, hypofractionated, and radiosurgical approaches achieves control rates between 84% and 100% in follow-up ranging from 5 to 10 years, and excellent facial and trigeminal nerve preservation (89.5%–100%, 90.5%–100%, respectively), but hearing preservation only in the range of 31%–42%. Additional studies are needed to determine if there are specific advantages to the use of proton beam in improving hearing outcomes, as well as the ideal timing of surveillance after treatment to detect recurrent disease.
Meningiomas
Epidemiology and presentation
Meningiomas are the most common extraaxial intracranial tumors and constitute 6.6% of all intracranial tumors. These benign tumors originate from nonneuroepithelial progenitor cells, the arachnoid cap cells of the leptomeninges that are imbedded in arachnoid villi of the skull vault and base. Though most common in the parasagittal area, meningiomas may arise anywhere in the skull, including the falx, cavernous sinus, tuberculum sellae, lamina cribrosa, foramen magnum, and torcular zone. The WHO classification system, revised in 2007, grades meningiomas as benign (I), atypical (II), and anaplastic (malignant) (III) on the basis on both histologic features and invasion of adjacent structures including brain, bone, sinus, and nerve.
Most often affecting middle-aged females, meningiomas progress asymptomatically until gradual enlargement leads to mass effect, compression of adjacent structures, and intracranial hypertension. Presenting symptoms depend on the location of tumor, and focal findings may be directly related to the size and location of tumor and presence of associated hydrocephalus. Common symptoms may include pain around the location of tumor, cranial nerve deficits, seizures, dizziness, behavioral disturbances, visual impairment, and eye proptosis. Parasagittal meningiomas of the frontal lobe may lead to Jacksonian seizures of the lower limbs or headache. Meningiomas of the anterior falx may present with headache and gradual personality changes with apathy and dementia. Suprasellar meningiomas may be heralded by hormonal disturbances , ; clinoidal meningiomas may lead to visual impairment, cranial nerve palsies, and exophthalmos, while peritorcular meningiomas may present with neurologic symptoms due to compression of the occipital lobe, such as headache, occipitally localized pain, papilledema and homonymous field deficits, ataxia, dysmetria, hypotonia, and nystagmus.
One of the most frequently found genetic defects of meningiomas are truncating mutations in the neurofibromatosis 2 gene (merlin) on chromosome 22q. Other somatic genetic defects have been characterized as well in meningiomas of both the sporadic and neurofibromatosis type 2 (NF2)-associated types. Meningiomas also elaborate a variety of soluble proteins such as the angiogenesis-promoting vascular endothelial growth factor (VEGF), which may contribute to the vascularized nature of these tumors and their prominent surrounding edema, and which may be used to predict recurrence. , Additionally, meningiomas contain estrogen and progesterone receptors, which may be connected to its increased incidence in women, its tendency to enlarge during pregnancy, and an association with breast cancer.
Imaging characteristics
MR is the preferred diagnostic modality for meningiomas, whereas CT may be helpful in the setting of patient contraindications to MR, and its ability to demonstrate adjacent bony reaction or infiltration and intratumoral calcifications. On CT, meningiomas are well-circumscribed, unilobular, extraaxial masses with smooth borders and broad-based dural attachments. On CT, they are most commonly isodense to cortex, and enhance homogenously and strongly after contrast administration. Meningiomas may exhibit a characteristic mottling in association with dural surfaces related to their hypervascularity. Bony reaction including adjacent hyperosteosis can appear in up to 18%–50% of cases as well. , These tumors may also contain punctate microscopic calcifications in up to a quarter of cases and may be calcified in up to 20% of cases.
Meanwhile, MR can provide the necessary detail for meningioma diagnosis and for subsequent treatment planning and prognosis ( Figure 23-8 ). The majority of tumors are hypointense to isointense relative to normal brain tissue on T1-weighted images due to hypercellularity and low water content. In contrast, they may appear isointense to hyperintense on T2-weighted images. The classic “dural tail sign,” the intense enhancement of the thickened dural attachment, is attributed to either reactive hyperemia, or tumor infiltration, or both, and has a reported sensitivity of 58% and specificity of 94%. Additional classic features include white matter buckling (an inward bowing of the gray and white matter junction), the presence of a “pseudo-capsule” that represents the vascular flow void at the dural base of the tumor, and the presence of a CSF attenuation cleft between the cortex and meningioma that dermarcates the tumor.
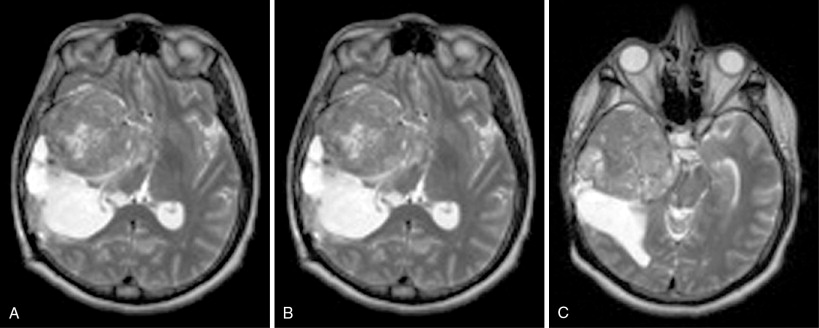
Imaging features that may affect the feasibility of surgical resection include enhancing tumor invasion into adjacent dura, invasion into dural venous sinuses, and degree of associated peritumoral edema, among other factors. In particular, peritumoral edema may be increased around malignant meningiomas, and thus correlated with increased risk of recurrence. The correlation of preoperative T2-weighted signal intensity patterns with postoperative histologic subtypes has been attempted, with conflicting results. , If reproducible, such radiographic–pathologic insights can be used to assist interventionalists in the selection of open resection versus minimally invasive intervention and radiotherapy.
Advanced imaging modalities such as DWI, diffusion tensor imaging (DTI), perfusion MR, MR spectroscopy, PET can enable the noninvasive acquisition of detailed information about tumor aggressiveness and histology that may be used to assist in planning for resection and/or radiotherapy.
DWI and DTI have been used to noninvasively characterize meningioma grade. Reduced water diffusion (and corresponding low ADC values) has been correlated with more aggressive atypical or malignant meningiomas, or dedifferentiation of a lower-grade to a higher-grade tumor. DWI-related biomarkers including ADC values also show promise to differentiate meningiomas from other enhancing extraaxial masses such as metastases, lymphomas, and schwannomas. Similarly, DTI has been employed to differentiate classic from atypical meningioma. DWI and DTI additionally may prove useful to follow tumor response to radiotherapy, surgery, or both, but validation of their application in these settings is still pending.
Dynamic susceptibility contrast (DSC) perfusion imaging, acquired by giving a bolus of contrast and determining the change in signal intensity as the contrast bolus passes through the brain, may be used define and target the vascular supply of meningiomas via intraarterial embolization. First, rCBV data from DSC perfusion imaging may be used to differentiate meningiomas (mean rCBV ratio = 6–9) and from schwannomas (mean rCBV ratio = 3) and lymphomas (mean rCBV ratio = 1). Second, perfusion imaging can delineate changes in vascular supply of these tumors that may correlate with more aggressive growth (such as the addition of the pial branches that supply the parenchyma and that are continuous with the blood–brain barrier to the dural branches from the external carotid artery). Enlarging more aggressive tumors may show elevated cerebral blood volume in tumors with a high volume of combined pial-cortical supply. Third, once the vascular supply to the tumor has been identified, DSC perfusion imaging can facilitate the preoperative endovascular embolization that can reduce intraoperative blood and be used to follow the response to embolization postoperatively.
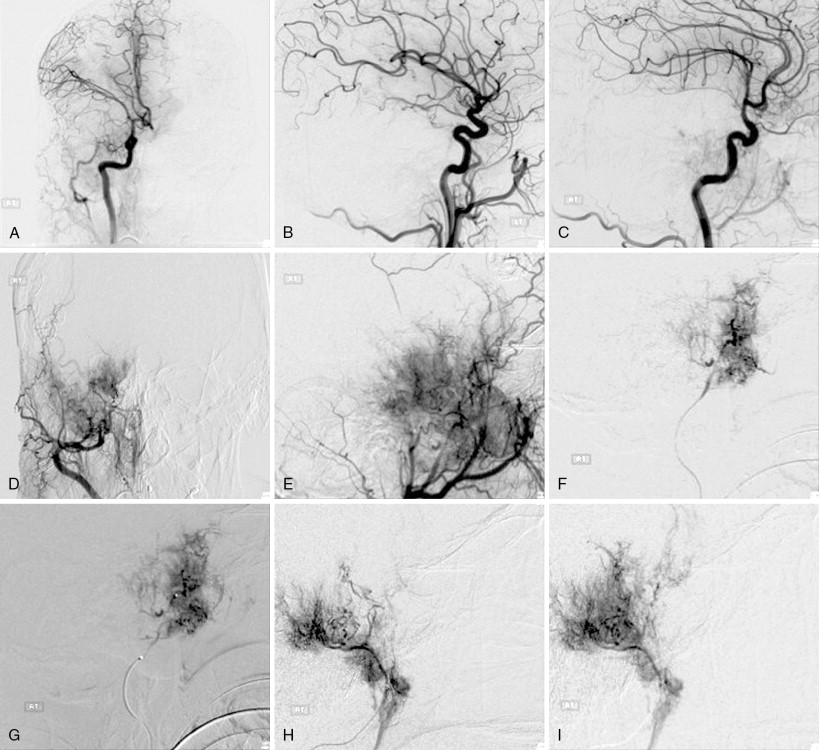
Embolization involves introduction of a microcatheter into the ECA and into the feeding dural vessels with angiography to confirm that it supplies the meningioma, followed by injection of polyvinyl alcohol (PVA) particles until stasis is achieved, followed by placement of pushable platinum coils more proximally in the feeding artery to achieve complete arterial occlusion. Both during and after embolization, advanced MR perfusion with intraarterial injections may be performed. The postembolization intraarterial MR perfusion studies can be used to evaluate for changes in tumor vascularity, and determine the volume of treated versus untreated tumor.
Noninvasive MR spectroscopy has been used both to define the metabolic signature of meningioma, and to identify metabolic biomarkers of tumor grade and aggressiveness. Meningiomas demonstrate the characteristic presence of alanine at 1.5 ppm, high choline and glutamate/glutamine metabolites peaks, and the absence or low levels of creatinine and N -acetyl-aspartate and lipids. MR spectroscopy of ex vivo meningioma specimens has demonstrated the ability for spectroscopy to distinguish between low- and high-grade meningiomas, primary versus recurrent disease using alanine/glycine and choline/glutamine ratios among other metabolic parameters. Analysis of 68 meningioma specimens with ex vivo MR spectroscopy demonstrated that low- and high-grade meningiomas were distinguished by differences in total alanine and total creatinine levels, that alanine and glycine/alanine ratios could be used to distinguish between primary disease and recurrence, and that rapid recurrence could be correlated with glycine/alanine, choline/glutamine ratios, among other parameters.
Though unable to independently differentiate meningiomas from other intracranial tumors, PET may be more useful in distinguishing malignant from benign meningiomas on the basis of hyper- versus isometabolism. Radiolabeled PET may be applied with MRI to delineate tumor volumes for intensity-modulated radiotherapy planning, and may also prove useful to assist with stereotactic biopsy guidance and therapy surveillance.
Treatment of meningiomas
Close follow-up with serial imaging is possible in patients with small, asymptomatic slowly progressing meningiomas, but requires particular vigilance in younger patients who are more likely to experience more rapid tumor growth.
Meanwhile, surgical resection is preferred for meningiomas causing symptoms and can achieve a complete cure in up to 80% of cases, if the tumor is superficial and bony invasion is not present. In patients who undergo gross total resection (GTR) who later experience recurrence, 12% of these cases recur within 5 years of GTR and 19% recur at 20 years. , Preoperative embolization may facilitate subsequent removal of highly vascularized tumor from adjacent tissue, and is associated with exceedingly low rates of ischemic and hemorrhagic complications ( Figures 23-9 and 23-10 ). The Simpson Criteria, a scale to evaluate completeness of resection, has been used to predict likelihood of recurrence or tumor regrowth after resection: grade I (complete removal with resection of underlying bone and associated dura) is associated with an ∼9% 10-year recurrence; grade II (complete removal with coagulation of dural attachments) is associated with ∼19% 10-year recurrence; grade III (complete removal without dural resection or coagulation) is associated with ∼29% 10-year recurrence; and grade IV (subtotal resection) is associated with ∼40% 10-year recurrence rate.