Frank R. DeLeo, William M. Nauseef
Granulocytic Phagocytes
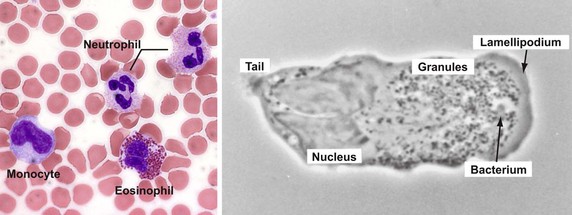
Vertebrate host defense against microbes represents the integration of the innate and acquired immune systems, which together respond to a diverse array of infectious threats.1,2 Innate (natural) immunity provides the host with the capacity to respond immediately to an infectious challenge, regardless of previous exposure to the specific invading agent, by using response elements encoded in germline genes. Elements of the innate system include phagocytic cells, namely polymorphonuclear leukocytes (PMNs), mononuclear phagocytes, and circulating soluble proteins, including components of the complement system (see Chapters 4 and 9). This sensitive system for the recognition of structural elements that are inherently and uniquely microbial has functional analogues in the immune systems of a wide variety of multicellular organisms, including plants and insects. As such, innate immune elements comprise an evolutionarily ancient system that provides a rapid and sensitive surveillance mechanism to protect the host when challenged with any invading microorganism. Granulocytes, the most numerous leukocytes in the peripheral circulation of humans, include neutrophils, eosinophils, and basophils. They represent the predominant cell type in the acute innate immune response and figure more broadly in the integration of innate and adaptive immunity.2,3 Structurally, these cells share with one another a multilobed nucleus and the presence of numerous membrane-bound, characteristically staining cytoplasmic granules, but functionally they differ significantly.
Neutrophils
Development
Neutrophils arise from pluripotent hematopoietic stem cells (HSCs) in the bone marrow through an orderly succession of phenotypically distinct cell types.4,5 From the HSCs arise multipotential progenitors (MPPs), a cell population with the capacity to differentiate into all hematopoietic lines but unable to multiply. The MPPs spawn common myeloid progenitors (CMPs) that serve as the source of precursors for the individual hematopoietic cell lines, including granulocyte/macrophage progenitors (GMPs). The complex procession from HSC to GMP and then to neutrophils is coordinated by specific transcription factors, including PU.1, CCAAT/enhancer-binding proteins (α, β, and ε), growth factor independent-1 (GFI-1), and interferon regulatory factor 8 (IRF8).5 Timely expression of such factors coordinates transcription of stage-specific genes that are responsible for the phenotypic and functional features that define myeloid intermediates along the differentiation pathway. In part, soluble proteins such as interleukin-17 (IL-17), IL-23, and granulocyte colony-stimulating factor (G-CSF) modulate the relative levels of transcriptional factors within myeloid cells and thus influence the fate of the cell.6 CSFs also alter the survival and direct the maturation and proliferation of myeloid cells. Each factor is named for the colony produced under its influence: GM-CSF, for granulocytes and macrophages; G-CSF, for granulocytes; M-CSF, for monocytes and macrophages; and multi-CSF (or IL-3), for a variety of colonies, including neutrophils, macrophages, eosinophils, megakaryocytes, and erythroid cells.
In addition to the granulopoiesis essential to maintain steady-state levels of circulating neutrophils, the hematopoietic system has the capacity to mobilize additional functioning neutrophils in response to the increased demand imposed by infection.7,8 Infection induces augmented production of cytokines, including G-CSF, GM-CSF, and IL-3, and these circulating proteins drive “emergency” granulopoiesis. IL-17, a cytokine produced by T helper (Th)17 cells,9 drives G-CSF production and promotes emergency granulopoiesis, as deduced from experimental models of chronic inflammation, but it does not contribute to homeostatic neutrophil production. G-CSF stimulates the production of granulocyte precursor cells, the proliferation of cells in the granulocyte lineage, and the survival of granulocyte precursors and neutrophils. Furthermore, G-CSF accelerates passage of granulocyte precursors through the bone marrow, thereby providing an immediate supply of young neutrophils into the circulation. Thus, the control of granulocyte production can be modulated not only to maintain homeostatic levels of neutrophils as aged cells are cleared from the circulation but also to respond to increased demands created by infectious or other challenges.
The cell populations during steady-state granulocyte development in the bone marrow can be divided into three pools: stem cell pool, a mitotic pool, and a postmitotic pool.10 The stem cell pool includes undifferentiated hematopoietic stem cells, whereas the mitotic pool encompasses cells that proliferate and mature sequentially from myeloblasts into promyelocytes and myelocytes. Maturation is associated with the appearance of the cytoplasmic granules characteristic of neutrophils, basophils, and eosinophils.11 The postmitotic phase of development includes metamyelocytes, band (or immature) neutrophils, and mature neutrophils, all cells held in reserve and ready for release.
Coincident with the appearance of morphologic changes, cells acquire the specific surface markers and functional properties of more mature cells.12 For example, Fc receptors appear as the cells develop into promyelocytes, competence for phagocytosis arises in the early myelocyte stage, and complement receptors surface in the late myelocyte and metamyelocyte stages. Oxygen-dependent microbicidal activity appears in the early metamyelocyte stage, and cells in the late metamyelocyte–band stage demonstrate increased adhesiveness, cell motility, and chemotactic responses.12 In addition, coordinated expression of genes encoding the granule proteins is synchronized with early stages of myeloid development, and normal granulocytic differentiation is intimately linked with expression of proteins localized in the specific granules.12
Morphologic and Structural Characteristics
The earliest histochemical studies of neutrophils classified the membrane-bound intracellular granules by their staining characteristics. Two populations of granules were distinguished based on staining with azure A: the positively staining azurophilic granules and the unstained specific granules. Sophisticated analyses of the composition of isolated neutrophil organelles have refined significantly our appreciation of the complexity and heterogeneity of neutrophil granules.13,14 Such studies have provided novel insights into the biologic roles of the various proteins in the matrix of the granule and have revealed functionally important proteins within the membranes of particular granule subsets.
At a first approximation, neutrophil granules can be categorized based on peroxidase staining. The peroxidase-positive granules are also known as primary granules, because they arise first in granulopoiesis, and as azurophilic granules, based on histochemical staining. Azurophilic granules contain myeloperoxidase (MPO)15; a variety of proteolytic enzymes, including cathepsin G, proteinase-3,16 and elastase17; and the antimicrobial defensins18 and bactericidal permeability-increasing protein (BPI) (Table 8-1).19 Because of the acid hydrolase activity of the azurophilic granule contents, this compartment had been considered lysosomal in nature. However, azurophilic granules lack lysosome-associated membrane protein,20 an identifying marker for lysosomes. Moreover, proteins such as MPO21 and the defensins22 segregate into the azurophilic granule independently of the mannose-6-phosphate receptor, a targeting system characteristic of lysosomal proteins. Taken together, these observations suggest that the azurophilic granule may be a specialized organelle that is distinctly different from conventional primary lysosomes.
TABLE 8-1
Contents of Neutrophil Granules and Secretory Vesicles
| GRANULE | MEMBRANE | MATRIX |
| Azurophilic (primary granule) | CD63,CD68, presenilin | Myeloperoxidase, elastase, cathepsin G, proteinase 3, defensins, BPI, lysozyme, sialidase, azurocidin, β-glucuronidase, azurocidin |
| Specific (secondary granule) | CD11b/CD18, CD66, CD67, gp91phox/p22phox, TNF receptor, SNAP-23, VAMP-2, stomatin | Collagenase, gelatinase, urokinase plasminogen activator, hCAP-18, NGAL, vitamin B12–binding protein, lysozyme, lactoferrin, haptoglobin, pentraxin 3, prodefensin, SLPI, orosomucoid, heparanase, β2-microglobulin, CRISP3 |
| Gelatinase (teritiary granule) | CD11b/CD18, CD67, gp91phox/p22phox, MMP25, TNF receptor, SNAP-23, VAMP-2, Nramp1 | Gelatinase, arginase 1, lysozyme, β2-microglobulin, CRISP3 |
| Secretory vesicles | CD11b/CD18, CD67, gp91phox/p22phox, MMP25, CD35, CD16, C1q receptor, CD14, fMLP receptor, SNAP-23, VAMP-2, Nramp1, alkaline phosphatase, DAF, CD10, CD13, cystic fibrosis transmembrane conductance regulator (CFTR) | Plasma proteins |
BPI, bactericidal permeability-increasing protein; CD, cluster of differentiation; CRISP3, cysteine-rich secretory protein 3; DAF, decay accelerating factor; fMLP, N-formyl-methionyl-leucyl-phenylalanine; hCAP-18, human cathelicidin protein-18; MMP25, matrix metalloproteinase 25; NGAL, neutrophil gelatinase–associated lipocalin; Nramp1, natural resistance-associated macrophage protein; SNAP-23, synaptosomal-associated protein-23; SLPI, secretory leukocyte protease inhibitor; TNF, tumor necrosis factor; VAMP-2, vesicle-associated membrane protein-2.
From Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33(5):657-670; and Borregaard N, Sorensen OE, Theilgaard-Mönch K. Neutrophil granules: a library of innate immunity proteins. Trends Immunol. 2007;28(8):340-345.
The peroxidase-negative granules include specific granules, gelatinase granules, and secretory vesicles.23 The contents of the specific and gelatinase granules overlap to a significant extent (see Table 8-1)24 but differ from those of azurophilic granules and secretory vesicles. More striking, however, is the distribution of functionally important plasma membrane proteins in the membranes of peroxidase-negative granules.25 These membranes contain flavocytochrome b558,26,27 an essential component of reduced nicotinamide adenine dinucleotide phosphate (NADPH)–dependent oxidase (discussed later); receptors for chemotactic peptides28; extracellular matrix proteins29; cytokines30; opsonins31; and adhesion proteins.32,33 The secretory vesicles are especially enriched for plasma membrane proteins34 and can be rapidly recruited to fuse with the plasma membrane, amplifying the potential of the neutrophil to respond to stimulation. They therefore represent an intracellular reservoir of functionally important membrane proteins that can be quickly recruited to the cell surface during neutrophil activation. The existence of such compartments is ideally suited to the role of neutrophils as the major circulating cell in the innate immune system; a reservoir of readily accessible functional proteins allows a rapid response without the delays that would be incurred by requirements for new protein synthesis. The functional consequences of this compartmentalization of proteins in the matrix and in the membrane of granules are discussed later.
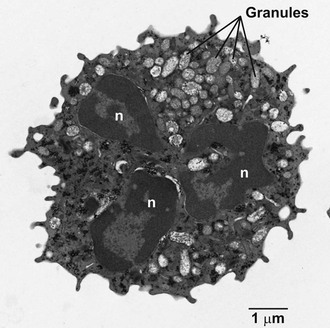
During granulocyte maturation, the nucleus becomes segmented (Fig. 8-1), and cytoskeletal elements, including microfilaments and microtubules, appear in the cytoplasm. A meshwork of microfilaments makes up the clear cortical veil that surrounds the cell and forms the lamellipodium of an advancing cell (see Fig. 8-1). These structures are polymers of actin, a protein representing 5% to 10% of the total cellular protein. Actin and its associated proteins constitute the contractile machinery necessary for cellular locomotion35 and phagocytosis.36 Actin monomers (G-actin), in the presence of actin-binding protein, polymerize to form cross-linked actin filaments (F-actin). Regulation of the length of the filaments and the degree of cross-linking provides the physicochemical dynamics of actin flux between the gel and sol states. Actin filaments are associated with the cytoskeleton or with the plasma membrane via membrane skeletal proteins.37 Stimulation of the cell with chemotactic factors causes an abrupt increase in the amount of actin associated with the cytoskeleton38 and a shift in microfilament organization from a parallel strand to a cross-hatched meshwork most evident at the leading edge of the directionally polarized cell.39 Microtubules appear to be necessary for the initial orientation of the cell in a chemotactic gradient, for the spatial organization of structures within the cell during locomotion, and in vesicle transport, degranulation, and the regulation of cell surface microviscosity during phagocytosis.
Mature neutrophils (Fig. 8-2; see Fig. 8-1) are characterized by a paucity of ribosomal material and mitochondria, in keeping with the relatively low levels of synthetic processes in these cells. However, studies over the past 2 decades have radically changed the image of neutrophils as biosynthetically inactive cells and have identified an array of proteins actively synthesized by neutrophils, both at rest and after stimulation. Among the proteins synthesized by neutrophils are major histocompatibility complex (MHC) class I molecules,40 complement receptors,41 CXC-chemokines, CC-chemokines, proinflammatory and anti-inflammatory cytokines, immunoregulatory molecules, colony-stimulating factors, tumor necrosis factor (TNF) superfamily members, and proteins important in angiogenesis and fibrogenesis.3 As presented later, the transcriptional profile of recruited neutrophils includes genes directed toward wound healing.42 Glycogen granules fill the cytoplasm and serve as a source of energy to support neutrophil activity.
As immune effector cells, neutrophils are equipped with surface receptors that sense extracellular signals and can recognize ligands to support the wide array of agonist-dependent activities within its functional repertoire. Surface receptors for immunoglobulin43 and complement fragments44 contribute to opsonin-dependent phagocytosis. Specific receptors on the plasma membrane initiate cell movement by recognizing interacting molecules on endothelial cells or extracellular matrix proteins, bacterially derived formylated proteins, chemotactic complement fragments C5a and C3a, platelet-activating factor, IL-8 and related chemokines, and leukotriene B4 (LTB4).45–49 These receptors are homogeneously distributed over the surface of the resting cell but undergo an asymmetrical clustering at the front of the cell when it polarizes in response to a chemotactic stimulus. The distribution of receptors with different ligand specificities can be independently regulated, even though stimulation via these receptors may evoke similar functional responses.50 Moreover, the various neutrophil functional activities exhibit differential requirements for receptor occupancy. For example, maximal degranulation requires brief receptor occupancy, whereas sustained oxidative responses depend on continuous ligand binding to the receptor.51 Neutrophils also possess membrane receptors that signal to evade (“don’t eat”) or promote (“eat me”) their own uptake by macrophages, a process known as efferocytosis (see later).52
Homeostasis of the Circulating Neutrophil Population
To maintain a stable number of circulating neutrophils, the production of new and functional cells must be balanced by clearance of those that are aged and spent. The daily production of mature PMNs in a healthy adult is remarkable, with approximately 109 cells/kg body weight entering the circulation from the bone marrow.53 During acute infection or other inflammatory stresses, neutrophils are mobilized from the marrow reserve, which is estimated to be ≈6.9 × 109 cells/kg.53 Even in the presence of persistent stimulation, this reserve can be depleted only if there is nutritional deficiency or another disorder (e.g., ethanol abuse) that compromises mechanisms for augmenting delivery to meet demands. Augmented stem cell input, increased mitoses during the mitotic stage of development, use of a store of cells whose maturation had been inhibited (so-called hiatal cells), and shortening of the maturation time within the marrow may all occur.10 Multiplication and differentiation of stem cells are stimulated by the CSF produced by peripheral blood monocytes, tissue macrophages, and stimulated lymphocytes.53,54
The total granulocyte pool (≈7 × 108 cells/kg body weight) includes two compartments of similar size: the intravascular circulating cells and the marginating cells. The distribution of the marginated pool varies with the size and flow of the capillary bed in an individual organ. All agree that liver, spleen, and bone marrow are included in this pool, but unsettled is the physiologic contribution of the pulmonary circulation.55 Whereas experimental data based on intravascular transit time suggest that the lung is the predominant site of marginated neutrophils, studies using radionuclide imaging demonstrate relatively little neutrophil margination in normal human lungs. Overall, the contribution of the pulmonary circulation to the marginated pool of normal neutrophils in healthy humans remains unsettled.
A dynamic equilibrium exists between neutrophils in marginated and circulating compartments, as cells marginate via transient endothelial interactions and then resume rapid flow, reflecting the balance between intercellular adherence and shear forces.56–58 The intravascular half-life of circulating neutrophils is normally 6 to 8 hours, whereas their persistence in extravascular sites ranges from a few hours to several days. A recent report using in vivo labeling with 2H2O suggests that normal human neutrophils have a life span in circulation of 5.4 days,59 more than 10-fold greater than previously thought. However, that conclusion has been challenged,60,61 and alternative interpretations of the same data yield estimates that agree with the long-standing accepted value of 6 to 8 hours. Granulocytosis, a common feature of acute inflammation, is a consequence of certain physiologic and pharmacologic stimuli that typically redistribute neutrophils among the various granulocyte pools as well as increase cell production. For example, the acute administration of corticosteroids or endotoxin, perhaps mimicking pathophysiologic events that occur in severe infection, promotes granulocyte release from the marrow reserve. Sustained steroid administration produces granulocytosis primarily by decreasing neutrophil adherence and shifting cells from the marginating to the circulating pool. Similarly, exercise, stress, epinephrine, hypoxia, aspirin, and alcohol cause granulocytosis by mobilizing marginating cells.
In the setting of acute inflammation, spent and apoptotic neutrophils are ingested by macrophages by the regulated process of efferocytosis.62 Neutrophils that are activated during their brief tour in circulation become senescent, a proapoptotic state characterized by increased surface expression of CXCR4 and being less responsive to N-formyl-methionyl-leucyl-phenylalanine (fMLP).63 Resident macrophages in liver, spleen, and likely bone marrow55 routinely ingest and thereby clear senescent neutrophils from circulation in a manner that is immunologically “silent,” that is, without release of proinflammatory cytokines.
Inflammatory Response
Inflammation represents a remarkably integrated cascade of events involving both cellular and soluble factors that are precisely orchestrated spatially and temporally. As such, it is best conceptualized as a complex network of signals that modulate the responses of different cells and circulating molecules that, in turn, interact and are subject to a variety of regulatory checkpoints operating by local, systemic, and neural mechanisms.3,64,65 Within the context of host response to invading microbes, the innate immune system sits poised to respond rapidly to perceived threats and in a stepwise fashion to recognize, contain, kill, and destroy potential pathogens. Because all successful biologic systems represent a balance among competing forces, the return of the host to homeostasis after an acute inflammatory response requires execution of a properly timed and appropriately proportioned anti-inflammatory cascade. Thus, the acute response of neutrophils requires a sensitive afferent limb to allow systemic recognition of a local threat at very low levels, as well as a targeted effector arm at the noxious source to contain, kill, and degrade potential pathogens.64 The coordinated response needs to occur before anti-inflammatory events supervene to trigger neutrophil apoptosis and removal en route to resolution of the inflammatory reaction.66 In addition, the fact that the noninflammatory homeostatic state is actively maintained by modulation of proinflammatory surveillance systems, rather than simply the absence of inflammatory stimuli, adds another layer of complexity and feedback signaling to an already intricate system.65–67
Circulating neutrophils are functionally heterogeneous, with the majority (80%) of, but not all, cells having the capacity to form immunoglobulin G (IgG) rosettes.68 Because release from the bone marrow is not synchronized, this heterogeneity probably reflects in part maturational differences within a single cell line, but more sophisticated analyses suggest that subsets of circulating neutrophils have distinct and important functional phenotypes.69–75 In contrast to the heterogeneity of circulating neutrophils, those in tissue are relatively homogeneous, and more than 96% are capable of IgG rosette formation.68 They contain fewer lysosomal granules and more glycogen than do their circulating counterparts because anaerobic glycolysis provides the energy for cell movement through the tissues.76 The phenotypic differences between circulating and tissue neutrophils could reflect determinants required for neutrophils to immigrate into tissue, influences of transmigration per se, elements in the tissue compartment, or other factors. In any case, the tissue neutrophil exhibits a phenotype different from that of the circulating, unstimulated neutrophil. For example, exudative neutrophils synthesize significantly more IL-8 and activate genes that collectively contribute to wound healing.42,77 Furthermore, neutrophils exposed to concentrations of mediators that are too low to stimulate directly nevertheless prepare the cell for an enhanced response to a second, unrelated stimulus, in a phenomenon known as priming.78,79
Priming
A broad array of proinflammatory mediators, including chemotactic factors, bacterial molecules, chemokines, cytokines, and certain lipids, can prime neutrophils, as can transmigration across the endothelium and migration into tissue. The primed state exists with respect to each of the major aspects of neutrophil function, persists for an extended period (longer than 20 minutes under experimental conditions in vitro) in relation to the response elicited by direct stimulation of the cell, and is reversible. It is not known whether priming agents share the same mechanism of action or if all the essential molecular events causing priming have been elucidated. Consistent with the diverse phenotypic features of primed neutrophils, partial assembly of the NADPH oxidase by phosphorylation and translocation of p47phox; secretory vesicle exocytosis and partial mobilization of specific granules to the plasma membrane, resulting in increased surface expression of flavocytochrome b558 (see later); reorganization of the plasma membrane and distribution of receptors and signaling molecules into lipid rafts; modulation of intracellular signaling intermediates; and transcription of several gene families have been implicated in contributing to the more responsive state of the cell.80–86 Most neutrophil priming agents also delay neutrophil apoptosis and thus prolong functional capacity, a phenomenon consistent with the potential for an enhanced proinflammatory response.87
Step 1: Neutrophil Recruitment
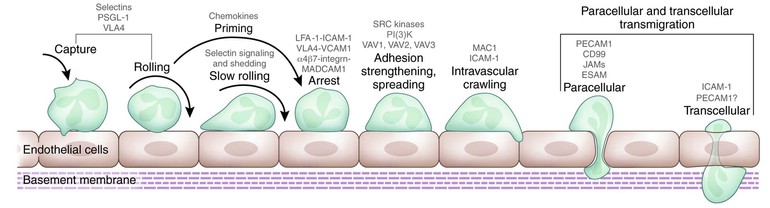
To combat invading microorganisms, neutrophils must emigrate from the circulation and into the extravascular tissue space, a process that represents both the responses of neutrophils to the shear stress in the circulation and the summation of coordinated interactions of cells, specific receptors, and soluble mediators.47,88,89 In fact, neutrophils in tissue, interacting with elements in the extracellular matrix, differ functionally from circulating neutrophils.90–92 The process of neutrophil transmigration out of the circulation involves at least four discrete steps: rolling adhesion, integrin activation, firm adhesion, and transmigration (Fig. 8-3).88 These events are mediated in turn by four classes of adhesion proteins: selectins, integrins, immunoglobulin-like proteins, and mucin-like selectin ligands. In addition to neutrophils and endothelial cells, platelets figure prominently in the initiation of the inflammatory response, co-localizing with neutrophils and participating in P-selectin–dependent leukocyte binding.89 The cooperation of several cell types and their secreted products culminates in events necessary to recruit circulating neutrophils to the site of inflammation, and the activation of autocrine and paracrine feedback loops modulates the extent of the host response.88,89