Global Epidemiology of Meningococcal Infections
Mark C. Steinhoff
Kenrad E. Nelson
INTRODUCTION
Meningococcal disease is a global problem, with an epidemiology that varies widely by region and causes an estimated 500,000 cases and 50,000 deaths each year according to the World Health Organization (WHO). The meningococcus bacteria cause severe disease with rapid onset and poor outcomes, even with treatment. Although the endemic disease is relatively rare, epidemic meningococcal disease occurs in outbreaks in the “meningitis belt” in sub-Saharan Africa as well as in other regions. Because of the varied and dynamic epidemiologic patterns of disease, control strategies are substantially different between geographic regions, and change within single countries over time.
This chapter describes meningococcal disease and control strategies related to it in both industrial and developing regions. Because of continued endemic meningitis and the potential for epidemics, vaccines are the mainstay of control efforts. Recent development and licensure of new vaccines in Europe and the United States, and the development of a vaccine tailored for the African meningitis belt, suggest better control can be expected in the future.
THE ORGANISM
Epidemic meningitis was described in Switzerland in 1805 and in the United States in 1806.1 The bacterium Neisseria meningitidis, also called meningococcus, was first isolated in 1887 at the beginning of the era of discovery of infectious organisms.2 Reviews of historical data from sub-Saharan Africa have shown large epidemics in that setting since the early 1900s,3 but likely not before then.
“Worldwide, approximately 500,000 cases of meningitis occur each year; in the United States, the incidence is approximately 3000 cases per year.4 Because of the success of vaccines against Haemophilus and pneumococcal meningitis, meningococcal meningitis is now the most important cause of endemic bacterial meningitis in children in the United States.5
The meningococcus is a gram-negative encapsulated aerobic bacterium. The capsular polysaccharide is a major virulence factor and defines 14 serogroups, among which 6 groups (A, B, C, W135, X, and Y) cause the most diseases. Meningococci are further classified by serotypes, defined by the PorB outer membrane proteins, and by serosubtype, defined by the PorA proteins. In addition, immunotypes related to lipopolysaccharides are used to classify strains. The genomes for isolates of group A and group B have been published,6, 7 and a group C genome is currently being sequenced. For epidemiologic purposes, many other strain classifications are used, including electropherotyping and multilocus sequence typing (MLST).8 Sequence typing focuses on 7 housekeeping genes that classify several hypervirulent lineages.
Meningococci have multiple mechanisms by which they generate new antigenic variations and evade host defenses; capsular switching and other genetic variance generate new epidemic strains.9, 10 Genetic sequence data suggest that genes have been acquired from other meningococci and other respiratory commensals such as H. influenzae, and the presence of many repeating sequences of DNA specifying surface proteins allows generation of antigenic variations.11
The meningococcus colonizes and infects humans, and there are no other hosts. It usually resides as a harmless commensal in the nasopharynx in
10% to 20% of adults. Transmission from person to person occurs either by direct contact with respiratory secretions (kissing), by indirect contact (sharing of eating utensils), or by aerosol droplets (coughing, sneezing). Only a small proportion of persons who are colonized in the nasopharynx develop invasive disease. Invasive disease is related both to virulence characteristics of the particular strain and to host factors.
10% to 20% of adults. Transmission from person to person occurs either by direct contact with respiratory secretions (kissing), by indirect contact (sharing of eating utensils), or by aerosol droplets (coughing, sneezing). Only a small proportion of persons who are colonized in the nasopharynx develop invasive disease. Invasive disease is related both to virulence characteristics of the particular strain and to host factors.
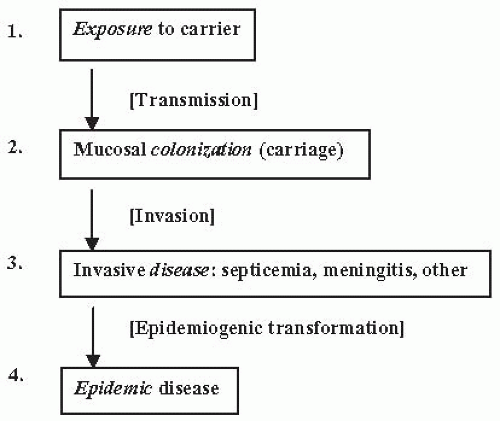
The major unknowns regarding meningococcal disease are the factors determining (1) acquisition and transmission of organisms, (2) the development of invasive disease in a small proportion of all persons who have nasopharyngeal colonization, (3) the development of epidemics, and (4) the shifting serogroups, subtypes, and clones that cause epidemics in various geographic settings. Figure 17-1 summarizes the processes and stages of meningococcal infection and epidemics. Recent genomic analyses of invasive strains compared to colonizing strains in a large collection in the Czech Republic showed that the invasive strains all had a specific phage DNA gene cluster, compared to only 10% of the carried strains. This finding suggests that the phage DNA mediates invasiveness and possibly determines epidemic clones.12
ACQUISITION AND CARRIAGE
Rates of meningococcal carriage in European children are generally less than 3%, increasing to 20% to 30% in the 15- to 24-year-old age group. Carriage rates among military recruits and contacts of cases are generally much higher, ranging from 20% to 70%. Respiratory infections by influenza and other viruses and exposure to tobacco smoke are associ-ated with increased carriage.13 The heterogeneity of subtypes and immunotypes is greater among carriers than among patients with invasive disease during epidemics. Colonization of the nasopharynx by meningococci usually produces an antibody response several weeks after acquisition of the organism. Because carriage of meningococcus and related nonpathogenic N. lactamica results in natural bactericidal antibody, 65% to 85% of adult individuals have naturally acquired protective bactericidal serum antibody titers.14, 15, 16 and 17
DISEASE
Invasive disease usually occurs within 1 to 4 days of acquisition. Approximately 60% of all patients presenting with acute cases of meningococcal disease have less than 24 hours of illness before hospitalization. In the endemic pattern disease, 30% of meningococcal disease is meningitis, which, even with therapy, has a mortality rate of 10% to 15% and neurologic and other sequelae in a high proportion of survivors. Septicemia without meningitis (meningococcemia) occurs in 10% to 20% of cases and can lead to loss of limbs and death. Pneumonia (5% to 10% of all meningococcal disease) and other syndromes including arthritis, pericarditis, endocarditis, pharyngitis, urethritis, and cervicitis have been described.5, 18
TREATMENT
Before the use of antibiotics, mortality from meningococcal disease was as high as 85%. Flexner and others showed that passive antibody treatment with specific animal sera was effective in reducing mortality.19 Treatment with penicillin is effective, because resistance to penicillin among meningococcal isolates is rare. Antibiotic treatment with broader-spectrum drugs, including ceftriaxone, to cover additional bacteria is often used for empirical treatment of bacterial meningitis and is effective. Chloremphenicol is also an effective therapy in many settings, though resistance has been reported.20 In addition to antibiotic treatment, good supportive care to treat shock and disseminated intravascular coagulation reduces mortality, though this rate remains between 10% and 15% in wealthy countries.21
In the setting of endemic disease, chemoprophylaxis can be effective to prevent illness in family members and other close contacts. In the United States, chemoprophylaxis of close contacts is recommended
for prevention of sporadic meningococcal disease. Close contacts are defined as household members, daycare center contacts, and anyone directly exposed to the oral secretions of the index case. Antimicrobial chemoprophylaxis should be administered to these contacts as soon as possible, because the risk of invasive disease is greatest within a few days of the index case. The Centers for Disease Control and Prevention (CDC) website provides information on the dose and duration of the recommended drugs, which include rifampin, ciprofloxacin, and ceftriaxone.22
for prevention of sporadic meningococcal disease. Close contacts are defined as household members, daycare center contacts, and anyone directly exposed to the oral secretions of the index case. Antimicrobial chemoprophylaxis should be administered to these contacts as soon as possible, because the risk of invasive disease is greatest within a few days of the index case. The Centers for Disease Control and Prevention (CDC) website provides information on the dose and duration of the recommended drugs, which include rifampin, ciprofloxacin, and ceftriaxone.22
RISK FACTORS
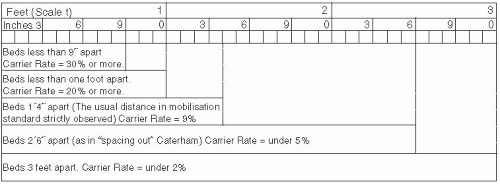
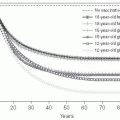
Important host factors for meningococcal disease are the absence of bactericidal antibodies in serum, the absence of a normal complement cascade, and the presence of certain mannose-binding lectins.23 A variety of human genetic polymorphisms are associated with severity of disease without apparently affecting the risk of acquisition.24 Other biological risk factors include complement deficiencies, anatomic or functional asplenia, and chronic disease. For meningococcal disease in the United States, risk factors include age younger than 11 months, household crowding, viral infection,13 active and passive smoking,25 residence in college dormitories, and chronic underlying illnesses. During outbreaks in the United States, bar or nightclub patronage and alcohol use have been associated with increased risk of acquisition.26 In African settings, risk factors include exposure to cooking fire smoke and sharing a bedroom with a case.27 During World War I, the importance of spacing of beds in sleeping quarters to reduce transmission and carriage of meningococci was understood and led to bed spacing regulations in military barracks (see Figure 17-2).
EPIDEMIOLOGY
In the past, meningococcus caused large epidemics in North America and Europe, but these types of outbreaks have not occurred in industrial countries (except for Norway and New Zealand) for the last 60 years. Rates of endemic meningococcal disease of all groups in the United States recently ranged from 1 to 1.5 per 100,000 population.22 In Europe, rates have been higher,28, 29 and 30 and childhood rates of 5 per 100,000 in Great Britain prompted universal use of vaccine.31 Table 17-1 compares the characteristics
of meningococcal disease in wealthier countries and in countries with limited resources. In general, the nature and quality of surveillance define the reported incidence of meningococcal disease.32
of meningococcal disease in wealthier countries and in countries with limited resources. In general, the nature and quality of surveillance define the reported incidence of meningococcal disease.32
Table 17-1 Comparison of Meningococcal Disease Epidemiology | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The epidemiology of meningococcal disease varies by the major serogroups, geography, and chronology. Group A historically has been the cause of large epidemic outbreaks and is associated with endemic and epidemic outbreaks in the meningitis belt of Africa. Group A disease is rare in the United States and Europe.33
Group B disease is the most important cause of endemic disease in developed countries, causing approximately 30% to 40% of cases in the United States and as many as 80% of cases in European countries. Norway, Cuba, and New Zealand have experienced epidemic group B disease in the recent past. Meningococcal group C disease has variable rates of endemic disease, and currently accounts for approximately 30% of disease in the United States and in Europe. Group Y has become more frequently found in the United States. Group W135 disease has become increasingly important and has been related to the Hajj pilgrimage since the late 1990s.34
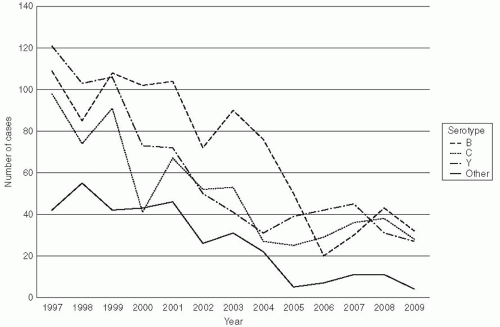
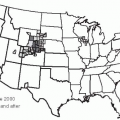
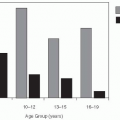
In the United States, in recent years serogroup Y has caused 39% of disease, serogroup C 31%, serogroup B 23%, and W135 2%; group A has not been found in cases (Figure 17-3).33 In Canada and Europe, serogroups B and C predominate (Figure 17-4), but in Africa serogroups A and W135 are most common. The distribution of serogroups in the United States changed during the 1990s.35 For example, group Y represented 9% of all cases between 1990 and 1992, but from 1997 to 2003, it accounted for 28% of all cases. Group B rates ranged from 43% to 34%.36 Epidemic meningitis was a feature in the North American and European countries until the middle of the last century. During the last decades, large epidemics have occurred in the African meningitis belt and within China.37
Serogroup A, subgroup III has caused two large global pandemics. The first occurred from the 1960s to 1970s in China, Russia, Scandinavia, and Brazil. A second group A(III) pandemic was associated with epidemics in the 1980s in China and an outbreak from 1982 to 1984 in Kathmandu, Nepal.37, 38 In 1985, epidemics occurred in New Delhi, India, and in Pakistan. During the 1987 Hajj in Mecca, Saudi
Arabia, the same strain caused an outbreak among pilgrims and later was found in epidemics in the meningitis belt in Africa, including the 1996 epidemic, which involved the largest number of cases of all recorded episodes. This A(III) ST5 strain caused epidemic disease in tropical regions not historically in the sub-Saharan belt, including Kenya, Tanzania, Zambia, and South Africa, as late as 1998.39, 40 and 41
Arabia, the same strain caused an outbreak among pilgrims and later was found in epidemics in the meningitis belt in Africa, including the 1996 epidemic, which involved the largest number of cases of all recorded episodes. This A(III) ST5 strain caused epidemic disease in tropical regions not historically in the sub-Saharan belt, including Kenya, Tanzania, Zambia, and South Africa, as late as 1998.39, 40 and 41
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree