Timothy R. Sterling, Richard E. Chaisson
General Clinical Manifestations of Human Immunodeficiency Virus Infection (Including Acute Retroviral Syndrome and Oral, Cutaneous, Renal, Ocular, Metabolic, and Cardiac Diseases)
Human immunodeficiency virus (HIV) infection results in a wide range of clinical consequences from asymptomatic infection despite active viral replication to severe immunodeficiency with life-threatening opportunistic disease. In persons infected with HIV, ongoing viral replication produces a steady decline in and eventual ablation of cell-mediated immunity, as well as marked immune activation and inflammation, all of which give rise to diverse manifestations of opportunistic disease. The acquired immunodeficiency syndrome (AIDS) is the most advanced stage of this illness, in which the infected host can no longer control opportunistic organisms or malignancies that rarely cause illness in immunocompetent individuals. The clinical features of HIV may vary according to the individual’s age, sex, race, geographic location, treatment status, and behavioral history. In this chapter selected clinical aspects of HIV infection, from the acquisition of the virus to death with AIDS, are reviewed and the classification and staging of this important viral infection and the acute retroviral syndrome and oral, cutaneous, renal, ocular, and cardiac diseases are discussed.
History
Disease caused by HIV-induced immunosuppression was first described in late 1980 and early 1981, when physicians in Los Angeles, New York, and San Francisco reported outbreaks of Kaposi sarcoma, a previously rare malignancy, and opportunistic infections in young homosexual men.1–6 These patients had a selective defect in cell-mediated immunity that was manifested by low numbers of CD4+ T lymphocytes and the development of a variety of opportunistic infections.
The occurrence of opportunistic diseases in homosexual men who had previously been healthy suggested that immunodeficiency developed because of an acquired rather than a congenital trait. In 1982, the Centers for Disease Control and Prevention (CDC) developed a case definition, based on the clinical, immunologic, and epidemiologic features of the first clusters of cases, for what was called the “acquired immunodeficiency syndrome.”7 AIDS was defined as the occurrence of a reliably diagnosed disease at least moderately indicative of underlying cellular immunodeficiency in a person without a condition known to be associated with an increased incidence of diseases related to cellular immunodeficiency. AIDS became a reportable condition in the United States in 1983. Soon after the initial case reports of AIDS, additional cases were observed in persons other than homosexual men. In 1981 and 1982, heterosexual injection drug users and immigrants from Haiti were reported to have AIDS.8–10,11,12 AIDS cases in hemophiliacs, recipients of blood transfusions, and Africans were soon reported.13,14
As the groups of persons at risk for AIDS expanded, clinicians noted an increasing spectrum of clinical manifestations of AIDS-associated immunodeficiency. Unexplained generalized lymphadenopathy, idiopathic thrombocytopenia, oral candidiasis, herpes zoster, and a constitutional wasting syndrome were observed in persons from AIDS risk groups who had deficits in cellular immunity.15–19 The term AIDS-related complex was coined to describe the signs and symptoms of immunodeficiency recognized with increasing frequency in persons at risk for AIDS.20 In 1982 to 1983, several investigators postulated an asymptomatic carrier state of the AIDS agent in healthy homosexual men, heterosexual partners of injection drug users, and Haitians who were noted to have laboratory evidence of impaired cellular immunity.21 After HIV was first described in 1983 to 1984,22–24 serologic tests to identify persons infected with HIV were developed that allowed large serologic surveys of at-risk populations to estimate the number of individuals infected with the virus and to delineate the spectrum of HIV-associated diseases.
Retrospective studies of serum and tissue indicated that the virus was present in humans in Africa as early as 1959 and that disease associated with HIV occurred in the United States in 1968.25–28 The CDC expanded its case definition of AIDS in 1985 and again in 1987 to accommodate the increased number of manifestations of impaired cellular immunity that had become associated with HIV infection.29,30 The World Health Organization (WHO) also promulgated a case definition for AIDS for use in developing countries that lacked sophisticated diagnostic resources.31 The AIDS case definition and HIV staging system were revised again in 1993 to include individuals with advanced immunodeficiency and with several other clinical manifestations of HIV disease.32 The surveillance case definitions for HIV and AIDS were again revised by the WHO in 2007.33 In 2008, the CDC revised the HIV classification system and the surveillance case definitions for HIV infection and AIDS in adults and adolescents and combined them into a single case definition for HIV infection.34 Both the WHO and CDC revised surveillance case definitions now require laboratory confirmation of HIV infection. Insights into the pathogenesis of HIV disease have emphasized the critical role of viral dynamics in the natural history of HIV infection,35–37 leading to clinical management schemata based largely on viral load and CD4+ cell levels (see later discussion).
Classification of HIV Infection
HIV infection represents an ongoing active viral process in most untreated individuals associated with progressive immunodeficiency that is likely to result in serious clinical consequences. Although there may be a prolonged state of clinical latency, during which many patients are unaware of their infection, HIV infection is usually not virologically latent, and infection is a chronic and progressive condition that without treatment may ultimately result in significant impairment and death. Although distinguishing between HIV infection and AIDS has been historically useful for epidemiologic purposes, the distinction is somewhat arbitrary and is less meaningful from a clinical perspective in an era of potent antiretroviral therapy (ART). As noted, current clinical staging approaches favor use of the CD4+ lymphocyte counts and plasma viral load assays.
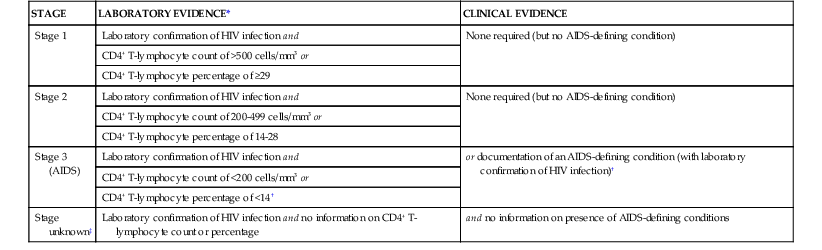
Several systems for classifying HIV infection and disease have developed and evolved over the past 3 decades. The 1986 CDC classification system placed HIV-infected persons into four categories: group I, acute infection; group II, asymptomatic infection; group III, persistent generalized lymphadenopathy (PGL); and group IV, symptomatic HIV disease.38 This system had limited prognostic usefulness and was supplanted by the 1993 classification system and revised case definition.32 The 1993 CDC classification system for HIV categorized HIV-infected individuals according to clinical and CD4+ cell count groupings. The clinical categories were as follows: group A, asymptomatic, acute HIV infection, or PGL; group B, symptomatic HIV disease; and group C, AIDS indicator conditions, encompassing the 1987 case definition with the addition of recurrent bacterial pneumonia, pulmonary tuberculosis, and invasive cervical cancer. The revised 2008 CDC surveillance case definition includes three stages based on CD4+ lymphocyte count and percentage and clinical evidence including AIDS-defining conditions (Table 124-1).34
TABLE 124-1
HIV Infection Staging Systems
| STAGE | LABORATORY EVIDENCE* | CLINICAL EVIDENCE |
| Stage 1 | Laboratory confirmation of HIV infection and | None required (but no AIDS-defining condition) |
| CD4+ T-lymphocyte count of >500 cells/mm3 or | ||
| CD4+ T-lymphocyte percentage of ≥29 | ||
| Stage 2 | Laboratory confirmation of HIV infection and | None required (but no AIDS-defining condition) |
| CD4+ T-lymphocyte count of 200-499 cells/mm3 or | ||
| CD4+ T-lymphocyte percentage of 14-28 | ||
| Stage 3 (AIDS) | Laboratory confirmation of HIV infection and | or documentation of an AIDS-defining condition (with laboratory confirmation of HIV infection)† |
| CD4+ T-lymphocyte count of <200 cells/mm3 or | ||
| CD4+ T-lymphocyte percentage of <14† | ||
| Stage unknown‡ | Laboratory confirmation of HIV infection and no information on CD4+ T-lymphocyte count or percentage | and no information on presence of AIDS-defining conditions |
* The CD4+ T-lymphocyte percentage is the percentage of total lymphocytes. If the CD4+ T-lymphocyte count and percentage do not correspond to the same HIV infection stage, select the more severe stage.
† Documentation of an AIDS-defining condition supersedes a CD4+ T-lymphocyte count of ≥200 cell/µL and a CD4+ T-lymphocyte percentage of total lymphocytes of ≥14.
‡ Although cases with no information on CD4+ T-lymphocyte count or percentage or on the presence of AIDS-defining conditions can be classified as stage unknown, every effort should be made to report CD4+ T-lymphocyte counts or percentages and the presence of AIDS-defining conditions at the time of diagnosis. Additional CD4+ T-lymphocyte counts or percentages and any identified AIDS-defining conditions can be reported as recommended.
Data from Schneider E, Whitmore S, Glynn KM, et al; Centers for Disease Control and Prevention. Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years—United States, 2008. MMWR Recomm Rep. 2008;57(RR-10):1-12.
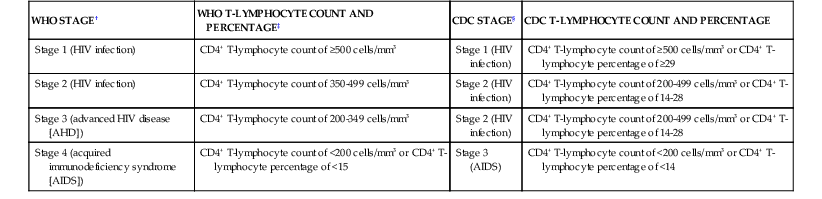
The CDC classification system for HIV infection recognizes the prognostic significance of the CD4+ cell count in individuals with HIV infection, but it is important to note that there is considerable variation in risk for opportunistic complications and prognosis in individuals with CD4+ cell counts below 200/mm3. Those with CD4+ counts below 50/mm3, for example, are generally considered to have advanced HIV disease and are at much higher risk for death and for the development of opportunistic infections such as cytomegalovirus (CMV) disease or disseminated Mycobacterium avium complex infection. The CDC classification system was initially developed at a time when the inevitable course of HIV infection was progression toward advanced immunodeficiency and death and when drug therapy was of limited and transient efficacy in stemming the course of the disease. According to the CDC classification system, HIV-infected individuals are classified on the basis of the most advanced stage that they have reached.34 In the present era, patients treated with combination ART often experience marked improvement in cellular immune function and may have a normal or near-normal life expectancy with dramatically lower risk for developing opportunistic disease than they had before receiving treatment.39 There is no current mechanism for reclassifying patients on the basis of immunologic and clinical improvement resulting from ART, a situation that understandably curtails use of the CDC and WHO systems. In areas in which combination ART is not widely used, these classification schemes more reliably reflect the maturity and status of the HIV epidemic within populations. The WHO classification system (Tables 124-2, 124-3, and 124-4) is used primarily in developing countries. However, there are limitations that make it difficult for this staging system to be uniformly implemented. Many of the classifications require the diagnosis of opportunistic infections that cannot be readily confirmed in most resource-poor settings; clinical criteria for establishing presumptive or definitive diagnoses might be useful. Estimates of weight loss and other constitutional symptoms are also difficult in such settings. A comparison of the CDC and WHO staging systems is presented in Table 124-5.
TABLE 124-2
World Health Organization (WHO) Clinical Staging of Established HIV Infection
| HIV-ASSOCIATED SYMPTOMS | WHO CLINICAL STAGE |
| Asymptomatic | 1 |
| Mild symptoms | 2 |
| Advanced symptoms | 3 |
| Severe symptoms | 4 |
TABLE 124-4
World Health Organization Clinical Staging of HIV/AIDS for Adults and Adolescents with Confirmed HIV Infection
Clinical Stage 1
Asymptomatic
Persistent generalized lymphadenopathy
Clinical Stage 2
Moderate unexplained weight loss (<10% of presumed or measured body weight)*
Recurrent respiratory tract infections (e.g., sinusitis, tonsillitis, otitis media, pharyngitis)
Herpes zoster
Angular cheilitis
Recurrent oral ulceration
Papular pruritic eruptions
Seborrheic dermatitis
Fungal nail infections
Clinical Stage 3
Unexplained* severe weight loss (>10% of presumed or measured body weight)
Unexplained chronic diarrhea for longer than 1 month
Unexplained persistent fever (>37.6° C [99.7° F]), intermittent or constant, for longer than 1 month)
Persistent oral candidiasis
Oral hairy leukoplakia
Pulmonary tuberculosis (current)
Severe bacterial infections (e.g., pneumonia, empyema, pyomyositis, bone or joint infection, meningitis, or bacteremia)
Acute necrotizing ulcerative stomatitis, gingivitis, or periodontitis
Unexplained anemia (<8 g/dL), neutropenia (<0.5 × 109/L), or chronic thrombocytopenia (<50 × 109/L)
Clinical Stage 4†
HIV wasting syndrome
Pneumocystis jirovecii
Recurrent severe bacterial pneumonia
Chronic herpes simplex infection (orolabial, genital or anorectal, longer than 1 month’s duration, or visceral at any site)
Esophageal candidiasis (or candidiasis of trachea, bronchi, or lungs)
Extrapulmonary tuberculosis
Kaposi sarcoma
Cytomegalovirus infection (retinitis or infection of other organs)
Central nervous system toxoplasmosis
HIV encephalopathy
Extrapulmonary cryptococcosis, including meningitis
Disseminated nontuberculous mycobacterial infection
Progressive multifocal leukoencephalopathy
Chronic cryptosporidiosis (with diarrhea)
Chronic isosporiasis
Disseminated mycosis (coccidioidomycosis or histoplasmosis)
Recurrent nontyphoidal Salmonella bacteremia
Lymphoma (cerebral or B-cell non-Hodgkin’s) or other solid HIV-associated tumors
Invasive cervical carcinoma
Atypical disseminated leishmaniasis
Symptomatic HIV-associated nephropathy or symptomatic HIV-associated cardiomyopathy
* Unexplained refers to when the condition is not explained by other causes.
† Some additional specific conditions can also be included in regional classifications (such as reactivation of American trypanosomiasis [meningoencephalitis and/or myocarditis]) in the World Health Organization region of the Americas and disseminated penicilliosis in Asia.
TABLE 124-5
Comparison of WHO and CDC Staging Systems*
| WHO STAGE† | WHO T-LYMPHOCYTE COUNT AND PERCENTAGE‡ | CDC STAGE§ | CDC T-LYMPHOCYTE COUNT AND PERCENTAGE |
| Stage 1 (HIV infection) | CD4+ T-lymphocyte count of ≥500 cells/mm3 | Stage 1 (HIV infection) | CD4+ T-lymphocyte count of ≥500 cells/mm3 or CD4+ T-lymphocyte percentage of ≥29 |
| Stage 2 (HIV infection) | CD4+ T-lymphocyte count of 350-499 cells/mm3 | Stage 2 (HIV infection) | CD4+ T-lymphocyte count of 200-499 cells/mm3 or CD4+ T-lymphocyte percentage of 14-28 |
| Stage 3 (advanced HIV disease [AHD]) | CD4+ T-lymphocyte count of 200-349 cells/mm3 | Stage 2 (HIV infection) | CD4+ T-lymphocyte count of 200-499 cells/mm3 or CD4+ T-lymphocyte percentage of 14-28 |
| Stage 4 (acquired immunodeficiency syndrome [AIDS]) | CD4+ T-lymphocyte count of <200 cells/mm3 or CD4+ T-lymphocyte percentage of <15 | Stage 3 (AIDS) | CD4+ T-lymphocyte count of <200 cells/mm3 or CD4+ T-lymphocyte percentage of <14 |
* For reporting purposes only.
† Among adults and children aged ≥5 years.
‡ Percentage applicable for stage 4 only.
§ Among adults and adolescents (ages ≥13 years). CDC also includes a fourth stage, stage unknown; laboratory confirmation of HIV infection but no information on CD4+ T-lymphocyte count or percentage and no information on AIDS-defining conditions.
CDC, Centers for Disease Control and Prevention; WHO, World Health Organization.
Natural History of HIV Infection
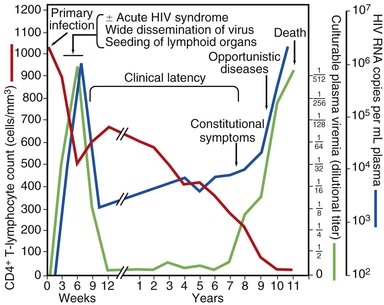
The clinical spectrum of HIV infection includes primary infection (the acute retroviral syndrome), asymptomatic infection, early symptomatic infection, and advanced immunodeficiency with opportunistic complications. Figure 124-1 shows a schematic diagram of the key immunologic, viral, and clinical features of HIV infection in untreated individuals. Viral load or viremia is monitored by measurement of HIV RNA in plasma, and immunologic status is reflected by the absolute number of CD4+ lymphocytes or the proportion of lymphocytes that express CD4+. Primary HIV infection is characterized by a high concentration of HIV RNA in plasma and suppression of the CD4+ cell count. Plasma viremia declines precipitously with antibody seroconversion and the development of an anti-HIV immune response, usually reaching a steady-state level within 6 to 12 months.40,41 In most untreated asymptomatic patients, the CD4+ cell count declines gradually over several years. The slope of decline is a function of the plasma viral load. Plasma viremia increases, accompanied by a more rapid decline in CD4+ count, before the onset of symptomatic disease. As the viral load rises and the CD4+ cell count falls, the risk for opportunistic infections, malignancies, wasting, neurologic complications, and death increases substantially.
There is considerable variation in the progression of HIV disease, with some individuals progressing from infection to AIDS in less than 5 years42 and so-called long-term nonprogressors remaining asymptomatic without treatment or evidence of immunologic decline for many years.43,44 Long-term nonprogressors appear to fall into at least two categories. Most have detectable viremia but maintain CD4+ cell levels that provide adequate protection from the development of opportunistic disease. These individuals generally have gradual loss of CD4+ lymphocytes, however, and eventually progress to advanced immunodeficiency. A much smaller group of individuals are so-called elite controllers, who have undetectable HIV viral loads and maintain normal CD4+ lymphocyte counts.45 This group is of considerable interest because of their ability to contain viral replication. Understanding of the mechanisms whereby they control HIV is of potential value for the development and evaluation of HIV vaccines.
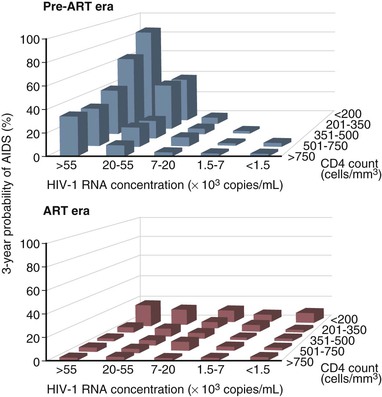
Before the availability of effective ART, the rate of progression from primary HIV infection to AIDS and from AIDS to death was estimated in a number of studies. Among homosexual men in San Francisco, the median time from seroconversion to AIDS by the 1987 CDC case definition was 9.8 years.46 Other studies estimated the period from infection to AIDS (1987 definition) to be 7 years for transfusion recipients, 10 years for hemophiliacs, 10 years for injection drug users, and 8 to 12 years for homosexual men.47 An important study of the natural history of HIV infection was the study of a cohort of homosexual and bisexual men by the San Francisco Department of Public Health and the CDC beginning early in the AIDS epidemic.48 These subjects were originally enrolled in a study of hepatitis B vaccine in 1978 and had serologic studies and clinical evaluations that dated from that time. Of the 489 men for whom the time of HIV seroconversion could be reliably estimated, 13% developed AIDS within 5 years, 51% within 10 years, and 54% at 11 years. In addition, of those who had not developed AIDS within 11 years of seroconversion, 19% had symptomatic disease and another 29% had CD4+ cell counts less than 200/mm3. Thus, after 11 years of follow-up, more than three fourths of HIV-infected homosexual men had severe immunodeficiency, had AIDS, or had died. A number of laboratory tests have been correlated with progressive immunodeficiency, the development of AIDS, and mortality. Taken together, however, the CD4+ lymphocyte count and plasma viral load are the best prognostic markers for subsequent disease course in an HIV-infected individual. The CD4+ lymphocyte count, a specific test for cellular immunocompetence, is a sensitive predictor of the development of symptomatic HIV infection and AIDS in the near term, because it reflects current immunologic capacity.49–52,53 Conversely, the plasma viral load (HIV-1 RNA) is an extremely useful predictor of disease course over a more extended period and is strongly associated with the rate of subsequent CD4+ cell count decline.54–58,59–62 A more rapid decline in CD4+ count, faster clinical progression, and decreased survival are all associated with a higher baseline viral load. In a study of HIV-infected gay or bisexual men enrolled in the Multicenter AIDS Cohort Study, the risk for progression to AIDS and death was highly correlated with plasma viral load at study entry, independent of CD4+ cell count.57,58 Baseline plasma viral load was a stronger predictor of progression and mortality than CD4+ count. In addition, the average annual decline in the CD4+ count of HIV-infected men varied according to their initial viral load, decreasing by 36 CD4+ cells/year in men with baseline HIV-1 RNA less than 500 copies/mL and by 77 CD4+ cells/year in men with baseline HIV-1 RNA higher than 30,000 copies/mL.58 Using the viral load and CD4+ count together, however, provides the best prognostic estimate of subsequent clinical course (Table 124-6; Fig. 124-2). Put in the context of HIV pathogenesis, the viral load measures the replicative rate of the infection and its destructive potential for the cellular immune system and the CD4+ count gauges the extent of immune compromise and the present risk for opportunistic disease.
TABLE 124-6
Probability of Developing AIDS* in 1604 Men in the Multicenter AIDS Cohort Study†
| BASELINE VIRAL LOAD‡ | BASELINE CD4+ COUNT§ | NO. STUDIED | NO. WITH AIDS | % AIDS AT 3 YEARS | % AIDS AT 6 YEARS | % AIDS AT 9 YEARS |
| <500 | >750 | 66 | 3 | 0 | 1.7 | 3.6 |
| <750 | 56 | 13 | 3.7 | 9.6 | 22.3 | |
| 501-3000 | Any | 257 | 90 | 2.0 | 16.6 | 35.4 |
| 3001-10,000 | >750 | 93 | 39 | 3.2 | 14.2 | 59.7 |
| <750 | 300 | 179 | 8.1 | 37.7 | 62.4 | |
| 10,001-30,000 | >750 | 64 | 42 | 9.5 | 36.7 | 62.4 |
| 351-750 | 259 | 194 | 16.1 | 54.9 | 76.3 | |
| ≤350 | 73 | 63 | 40.1 | 72.9 | 86.2 | |
| >30,000 | >500 | 141 | 105 | 32.6 | 66.8 | 76.3 |
| 351-500 | 121 | 111 | 47.9 | 77.7 | 94.4 | |
| 201-350 | 104 | 92 | 64.4 | 89.3 | 92.9 | |
| <200 | 70 | 67 | 85.5 | 97.9 | 100 |
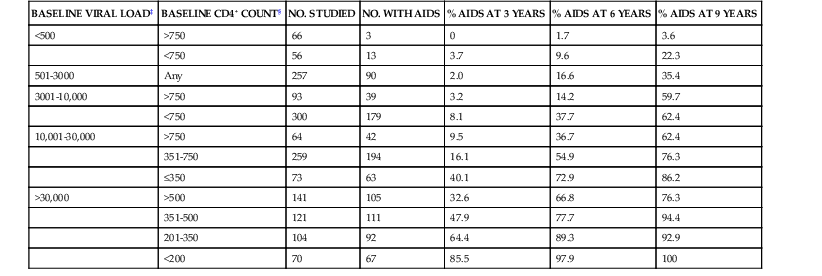
* 1987 Centers for Disease Control and Prevention case definition.
† Based on baseline HIV branched-chain DNA viral load and CD4+ cell count.
‡ HIV RNA copies/mL of plasma by branched-chain DNA. Viral load determined by reverse-transcriptase polymerase chain reaction approximately twofold greater.
§ CD4+ cells/mm3.
Data from Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946-954.
In the absence of treatment, survival is short after the diagnosis of clinically defined AIDS. Studies of survival of the first patients with AIDS in San Francisco and New York found a median survival of 9 to 12 months, with most patients dead within 2 years.63,64 Patients diagnosed with an opportunistic infection had the most rapid mortality, whereas survival was significantly longer in patients initially diagnosed with Kaposi sarcoma. Subsequent studies revealed that survival after diagnosis of AIDS was directly related to the CD4+ count at diagnosis. In most studies before the availability of combination ART, median survival after the diagnosis of AIDS was estimated to be between 12 and 18 months.65 The mean survival time after a CD4+ count of 200/mm3 was 38 to 40 months.66,67
The rate of progression of HIV infection in population-based studies varies depending on age, with older individuals generally having a more rapidly progressive course.68–72 Whether age differences in the pace of progression of HIV infection are the result of differences in viral setpoints, host immune responses, or both is unclear. Patients who experience more severe or long-lasting symptoms during the acute retroviral syndrome tend to have higher viral loads after seroconversion and progress more rapidly than those who seroconvert without symptoms.73 Women have approximately half-log10 lower HIV-1 RNA than men after seroconversion, but this difference diminishes with time from seroconversion.74–76 Although HIV-1 RNA is an important predictor of subsequent disease progression in both women and men,58,77,78 there is no gender difference in HIV disease progression, particularly when women and men have equal access to care.79–81 There do not appear to be racial differences in HIV-1 RNA levels82 or the natural history of HIV disease progression.80,83
Other laboratory studies that predict the development of AIDS in a seropositive individual include a total lymphocyte count less than 1000/mm3, a total white blood cell count less than 4000/mm3, a hematocrit less than 40 mL/dL, and a low percentage of CD4+ lymphocytes. Because the CD4+ percentage has a narrower range of variation in most clinical laboratories than the absolute CD4+ cell count, many clinicians favor using this measure for staging and monitoring of patients.84 Other markers of HIV disease progression that have been validated in clinical studies include the HIV p24 antigen, serum β2-microglobulin, neopterin, acid-labile interferon-α, anti-p24 antibody, and soluble CD8. These so-called surrogate markers are measures of virus presence or host immune responses to HIV. Many of these measures do not provide prognostic information independent of the viral load and have therefore been supplanted by quantitative plasma HIV-1 RNA monitoring in developed countries. Heat-denatured p24 antigen assay provides prognostic information independent of HIV-1 RNA85,86 but is unlikely to supplant the CD4+ count or HIV viral load as a clinical monitoring tool. Low-cost alternatives to flow cytometric quantification of CD4+ lymphocytes for application in resource-poor settings are now available and include manual assays that use enzyme-linked immunosorbent assay or bead-based formats.87 Other low-cost predictors of disease progression include total lymphocyte count and hemoglobin, although these are relatively nonspecific.88–90 Of note, coinfection with GB virus C, a close relative of hepatitis C virus, may be associated with a decreased risk for HIV disease progression.91–93
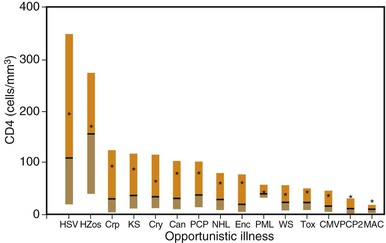
The probability of an HIV-infected individual developing opportunistic disease is influenced by several factors. First, immunocompetence is a critical determinant of whether an infected individual can contain a potential pathogen. As discussed later, the CD4+ cell count appears to be the most clinically useful measure of host cellular immunocompetence and plays a central role in the staging of HIV disease. Second, exposure to potential pathogens is required before disease can result. Although some opportunistic pathogens are ubiquitous, resulting in latent or continuous infection in a large proportion of HIV-infected persons (e.g., Pneumocystis jirovecii, CMV), others are prevalent in a smaller proportion of individuals and cause disease less often (e.g., Toxoplasma gondii). Other opportunistic pathogens do not appear to be associated with latent reactivation but rather cause disease when a sufficiently immunocompromised host acquires new infection (e.g., Cryptococcus neoformans, M. avium complex). Third, the relative virulence of a potential pathogen is a factor that may determine which disease is likely to occur. For example, more virulent organisms such as Mycobacterium tuberculosis or Streptococcus pneumoniae cause clinical illness in patients with less severe immunodeficiency, whereas less virulent organisms such as P. jirovecii or CMV cause illness in those with more severe immunodeficiency.94–96 Finally, whether a patient is taking chemoprophylactic agents with activity against specific pathogens influences the risk for disease. Figure 124-3 shows CD4+ cell counts at the time of diagnosis of opportunistic diseases in patients with CD4+ cell counts of 300/mm3 or less before 1996.97,98 Although the range of CD4+ cell counts for some conditions is broad, most patients with truly opportunistic infections had CD4+ counts less than 100/mm3.
Although the clinical manifestations of HIV infection do not vary according to HIV subtype, the incidence of specific opportunistic infections is profoundly influenced by geography and the prevalence of infectious diseases in particular regions. HIV-1 infection increases susceptibility to tuberculosis, and the incidence of tuberculosis in HIV-infected persons is extremely high in sub-Saharan Africa, where tuberculosis is endemic. In this setting, tuberculosis is the most common opportunistic infection in people with HIV infection and the leading cause of death. Approximately 25% of all deaths from tuberculosis worldwide are associated with HIV disease, mostly in sub-Saharan Africa.99 Malaria is also endemic in many developing countries and occurs with increased frequency and severity in HIV-infected persons, particularly during pregnancy.100 Opportunistic infections such as P. jirovecii pneumonia, M. avium complex disease, CMV retinitis, non-Hodgkin’s lymphoma, and HIV encephalopathy, which are relatively common in developed countries, are uncommon in developing countries, such as those in West Africa.101 In regions where it is endemic (e.g., the Mediterranean, Central America, South America, Africa, and Asia), leishmaniasis occurs with increased frequency among HIV-infected persons. Similarly, Trypanosoma cruzi (South America), histoplasmosis (Ohio and Mississippi river valleys), and Penicillium marneffei (Thailand, China, Hong Kong) occur with increased frequency in certain regions.
The incidence of specific opportunistic diseases has been determined for several large cohorts of HIV-infected individuals. In a cohort of more than 1200 patients with CD4+ cell counts less than 300/mm3 prior to the availability of ART, the most common opportunistic infection was Candida esophagitis (13.3 cases/100 person-years). P. jirovecii pneumonia, disseminated M. avium complex, CMV disease, and the AIDS dementia complex occurred at rates of 5 to 9 cases/100 person-years. The relatively lower incidence of P. jirovecii pneumonia reflects the use of specific prophylaxis with trimethoprim-sulfamethoxazole or aerosolized pentamidine, which dramatically lowers the risk for this infection, even in the absence of ART.71,97 Less common were toxoplasmosis, cryptococcal meningitis, herpes zoster, the wasting syndrome, and Kaposi sarcoma (2 to 4 cases/100 person-years). The least common complications were non-Hodgkin’s lymphoma, tuberculosis, progressive multifocal leukoencephalopathy, and cryptosporidiosis (1 to 2 cases/100 person-years). Similar results have been found in other cohorts of patients in developed country settings.102 Considerably less information is available on the natural history of HIV infection in developing countries, but the spectrum of disease in patients presenting with HIV-related illnesses is different, with tuberculosis, cryptococcosis, bacterial sepsis and pneumonia, herpes zoster, and gastroenteritis predominating.103
Early clinical findings may also predict disease progression in HIV-infected individuals who have not developed opportunistic disease. Oral candidiasis and oral hairy leukoplakia are markers of immunosuppression and herald the development of AIDS in many patients.104–106 Generalized lymphadenopathy is a common clinical finding in early HIV infection but does not predict progression to AIDS.107 The occurrence of an opportunistic disease increases the risk for death independently of the CD4+ cell count.108,109 This may be caused not only by morbidity related to the complication itself but also by an increase in immune activation and inflammatory responses leading to upregulation of HIV replication, with acceleration of HIV disease progression. A number of studies have demonstrated increases in HIV viral load in patients with acute opportunistic infections.110–113 Although viral load generally decreases somewhat after the acute illness, it generally does not return to premorbid levels.
Effect of Antiretroviral Therapy on Natural History of HIV Infection
Even before the era of effective combination ART, it was clear that nonsuppressive ART and prophylaxis against P. jirovecii pneumonia had substantially altered the natural history of AIDS, prolonging the median survival of treated AIDS patients to 2 to 3 years.114–118 Antiretroviral therapy and prophylaxis against P. jirovecii pneumonia and M. avium complex also prolonged the time from HIV infection to AIDS, decreased the incidence of opportunistic complications, and improved overall survival.119–126 Changes in plasma HIV-1 RNA and CD4+ lymphocyte counts resulting from ART have been shown to be strong predictors of clinical progression (or regression) of HIV disease.127–129
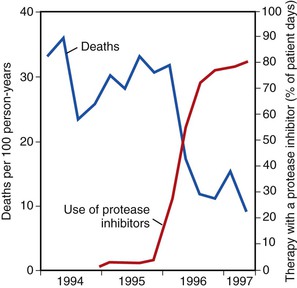
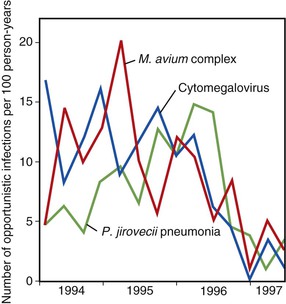
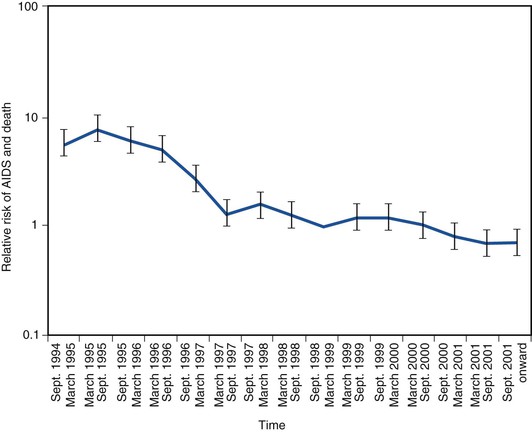
The use of combination ART and the introduction of protease inhibitors in 1995 and 1996 led to a dramatic change in the natural history of treated HIV disease.130–134 In the HIV Outpatient Study, mortality declined from 29.4 deaths/100 person-years in 1995 to 8.8 deaths/100 person-years in the second quarter of 1997 (Fig. 124-4).135 This decline in mortality was accompanied by marked decreases in the incidence of P. jirovecii pneumonia, M. avium complex disease, and CMV retinitis; the incidence of any one of those three infections declined from 21.9/100 person-years in 1994 to 3.7/100 person-years by mid-1997 (Fig. 124-5). The degree of benefit was associated with the intensity of ART; combination therapy resulted in an improved prognosis compared with monotherapy. In the United States as a whole, deaths attributed to AIDS decreased by 23% in 1996 and by 44% in 1997.131,132 Subsequent studies among cohorts of HIV-infected persons have continued to demonstrate the beneficial effect of ART on clinical disease progression and death (Fig. 124-6).136 Improvements in survival have also been documented in patients receiving ART in resource-poor settings, although the incidence of death remains higher in these areas than in developed countries.137,138 In developed countries in particular, death among persons with AIDS or advanced HIV infection is now more frequently caused by chronic diseases not traditionally classified as related to HIV infection. In a follow-up to the earlier HIV outpatient study, Pallela and colleagues found continued declines in AIDS-related mortality between 1997 and 2004, with relative (but not absolute) increases in deaths due to non-AIDS-related causes, such as liver disease, non-AIDS cancers, and cardiovascular disease.139 The Antiretroviral Therapy Cohort Collaboration reported that among more than 1800 deaths in 13 HIV clinical cohorts in the ART era, about half were due to AIDS and the remainder due to non-AIDS cancers, cardiovascular disease, trauma, liver disease, and other causes; the rate of AIDS-related deaths fell steadily with increased time on ART.140 A study from Switzerland has indicated that patients receiving effective ART have a risk for death similar to that in patients with cured cancer.141 Interruption of ART increases the risk for opportunistic disease and death from any cause, as well as major cardiovascular, renal, and hepatic disease.142,143 This suggests that lower counts of CD4+ lymphocytes and higher HIV-1 RNA increase the risk for non-AIDS events as well as AIDS-related events in HIV-infected persons.144
Effective therapy has not only decreased the incidence of new opportunistic infections but has also led to the resolution of preexisting conditions.145 In some cases, the immune restoration resulting from ART can alter the clinical presentation of specific opportunistic infections, as in the case of focal mycobacterial lymphadenitis or CMV vitritis.146 It may also unmask opportunistic infections that were not evident prior to ART initiation, such as tuberculosis.147,148 It is becoming increasingly clear that the immunologic changes resulting from ART represent at least a partial immune reconstitution, although the recovery of antigen-specific immunity appears to lag behind CD4+ cell count increases.149–152 The clinical manifestations of immune reconstitution syndromes are discussed at the end of this chapter. The incidence of new opportunistic infections in patients who have had satisfactory virologic and immunologic responses to ART is extremely low, even when primary prophylaxis has been discontinued.153,154 Moreover, reactivation of previously diagnosed opportunistic infections, such as M. avium complex infections and CMV retinitis, appears to be uncommon in patients with immune recovery who discontinue maintenance therapy.155,156
Thus, over the past 3 decades, the natural history of HIV infection has undergone considerable change, as has our understanding of it. The clinical course of HIV disease in those receiving ART is likely to evolve further in the coming years, with additional manifestations and complications of long-standing infection and use of antiretroviral drugs becoming apparent as larger numbers of patients are treated for longer periods of time.
Clinical Manifestations
HIV infection causes disease manifestations of three principal types: (1) an acute viral illness seen in the initial weeks of infection and associated with a high viral load and an intense host immune response; (2) immunologically mediated processes related to host responses to chronic viral infection, including inflammation (e.g., lymphadenopathy, thrombocytopenia, HIV-related dementia, and cardiovascular disease accelerated by proinflammatory responses); and (3) opportunistic diseases resulting from impaired host responses as the cellular immune system is damaged or ablated. The major clinical syndromes most frequently seen in HIV-infected individuals fall into the last category, that is, opportunistic diseases that arise as a consequence of impaired cellular immunity in late-stage HIV infection. Potent ART has added two new categories of clinical manifestations that may be commonly encountered in patients with HIV infection: (1) immune reconstitution syndromes with exacerbations of previously silent or adequately treated infections, especially mycobacterial infections,157,158 and (2) a syndrome of lipodystrophy with fat loss and redistribution, elevated serum triglyceride and cholesterol levels, and insulin resistance seen in patients receiving ART, especially with protease inhibitors.159,160 The clinical features of immune reconstitution syndromes are discussed later in this chapter, and the manifestations of drug toxicity related to the treatment of HIV are discussed in Chapter 130.
Acute Retroviral Syndrome
The initial manifestation of HIV infection in one half to two thirds of recently infected individuals is a mononucleosis-like illness referred to as the acute retroviral syndrome. The syndrome was first described in 1985 by Cooper and colleagues161 as an acute mononucleosis-like syndrome in 11 of 12 homosexual men who seroconverted for HIV antibodies. In a follow-up study, 36 of 39 (92%) homosexual men with recent HIV infection recalled an illness consistent with the acute retroviral syndrome during the time when their tests showed seroconversion162 but 40% of a seronegative control group also reported a mononucleosis-like illness. Similar descriptions of a characteristic syndrome have been reported in people infected with HIV through parenteral exposures, including health care workers exposed to accidental parenteral inoculation of HIV.163
The incidence of the acute retroviral syndrome is not precisely known. Retrospective studies of homosexual men infected with HIV found a low frequency of seroconversion illness.164,165 A prospective study of homosexual men showed a 55% incidence of a mononucleosis-like illness in 22 subjects who became antibody positive compared with 21% in 44 nonconverting control subjects.166 In one study of 378 persons with acute retroviral syndrome, injection drug users had or reported symptoms less frequently than persons who acquired HIV through sexual transmission.167 Most health care workers with occupationally acquired HIV had the acute retroviral syndrome after exposure.163,168 Overall, this syndrome is probably underreported and underdiagnosed, as noted in two series of patients, most of whom were not initially thought to have acute HIV infection.169,170
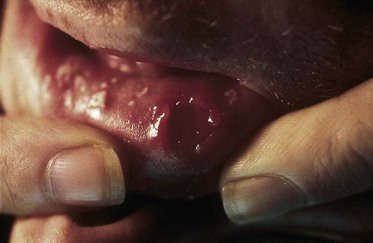
The clinical features of the acute retroviral syndrome are nonspecific and variable.171–173 The onset of the illness ranges from 1 to 6 weeks after exposure to the virus but peaks at 3 weeks. Table 124-7 shows the signs and symptoms of the acute retroviral syndrome reported in 209 cases, reviewed by Niu and co-workers.174 Fever, sweats, malaise, myalgias, anorexia, nausea, diarrhea, and a nonexudative pharyngitis are prominent symptoms.174,175,176,177–181 Many patients report headaches, photophobia, and meningismus. Two thirds of patients may have a truncal exanthem that may be maculopapular, roseola-like, or urticarial. Findings of skin biopsies are nonspecific, with perivascular lymphocytic infiltrates and dermal mononuclear cell infiltrates.182 In addition to aseptic meningitis, neurologic symptoms occur in a minority of patients and may include encephalitis, peripheral neuropathy, and an acute ascending polyneuropathy (Guillain-Barré syndrome).183 Physical examination frequently reveals cervical, occipital, or axillary lymphadenopathy, rash, and, less commonly, hepatosplenomegaly. Oral aphthous ulcerations (Fig. 124-7) have been reported in several cases; these may involve the esophagus. Oral and esophageal candidiasis during the seroconversion illness has been reported. The remainder of the physical examination is usually unremarkable. Symptoms generally resolve in 10 to 15 days. A wide range of acute opportunistic infections have been reported in patients with the acute retroviral syndrome, including P. jirovecii pneumonia, cryptococcal meningitis, and Candida esophagitis. Their occurrence is probably caused by the depression of the CD4+ cell count that generally accompanies acute HIV infection.
TABLE 124-7
Symptoms and Signs of the Acute Retroviral Syndrome in 209 Patients
| SYMPTOM OR SIGN | NO. WITH FINDING | FREQUENCY (%) |
| Fever | 200 | 96 |
| Adenopathy | 154 | 74 |
| Pharyngitis | 146 | 70 |
| Rash | 146 | 70 |
| Myalgia or arthralgia | 112 | 54 |
| Thrombocytopenia | 94 | 45 |
| Leukopenia | 80 | 38 |
| Diarrhea | 67 | 32 |
| Headache | 66 | 32 |
| Nausea, vomiting | 56 | 27 |
| Elevated aminotransferase levels* | 38 | 21 |
| Hepatosplenomegaly | 30 | 14 |
| Thrush | 24 | 12 |
| Neuropathy | 13 | 6 |
| Encephalopathy | 12 | 6 |
* Based on 178 subjects.
Modified from Niu MT, Stein DS, Schnittman SM. Primary human immunodeficiency virus type 1 infection: review of pathogenesis and early treatment intervention in human and animal retrovirus infections. J Infect Dis. 1993;168:1490-1501.
Laboratory evaluation of patients with the syndrome reveals a reduced total lymphocyte count, elevated sedimentation rate, negative heterophil antibody test, and elevated aminotransferase and alkaline phosphatase levels.174 When lymphocyte phenotyping is performed, a characteristic pattern is observed.184 Initially, the total lymphocyte count, including both CD4+ and CD8+ T lymphocytes, decreases, with a normal ratio of CD4+ to CD8+ cells. Within several weeks, both the CD4+ and CD8+ cell populations begin to increase. The rise in CD8+ cell numbers is relatively greater than that in CD4+ cells, and the CD4/CD8 ratio is inverted. In the weeks that follow, the CD8+ cell population increases rather markedly because of HIV-specific CD8+ T lymphocytes. The ratio of CD4+ to CD8+ cells usually remains inverted as the acute illness resolves, primarily because of excess numbers of CD8+ cells. In patients with neurologic symptoms, the cerebrospinal fluid may show a lymphocytic pleocytosis with normal levels of protein and glucose.185
Tests for detecting acute HIV infection iinclude plasma HIV RNA, which becomes positive at about 5 days after infection and HIV p24 antigen, which may be detected after 10 days, whereas antibody reactivity on enzyme immunoassay testing is not found until 14 to 21 days.186 HIV p24 antigen may be detected in the serum and cerebrospinal fluid in about 75% of patients with primary HIV infection within 2 weeks of exposure, often coincidentally with the onset of symptoms.185,186 Antigenemia can persist for several weeks or months and generally resolves when antibodies to p24 are produced in sufficient quantity to form complexes with free antigen. The most sensitive marker for acute HIV infection, however, is plasma HIV RNA, which is markedly elevated in most patients.187 Typical RNA levels range from 105 to more than 106 copies/mL of plasma, and the titers decline as the CD8+ cytotoxic T-cell and antibody responses increase subsequently. Low-level (<104) false-positive HIV RNA tests may occur, but high-level viremia is diagnostic of acute infection in the absence of anti-HIV antibodies. The enzyme immunoassay for HIV antibodies remains negative for an average of 2 to 6 weeks after the onset of symptoms, despite the appearance of specific antibodies on a Western blot of the patient’s serum. Anti-p24 appears on the Western blot shortly before seroconversion is detected by enzyme-linked immunosorbent assay and by the appearance of antibodies to other antigens. To increase sensitivity among antibody-negative persons, and to overcome the costs, labor, and false-positive results of HIV-1 RNA tests, pooled-sample group testing can be used.188,189
The differential diagnosis of the acute retroviral syndrome includes a number of other illnesses—infectious mononucleosis and other viral infections such as influenza, viral hepatitis, measles, rubella, primary herpes simplex virus (HSV) infection, cytomegalovirus, and secondary syphilis. Evaluation of patients presenting with an illness consistent with acute retroviral infection should include a careful history to elicit risks for HIV infection, laboratory tests to rule out mononucleosis, cytomegalovirus, and syphilis, HIV antibody and plasma RNA tests, and complete blood cell counts and differential. There is potential benefit in treating acute HIV with combination ART because there is evidence that this may lower the viral setpoint, lead to enhanced CD4+ and CD8+ HIV-specific responses, and decrease the severity of acute disease.190 However, early treatment does not appear to prevent establishment of reservoirs of latently infected resting CD4+ cells (although it may decrease the size of the reservoirs) and may not provide any long-term benefit.191 There are also concerns about the potential toxicity of long-term therapy and the risk for developing drug resistance. Nonetheless, because recommendations for ART are moving toward treatment for all HIV-infected persons, it is also recommended for persons with early HIV infection—the acute phase of infection up to 6 months after infection192 (see Chapter 130).
Persistent Generalized Lymphadenopathy
Infection with HIV is associated with a high prevalence of generalized lymphadenopathy, often beginning with the acute retroviral syndrome. In the early 1980s, PGL was recognized as a prodromal state to the development of AIDS in homosexual men who were otherwise healthy.15,17 The pathogenesis of generalized lymphadenopathy is related to the rapid infection of CD4+ cells in lymph nodes by HIV after initial infection. The syndrome of PGL is defined as the presence of two or more extrainguinal sites of lymphadenopathy for a minimum of 3 to 6 months for which no other explanation can be found. Biopsy specimens of lymph nodes from such patients usually reveal a follicular hyperplasia without specific pathogens.
PGL develops in 50% to 70% of HIV-infected individuals. The most frequently involved node groups are the posterior and anterior cervical, submandibular, occipital, and axillary chains; epitrochlear and femoral nodes may also be enlarged. Physical examination usually reveals symmetrical, mobile, rubbery lymph nodes ranging from 0.5 to 2 cm. Pain and tenderness are uncommon. Localized (i.e., asymmetrical) adenopathy and rapid nodal enlargement are not characteristic and suggest an infectious or malignant process. The remainder of the physical examination is often unremarkable, although other complications of HIV infection may be found, such as thrush or hairy leukoplakia. Mediastinal and hilar adenopathy is not characteristic of the syndrome; however, abdominal computed tomography (CT) often reveals enlarged mesenteric and retroperitoneal adenopathy in HIV-infected persons. The natural history of HIV infection in those with PGL does not differ significantly from that of HIV infection without PGL.107,193 Involution of enlarged lymph nodes, with degeneration of follicular germinal centers and loss of hyperplasia, often accompanies progression of HIV infection to advanced disease.
In patients treated with ART, previously involuted lymph nodes may again enlarge as HIV-specific and other T cells are replenished. In addition, focal lymphadenitis with constitutional symptoms may occur in patients with previously silent mycobacterial infections 1 to 2 months after starting ART. These reversal or unmasking reactions, or immune reconstitution syndromes, are reminiscent of reversal reactions seen in multibacillary forms of leprosy, heralding a return of pathogen-specific T-cell responses.
The differential diagnosis of PGL includes HIV infection and a wide variety of other processes associated with generalized lymphadenopathy, such as sarcoid, secondary syphilis, and Hodgkin’s disease. In patients with HIV infection, lymphadenopathy may also be caused by mycobacterial infections, Kaposi sarcoma, and lymphoma.194 An unusual cause of lymphadenopathy in patients with HIV infection is multicentric Castleman disease.195,196 Castleman disease is an angioproliferative, hyperplastic process of lymph nodes and other lymphoid tissues showing characteristic histologic findings, with either hyaline vascular or plasma cell variants. In patients with HIV in particular, multicentric Castleman disease is the most common presentation, with involvement of lymph nodes, liver, spleen, and other organs. Although the pathogenesis of Castleman disease is not fully understood, infection with Kaposi sarcoma–associated herpesvirus (human herpesvirus type 8) is believed to underlie a large proportion of cases.197,198 Unlike PGL, multicentric Castleman disease is associated with constitutional symptoms and multiorgan involvement in most HIV-infected patients. The diagnosis is established histopathologically. Treatment with the anti-CD20 monoclonal antibody rituximab has shown promise.199
In patients with clinical findings suggesting opportunistic disease, needle aspiration of lymph nodes may help establish a specific diagnosis.200 Examination of aspirates with cytologic, acid-fast, and Gram stains and with flow cytometry is valuable in identifying infection or malignancy. If a specific diagnosis is not determined after staining and culture of node aspirates, lymph node biopsy is indicated. Aspiration of lymph nodes in patients with PGL usually reveals benign cells. Biopsy specimens show follicular hyperplasia, with the normal architecture distorted by greatly expanded germinal centers composed of B lymphocytes. It is now known that active viral replication is occurring in these follicular cells and that virus is trapped in dendritic cells, although the patient may appear well clinically.201
Most patients with PGL require no invasive evaluation and can be managed according to standard guidelines for HIV infection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree