INTRODUCTION
SUMMARY
Natural killer (NK) cells, with a predominant morphology of large granular lymphocytes, represent a lineage of lymphoid cells with constitutive ability to mediate cytotoxicity toward pathologic target cells and secrete cytokines. NK cells participate in the innate resistance to microbial pathogens and malignancies; opposing effects of activating and inhibitory receptors regulate NK cell activity. Malignant expansions of NK cells, either acute or chronic, are rare, but represent well-identified clinical entities.
Acronyms and Abbreviations
ADCC, antibody-dependent cell-mediated cytotoxicity; AML, acute myelogenous leukemia; CAR, chimeric antigen receptor; CTL, cytotoxic T lymphocyte; GM-CSF, granulocyte-macrophage colony-stimulating factor; HLA, human leukocyte antigen; IFN, interferon; Ig, immunoglobulin; IL, interleukin; ILC, innate lymphoid cell; iNKT, invariant natural killer T cell; ITIM, immunoreceptor tyrosine-based inhibitory motif; KIR, killer cell Ig-like receptor; LCMV, lymphocytic choriomeningitis virus; LGL, large granular lymphocyte; MCMV, mouse cytomegalovirus; MHC, major histocompatibility complex; NK, natural killer; TCR, T-cell antigen receptor; TNF, tumor necrosis factor; TRAIL, TNF-related apoptosis-inducing ligand.
IDENTIFICATION AND DEFINITION OF NATURAL KILLER CELLS
Natural killer (NK) cells were identified in the blood and lymphoid organs of humans and experimental animals as cells capable of killing tumors, virus-infected cells, and, in some instances, normal cells, in the absence of previous deliberate or known sensitization.1,2 NK cells are now considered to belong to the cellular subgroup that is characterized by the production of interferon (IFN)-γ within the family of innate lymphoid cells (ILCs), a family of developmentally related cells involved in innate immunity and tissue development.3 NK cells are defined as cytotoxic cells with the predominant morphology of large granular lymphocytes (LGLs) that: (1) neither productively rearrange any of the genes encoding the T-cell receptor (TCR) chains nor express on their surface the CD3-TCR complex; (2) express the CD56 (N-CAM), CD335 (NKp46), and CD16 (FcγRIIIA), antigens in humans, the NK1.1 (NKR-P1C), NKp46, and DX5 (VLA-2/CD49d) antigens in mice, and the NKR-P1 antigen in rats; and (3) can kill cells not expressing major histocompatibility complex (MHC) class I or class II antigens. Thus, target-cell recognition by NK cells is distinct from cytotoxic T lymphocytes (CTLs), which recognize specific antigenic peptides bound to MHC class I molecules. Nonetheless, the presence of MHC class I on target cells affects NK cell recognition, in some cases inhibiting a NK cell response.
Certain T lymphocytes that express either αβ or a γδ TCR may exhibit, particularly upon activation, TCR-independent cytolytic activity that resembles that of NK cells and often express many of the same surface receptors as NK cells. Among the T lymphocytes in humans and mice that coexpress many of the NK cell antigens, invariant natural killer T (iNKT) cells express an invariant TCR that recognizes glycolipids presented by CD1d, a nonclassical MHC molecule, and upon stimulation rapidly produce large amounts of IFN-γ, granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-4, and IL-13.4
Human LGLs are medium- to large-size lymphocytes with round or indented nuclei, condensed chromatin, and usually prominent nucleoli. The cytoplasm is abundant and contains a variety of organelles and granules (primary lysosomes) that, in addition to lysosomal enzymes, contain proteins important for cytotoxic function, such as serine esterases (granzymes) and pore-forming proteins (perforin).5 Although many NK cells have the morphology typical of LGL, a significant proportion of NK cells are agranular and indistinguishable from other lymphocytes.6
NK cells originate in the marrow from the common lymphoid progenitor cell.7 Most have a life span ranging from a few days to a few weeks,8 but some may persist for months after exposure to viral challenge.9 In mice, the cytokine IL-15 plays a particularly important role in the differentiation and expansion of NK cells.10 NK cell differentiation does not require the thymus, although NK cell progenitors can differentiate in the thymus from precursors expressing IL-7Rα CD127.11 Secondary lymphoid tissues may also be a site of NK cell development in humans.12 The increased number of NK cells and altered anatomical distribution in response to infection or other stimuli are the result of increased NK cell production in the marrow and proliferation of peripheral NK cells.
NK cells represent approximately 5 to 20 percent of blood lymphocytes.13 Immature NK cells express high amounts of CD56, lack CD16, and have low cytolytic capacity, whereas mature NK cells in blood express low amounts of CD56, high levels of CD16, and mediate potent lytic activity.14 NK cells are present in the red pulp of the spleen and are found at a low frequency in other lymphoid organs and marrow.2 NK cells with a phenotype resembling the CD56high peripheral subset have been detected in lymph nodes.15 Small numbers of NK cells can be identified in the liver (pit cells), lung, and intestinal mucosa.16,17 In response to type I IFN or viral or bacterial infections, NK cells accumulate in organs in which they normally are rare, particularly the liver, marrow, and lymph nodes where they may produce large amounts of cytokines.18 CD56bright CD16– NK cells are the predominant cell type present in the human early pregnancy decidua.19 Decidual NK cells produce cytokines that have tissue remodeling capacity, facilitate embryonic implantation, monitor mucosal integrity throughout the menstrual cycle, control trophoblast invasion during pregnancy, and modulate the maternal immune response against embryo antigens.19
MECHANISMS OF NATURAL KILLER CELL FUNCTIONS
Cytotoxicity mediated by NK cells depends on binding to the target cells, followed by activation of the lytic mechanism, which usually involves release of perforin and granzymes from the granules.5 Cytotoxicity can also be mediated through the interaction of surface molecules, for example, the interaction of Fas ligand, membrane tumor necrosis factor (TNF), or TNF-related apoptosis-inducing ligand (TRAIL) on NK cells with their death-inducing receptors on target cells. Lysis of the target cells results from alteration of membrane permeability and induction of apoptosis.5
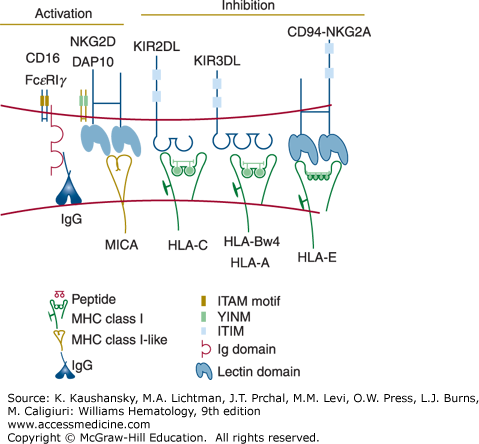
Several surface molecules on NK cells have been identified that activate the cytotoxic mechanism and induce cytokine secretion (Fig. 77–1).20 One of these molecules is the low-affinity receptor for the Fc fragment of immunoglobulin (Ig) G (FcγRIIIA or CD16), which is expressed on most human circulating NK cells in association with the signal-transducing CD3ξ or FcεRIγ chains. When CD16 is crosslinked by IgG antibodies bound to a target cell surface, it triggers antibody-dependent cell-mediated cytotoxicity (ADCC). Natural killing can also be activated by several other receptors that recognize relevant ligands on the potential target cell. NKG2D, a receptor expressed on all NK cells, has been implicated in NK cell recognition of transformed and virus-infected cells.21 This receptor recognizes a family of MHC class I-related glycoproteins (including MICA, MICB, ULBP1–ULBP6), which are absent or expressed at only low levels on healthy cells but are induced or upregulated upon cell transformation or viral infection.21 Viruses, such as cytomegalovirus, have devised strategies to prevent the expression of NKG2D ligands in the infected cells,22 presumably to escape NK cell-mediated immunity. NK cells express many other activating receptors that have been implicated in their recognition of tumors, including DNAM-1 (CD226) and the “natural cytotoxicity” receptors NKp30, NKp44, and NKp46.20
NK cells preferentially kill certain tumor cells lacking expression of MHC class I molecules.23 NK cells are regulated by positive signals initiated by activating receptors and negative signals transmitted by interactions between inhibitory receptors for MHC class I on the NK cells and autologous MHC class I molecules on potential target cells. NK cells may mediate immune surveillance against cells that lose expression of MHC class I. Numerous viruses inhibit the synthesis or transport of MHC class I proteins, presumably to avoid detection by CTL.24 In addition, frequent loss of MHC class I expression on tumor cells has been documented.25 However, NK cells are capable of killing cells expressing MHC class I if they receive sufficiently strong activation signals.
Two families of NK cell receptors for MHC class I have been identified in humans. The Killer cell Ig-like receptors (KIRs) are encoded by approximately 15 genes present on human chromosome 19q13.4.26 KIR genes are highly polymorphic and evolve rapidly, diversifying by gene duplication and conversion events. Certain KIRs bind human leukocyte antigen (HLA)-C ligands, whereas other KIRs recognize certain alleles of HLA-B or HLA-A. Another class of NK cell receptors for MHC class I are heterodimeric glycoproteins composed of a CD94 subunit that is disulfide bonded to an NKG2A molecule.20 The genes encoding CD94 (KLRD1) and NKG2A (KLRC1) are on human chromosome 12p12-p13 and are members of the C-type lectin superfamily. The CD94-NKG2A receptors bind to HLA-E, a unique MHC class I protein that displays peptides derived from leader segments from HLA-A, HLA-B, HLA-C, or HLA-G proteins.27 When synthesis of HLA-A, HLA-B, HLA-C, or HLA-G is disrupted, possibly by viral infection or transformation of the host cell, HLA-E cannot be transported to the cell surface for presentation to the CD94-NKG2A receptor. The various KIR and CD94-NKG2A receptors are expressed on overlapping subsets within the NK cell population and on certain memory T cells, usually CD8+ T cells, although a minor subset of CD4+ T cells also express KIR. The inhibitory KIR and CD94-NKG2A receptors have an immunoreceptor tyrosine-based inhibitory motif (ITIM) sequence in their cytoplasmic domains, which binds to the cytoplasmic tyrosine phosphatase SHP-1, resulting in suppression of cytotoxicity and cytokine secretion.20 Therefore, the functional behavior of NK and T cells expressing KIR or CD94-NKG2A is regulated by the balance of positive signals transmitted by a variety of activating receptors and negative signals provided by the inhibitory MHC class I receptors. Although the expression of NK cell inhibitory receptors is variegated and polymorphic, most NK cells express at least one inhibitory receptor recognizing self MHC and thus are not self-reactive. This is accomplished at least in part by the requirement of interaction of an inhibitory receptor with its ligands during NK cell development for full functional maturation and possibly expansion (NK cell “licensing”).28
Certain receptors of the KIR and CD94-NKG2 families do not possess ITIM sequences and activate, rather than suppress, NK- and T-cell responses.20 These receptors noncovalently associate with the homodimeric adapter protein DAP12.29 Like the CD3ξ and the FcεRIγ subunits, DAP12 contains an immunoreceptor tyrosine-based activation motif (ITAM) in the cytoplasmic domain. Upon receptor ligation, DAP12 becomes tyrosine phosphorylated, recruits the ZAP70 and Syk cytoplasmic tyrosine kinases, and induces cellular activation.29 The physiologic role of activating NK cell receptors for MHC class I has not been determined, but these receptors may have consequences in allogeneic marrow transplantation. In mice, an activating receptor in the Ly49 family (the functional counterpart of KIRs in humans) has been shown to recognize a viral glycoprotein encoded by cytomegalovirus and protects the mice from this pathogen.30,31 This finding suggests that certain activating KIRs in humans may also recognize pathogens.
Although resting blood NK cells are cytotoxic, their activity can be greatly enhanced both in vivo and in vitro by exposure to cytokines such as IFN-α/β, IL-2, IL-12, IL-15, and IL-18.32,33,34 Resting NK cells constitutively express intermediate-affinity IL-2 receptors, and IL-2 induces the progression of most NK cells into the cell cycle.35
Many of the physiologic functions of NK cells are mediated at least partly by their ability to secrete cytokines. NK cells are powerful producers of IFN-γ and GM-CSF, and other cytokines and chemokines. Stimulation by cytokines, such as IL-2, IL-12, IL-18, TNF-α, and IL-1,2,33,36,37 and triggering by activating receptors, such as CD16 interacting with immune complexes, are among the stimuli that, acting individually or often in synergistic combination, induce NK cells to produce cytokines.2,38,39
PHYSIOLOGIC ROLES OF NATURAL KILLER CELLS