INTRODUCTION
SUMMARY
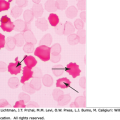
Erythrocyte fragmentation and hemolysis occur when red cells are forced at high shear stress through partial vascular occlusions or over abnormal vascular surfaces. “Split” red cells, or schistocytes, are prominent on blood films under these conditions, and considerable quantities of lactate dehydrogenase are released into the blood from traumatized red cells. In the high-flow (high-shear) microvascular (arteriolar/capillary) or arterial circulation, partial vascular obstructions are caused by platelet aggregates in the systemic microvasculature during episodes of thrombotic thrombocytopenic purpura by platelet-fibrin thrombi in the renal microvasculature in the hemolytic uremic syndrome; and by malfunction of a cardiac prosthetic valve in valve-related hemolysis. Less-extensive red cell fragmentation, hemolysis, and schistocytosis occur under conditions of more moderate vascular occlusion or endothelial surface abnormalities, sometimes under conditions of lower shear stress. These latter entities include excessive platelet aggregation, fibrin polymer formation, and secondary fibrinolysis in the arterial or venous microcirculation (disseminated intravascular coagulation); in the placental vasculature in preeclampsia/eclampsia and the syndrome of hemolysis, elevated liver enzymes and low platelets (HELLP) in march hemoglobinuria; and in giant cavernous hemangiomas (the Kasabach-Merritt phenomenon).
PREECLAMPSIA/ECLAMPSIA AND HELLP SYNDROME
A life-threatening condition of pregnancy denoted by eclampsia, hemolysis, and thrombocytopenia was first noted in the German literature by Stahnke in 1922.1 Subsequently, Pritchard and coworkers described three cases in English and suggested that an immunologic process might account for both the preeclampsia or eclampsia and the hematologic abnormalities.2 Although initially known as edema-proteinuria-hypertension gestosis type B,3 a catchier phrase, HELLP syndrome (H for hemolysis, EL for elevated liver function tests, and LP for low platelet counts), was later applied by Louis Weinstein in 1982.4
Acronyms and Abbreviations:
ADAMTS13, a disintegrin and metalloproteinase with thrombospondin domain 13; ALT, alanine transaminase; aPTT, activated partial thromboplastin time; AST, aspartic acid transaminase; AT, antithrombin; DIC, disseminated intravascular coagulation; HELLP, hemolysis, elevated liver enzymes, and low platelet count; LDH, lactate dehydrogenase; MAHA, microangiopathic hemolytic anemia; NO, nitrous oxide; PGF, placental growth factor; PGI2, prostaglandin I2; PT, prothrombin time; PTT, partial thromboplastin time; sEng, soluble endoglin; sFlt-1, soluble form of fms-like tyrosine kinase 1; sVEGFR-1, soluble vascular endothelial growth factor receptor-1; TGF-β, transforming growth factor-β; TTP, thrombotic thrombocytopenic purpura; VEGF, vascular endothelial growth factor; VWF, von Willebrand factor.
HELLP syndrome occurs in approximately 0.5 percent of pregnancies overall,5 in 4 to 12 percent of those complicated by preeclampsia (hypertension + proteinuria), and in 30 to 50 percent of those complicated by eclampsia (hypertension + proteinuria + seizures); however, approximately 15 percent of patients ultimately diagnosed with HELLP syndrome present with neither hypertension nor proteinuria.6 Two-thirds of HELLP patients are diagnosed antepartum, usually between 27 and 37 weeks. The remaining one-third are diagnosed in the postpartum period, from a few to 48 hours following delivery (occasionally as long as 6 days).7,8 Risk factors for HELLP syndrome include European ancestry, multiparity, older maternal age (older than age 34 years), and a personal or familial history of the disorder.5 Although the presence of homozygosity for the 677 (C→T) polymorphism of the methylenetetrahydrofolate reductase gene may be a modest risk factor for the development of preeclampsia, this weak association does not exist for HELLP syndrome.9 Whether or not the factor V Leiden or prothrombin 20210 gene mutations are risk factors for HELLP syndrome remains controversial.10,11,12
A developing embryo must acquire a supply of maternal blood to survive. During a normal pregnancy, the first wave of trophoblastic invasion into the decidua occurs at 10 to 12 days. This is followed by a second wave at 16 to 22 weeks, when these specialized placental epithelial cells replace the endothelium of the uterine spiral arteries and intercalate within the muscular tunica, increasing the vessels’ diameters and decreasing their resistance. As a result, the spiral arteries are remodeled into unique hybrid vessels composed of fetal and maternal cells, and the vasculature is converted into a high-flow–low-resistance system resistant to vasoconstrictors circulating in the maternal blood.13 In a preeclamptic pregnancy, the second wave fails to penetrate adequately the spiral arteries of the uterus, perhaps as a result of reduced placental expression of syncytin and subsequent altered cell fusion processes during placentogenesis.14 The resultant poorly perfused, hypoxic placenta then releases the extracellular domain (soluble) form of fms-like tyrosine kinase 1 (sFLT-1), also known as soluble vascular endothelial growth factor receptor-1 (sVEGF receptor-1, or sVEGFR-1). sVEGFR-1 functions as an antiangiogenic protein because it binds to vascular endothelial growth factor (VEGF) and placental growth factor (PGF), and prevents their interaction with endothelial cell receptors. The result is glomerular endothelial cell and placental dysfunction.15,16,17 Direct and indirect sequelae include increased vascular tone, hypertension, proteinuria, enhanced platelet activation and aggregation, and decreased levels of the vasodilators prostaglandin I2 (PGI2) and nitrous oxide (NO).5,17 Concurrent activation of the coagulation cascade results in platelet-fibrin deposition in the capillaries, multiorgan microvascular injury, microangiopathic hemolytic anemia, elevated liver enzymes because of hepatic necrosis, and thrombocytopenia because of peripheral consumption of platelets.5
Another antiangiogenic molecule, a soluble form of endoglin (sEng), also increases in patient serum during early and severe preeclampsia.18 Endoglin is part of the transforming growth factor-β (TGF-β) complex, and is expressed on vascular endothelial cells and syncytiotrophoblasts. The shed extracellular domain of endoglin, sEng, is capable of binding to and inactivating the proangiogenic growth factors, TGF-β1 and TGF-β3. The presence of elevated serum levels of both sFLT-1 (sVEGFR-1) and sEng may be associated with the progression of preeclampsia to HELLP.17,18
Ninety percent of patients with HELLP syndrome present with malaise and right upper quadrant or epigastric pain. Between 45 and 86 percent have nausea or vomiting, 55 to 67 percent have edema, 31 to 50 percent have headache, and a smaller percentage complain of visual changes. Fever is not typically seen. Although hypertension is found in 85 percent of affected patients, 15 percent of those with HELLP syndrome do not develop either hypertension or proteinuria.6
There is no consensus regarding the laboratory criteria necessary to diagnose HELLP syndrome, so clinical judgment in conjunction with judicious interpretation of a variety of laboratory tests constitute the diagnostic standard. In 54 to 86 percent of patients, the blood film has schistocytes, helmet cells, and burr cells consistent with microangiopathic hemolytic anemia. Reticulocytosis can be present. Low haptoglobin levels are both sensitive (83 percent) and specific (96 percent) for confirming the presence of hemolysis, and return to normal within 24 to 30 hours postpartum.6
Lactate dehydrogenase (LDH) levels are usually above normal. The ratio of LDH-5 (an isoenzyme found specifically in the liver) to total LDH is elevated in proportion to the severity of HELLP. The high LDH seen in HELLP is most likely the result, principally, of liver damage rather than hemolysis. Serum levels of aspartic acid transaminase (AST) and alanine transaminase (ALT) can be more than 100 times normal, whereas alkaline phosphatase values are typically only about twice normal and total bilirubin ranges between 1.2 and 5.0 mg/dL. Liver enzymes usually return to normal within 3 to 5 days postpartum.6
The degree of thrombocytopenia has been used in a classification system to predict maternal morbidity and mortality, the rapidity of postpartum recovery, the risk of disease recurrence, and perinatal outcome. This Mississippi triple-class system places those patients with platelet counts less than 50 × 109/L in class 1 (approximately 13 percent incidence of bleeding); those with platelet counts between 50 and 100 × 109/L in class 2 (approximately 8 percent incidence of bleeding); and those with a platelet count greater than 100 × 109/L in class 3 (no increased bleeding risk). Patients with class 1 HELLP syndrome suffer the highest incidence of perinatal morbidity and mortality, and have the most protracted recovery periods postpartum.19 There is a direct correlation between the extent of thrombocytopenia and measurements of liver function,20 but the same cannot be said for the severity of associated hepatic histopathologic changes.21 If a marrow aspiration and biopsy are performed, abundant megakaryocytes are found consistent with a consumptive thrombocytopenia causing reduction of the normal platelet life span of approximately 10 days to 3 to 5 days.19 The platelet count nadir occurs 23 to 29 hours postpartum, with subsequent normalization within 6 to 11 days.7
The prothrombin time (PT) and activated partial thromboplastin time (aPTT) are usually within normal limits, although one report cited a prolonged aPTT in 50 percent of patients.22 Although low fibrinogen levels are inconsistently found, other measures of increased coagulation and secondary fibrinolysis may be present. These include decreased protein C and antithrombin III (AT III) levels, and increased D-dimer and thrombin-AT III values. von Willebrand factor (VWF) antigen levels increase in proportion to the severity of the disease, reflecting the extent of endothelial damage; however, no unusually large VWF multimers are present in plasma23 and ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin domains-13) levels are within a broad normal range (ADAMTS13 normally declines moderately during pregnancy).24,25 This is in contrast to the severe deficiency of ADAMTS13 in familial and autoantibody-mediated types of thrombotic thrombocytopenic purpura (TTP).26 Unlike TTP, the thrombi found in organs involved in the HELLP syndrome contain increased amounts of fibrin and low levels of VWF.23
In patients with severe liver involvement, hepatic ultrasonography shows large, irregular, well-demarcated (or “geographical”) areas of increased echogenicity.27 Liver biopsy shows periportal or focal necrosis, platelet-fibrin deposits in the sinusoids, and vascular microthrombi. As the disease progresses, large areas of necrosis can coalesce and dissect into the liver capsule. This produces a subcapsular hematoma and the risk of hepatic rupture.5
Other complications of pregnancy that can be confused with HELLP include TTP28 and the hemolytic uremic syndrome, sepsis, disseminated intravascular coagulation (DIC), connective tissue disease, antiphospholipid antibody syndrome, and acute fatty liver of pregnancy. This latter entity is also seen in the last trimester or postpartum and presents with thrombocytopenia and right upper quadrant pain, but the levels of AST and ALT only rise to 1 to 5 times normal and the PT and partial thromboplastin time (PTT) are both prolonged. Oil-red-O staining of liver biopsies demonstrates fat in the cytoplasm of centrilobular hepatocytes, and routine stains show inflammation and patchy hepatocellular necrosis as a result of the HELLP syndrome. Because it causes right upper quadrant pain and nausea, HELLP has also been misdiagnosed as viral hepatitis, biliary colic, esophageal reflux, cholecystitis, and gastric cancer. Conversely, other conditions misdiagnosed as HELLP syndrome include cardiomyopathy, dissecting aortic aneurysm, acute cocaine intoxication, essential hypertension and renal disease, and alcoholic liver disease.19
Supportive care of HELLP includes intravenous administration of magnesium sulfate to control hypertension and prevent eclamptic seizures, management of fluids and electrolytes, judicious transfusion of blood products, stimulation of fetal lung maturation with beclomethasone, and delivery of the fetus as soon as possible.19 Indications for delivery include a severe disease presentation, maternal DIC, fetal distress, and a gestational age greater than 32 weeks with evidence of lung maturity.6 Cesarean section under general anesthesia is used in 60 to 97 percent of cases, but vaginal delivery after induction can be attempted if the fetus is older than 32 weeks of age and the mother’s cervical anatomy is favorable. Postpartum curettage is helpful in lowering the mean arterial pressure and increasing the urine output and platelet count. Transfusion therapy with packed red cells, platelets, or fresh-frozen plasma is indicated in cases complicated by severe anemia or bleeding because of coagulopathy.
Although previously thought to be beneficial based upon the results of observational studies and small randomized trials, the use of dexamethasone has fallen out of favor after large randomized trials found that it didn’t reduce the duration of hospitalization, amount of blood products transfused, maternal complications, or time to normalization of laboratory abnormalities.29
Plasma exchange cannot arrest or reverse HELLP syndrome when used antepartum, but may minimize hemorrhage and morbidity when used peripartum. It can also be tried postpartum in the 5 percent of patients who fail to improve within 72 to 96 hours of delivery. These women are more likely to be younger than 20 years of age or nulliparous.7 Whether or not plasma exchange can effectively lower circulating levels of sVEGF and/or sEng is not known. Liver transplantation may be necessary in occasional patients with HELLP complicated by large hematomas or total hepatic necrosis. It is not yet known if replacement with some (possibly modified) form of VEGF and/or TGF-β may have future therapeutic use in preeclampsia or HELLP. A single case report describes the successful use of eculizumab to prolong by 17 days a pregnancy affected by severe HELLP, without associated maternal or fetal morbidity or mortality.30
Most patients stabilize within 24 to 48 hours following delivery; however, maternal death still occurs in 3 to 5 percent even with best supportive care. Mortality rates as high as 25 percent were reported prior to 1980. Events leading to maternal death include cerebral hemorrhage, cardiopulmonary arrest, DIC, adult respiratory distress syndrome, and hypoxic ischemic encephalopathy.5 Other complications include infection, placenta abruptio, postpartum hemorrhage, intraabdominal bleeding, and subcapsular liver hematomas with resultant rupture (a fatal event in 50 percent of those in whom it occurs).6 The latter patients complain of right-sided shoulder pain and are found to be in shock with ascites or pleural effusions. The hematoma is usually present in the anterior superior portion of the right lobe of the liver.5 If the liver remains intact when discovered, abdominal palpation, seizures, and emesis should be avoided or prevented. Emergency surgery is required for hepatic artery embolization or ligation, hepatic lobectomy, or even liver transplantation in patients with total hepatic necrosis.5,19
Renal complications of HELLP include acute renal failure, hyponatremia, and nephrogenic diabetes insipidus as a result of impaired hepatic metabolism of vasopressinase and resultant “resistance to vasopressin” (antidiuretic hormone). Pulmonary complications of HELLP include of pleural effusions, pulmonary edema, and adult respiratory distress syndrome. Neurologic sequelae of HELLP not mentioned above include retinal detachment, postictal cortical blindness, and hypoglycemic coma.31
Fetal morbidity and mortality are between 9 and 24 percent.6 Complications arise as a result of prematurity, placental abruption, and intrauterine asphyxia. Intrauterine growth retardation is seen in 39 percent of infants. One-third of all babies born to mothers with HELLP have thrombocytopenia, but intraventricular hemorrhage is seen in only 4 percent of thrombocytopenic infants.32
HELLP syndrome complicates 2 to 5 percent of all pregnancies,5 and can recur in as many as 27 percent of those affected during subsequent pregnancies.33 Other hypertensive disorders of pregnancy (preeclampsia or pregnancy-induced hypertension) are also relatively common in future pregnancies (27 percent of second and subsequent pregnancies).34 Women who recover from preeclampsia/HELLP may also be more likely to develop subsequent hypertension and cardiovascular disorders, possibly because of some persistent abnormal balance between proangiogenic and antiangiogenic factors.17
DISSEMINATED MALIGNANCY
The association between widespread malignancy and hemolytic anemia associated with pathologic changes in small blood vessels was first noted by Brain and colleagues in 1962.35
Cancer-associated microangiopathic hemolytic anemia (MAHA) has been described in a wide variety of malignancies (Table 51–1). MAHA is more likely to be associated with metastatic malignant disease than with localized cancers or benign tumors.36 Approximately 80 percent of the tumors are mucinous adenocarcinomas of either the stomach (55 percent), breast (13 percent), or lung (10 percent). The median age at diagnosis is 50 years, with a slight male predominance.37
MAHA as a result of malignancy can be caused by either of two distinct mechanisms: (1) DIC with intravascular occlusions (often partial) of small vessels by platelet-fibrin thrombi; or (2) intravascular tumor emboli.35,38 In the first mechanism,1 intravascular activation of coagulation may occur from excessive exposure of tissue factor on phagocytes, activated endothelial cells, or tumor cells. Alternatively, a protease in the mucin secreted by adenocarcinomas may directly activate factor X.39 Subsequent activation of coagulation factors, thrombin generation, fibrin polymer deposition, and platelet aggregation result in the formation of intravascular platelet-fibrin thrombi, and the shearing of red cells attempting to maneuver past the partial platelet-fibrin occlusions in the high-flow microvasculature. Also, circulating carcinoma mucins may interact with leukocyte L-selectin and platelet P-selectin, causing the rapid generation of platelet-rich microthrombi.40 In the second mechanism,2 intravascular tumor emboli partially occlude small vessels, mechanically or chemically disrupt the endothelium and promote platelet adherence to exposed subendothelium, coagulation activation and fibrin polymer formation, intimal hyperplasia, and vascular hypertrophy.35,37,38
Patients with cancer-associated DIC/MAHA present with moderate-to-severe anemia. The blood film reveals schistocytes (accounting for approximately 5 to 21 percent of the red cells), burr cells, microspherocytes, reticulocytes/polychromasia, and nucleated red cells.38 Although the reticulocyte count can be high, it is an unreliable measure of hemolysis because extensive replacement of the marrow by metastatic tumor (Chap. 45) may prevent the reticulocytosis expected with MAHA. Other indicators of hemolysis that could be more reliable include increased levels of serum unconjugated bilirubin and LDH, the presence of plasma hemoglobin, and elevated urine urobilinogen and hemoglobinuria (as αβ dimers).37
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree