INTRODUCTION
SUMMARY
Erythrocyte disorders from physical or chemical agents occur via such processes as red cell volume expansion within hypotonic solutions, erythrocyte membrane damage from biotoxins, damage to the spectrin skeleton from insults such as heat, and eryptosis associated with oxidizing agents such as oxygen, arsine gas, and chlorates. Erythrocyte damage also can be induced by other agents that lack well defined mechanisms of action (see Table 52–1). These processes include erythrocyte damage caused by lead, copper, and radiation, as well as neocytolysis, a phenomenon once thought unique to microgravity, but subsequently observed in individuals demonstrating altitude induced polycythemia upon transition to normoxic conditions.
Abbreviations and Acronyms:
AsH3, arsenic hydride (arsine gas); EDTA, ethylenediaminetetraacetic acid; G6PD, glucose-6-phosphate dehydrogenase; NADPH, reduced nicotinamide adenine dinucleotide phosphate.
MECHANISTICALLY DESCRIBED ERYTHROCYTE DAMAGE
Chemical and physical agents causing erythrocyte disorders within the context of enzyme deficiency, unstable hemoglobins, cell fragmentation or immune dysfunction are discussed in Chaps. 46 to 51 and 54. The present chapter deals with drugs, toxins, and other physical agents that can cause red cell disorders, which are not discussed elsewhere within this text.
When large amounts of distilled water gain access to the systemic circulation, either by intravenous injection or when used as an irrigating solution during surgery, hemolysis will occur.1 Severe hemolysis may also result from water inhalation in near-drowning.2 Occasionally self-induced hypotonic lysis secondary to water intoxication from polydipsia in the setting of psychiatric illness or hazing rituals occurs.3 In all cases, hemolysis follows expansion of the erythrocyte volume, transition to a spherical shape and ultimately cell rupture.4
Bee5,6 and wasp7,8,9 stings, as well as contact with caterpillar bristle from Lonomia obliqua,10 are associated with severe hemolysis. In addition, spider and scorpion bites occasionally are followed by hemolytic anemia and hemoglobinuria.11,12,13,14,15,16 The spiders usually responsible are Loxosceles laeta and Loxosceles recluse. In such cases, sphingomyelinase D is one of the causative toxins. The venom preferentially hydrolyzes band 3 of the red cell membrane protein.17 Band 3 has dual functions of ion exchange and anchoring of the cell membrane to the underlying cellular skeleton.18 It appears disruption of the structural role is responsible for cell lysis.
One of the most intriguing mechanisms of membrane damage is that induced by a class of pore-forming cytotoxins, usually from Bacillus cereus.19 Toxins using similar mechanisms of hemolysis are found in marine organisms including jelly fish (Chironex fleckeri),20 sea cucumbers (Cucumaria echinata),21 and sea anemones (Stichodactyla helianthus).22 X-ray crystallography reveals these toxins to be composed of proteins that associate to span the erythrocyte membrane forming an ion permeable pore.21 Aged cells appear preferentially damaged.
Additional discussion of hemolysis associated with microorganism-produced toxins, including Clostridium-induced spherocytosis and massive hemolysis, is found in Chap. 53.
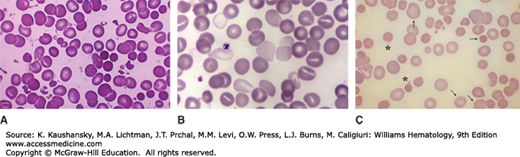
Gross hemoglobinemia was observed in 11 of 40 patients with second- and third-degree burns involving 15 to 65 percent of body surface area.23 Within the first 24 hours following a burn, hemolytic anemia results from the direct effect of heat on circulating erythrocytes. Blood heated to temperatures above 49°C demonstrates morphologically similar damage (Fig. 52–1A), consistent with increased osmotic and mechanical fragility.24,25
Figure 52–1.
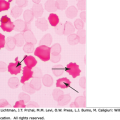
A. Blood film prepared at admission from a patient who had suffered severe burn injury involving a large percentage of the body surface. Note the presence of normal erythrocytes (apparently from vessels not exposed to heat damage) along with populations of normocytic and microcytic spherocytes. In addition, there are numerous red cell fragments, some smaller than platelets. B. Blood film prepared from a patient exposed to arsenic hydride. Note the very pale red cells resulting from partial hemoglobin loss secondary to membrane damage. An extreme example, represented by the virtual ghost thinly rimmed with scant residual hemoglobin, can be found in the upper left-hand corner. C. Wilson disease. In this image from a patient with Wilson disease, there are numerous visible sequelae of oxidative damage caused by excess copper. The striking microspherocytosis indicates damage to the membrane. Damage to hemoglobin is demonstrated by the Heinz bodies projecting from red cells (asterisks show two examples). The horizontal arrow points to one of several spherocytes. The vertical arrow points to a macrocyte (reticulocyte). An occasional cell shows damage to both membrane and hemoglobin. The presence of echinocytes (oblique arrows show two examples) suggests that the liver is also affected. (A & B, reproduced with permission from Lichtman’s Atlas of Hematology, www.accessmedicine.com. C, used with permission of Barbara J. Bain, Imperial College, London, UK.)
In addition to acute damage, heat decreases erythrocyte resilience. A normal erythrocyte in liquid behaves physically as a drop of fluid because the flexible membrane allows the surface of the cell to rotate around the intracellular contents.26 These fluid-like properties couple collisional energy between the erythrocyte membrane and the viscous hemoglobin solution within the cell, allowing dissipation of collisional energy through the entire cell and ultimately protecting the cell membrane. When heated, the spectrin comprising the erythrocyte skeleton melts, and upon cooling becomes rigid. This rigidity prevents collisional energy dissipation, making such cells particularly susceptible to membrane damage.27 The ensuing damage to the erythrocyte membrane structure results in splenic sequestration and cell removal.28
Although oxygen is a powerful oxidizing agent, quantum mechanical properties of the oxygen molecule prevent spontaneous oxidation of biologic membranes.29 When bound to hemoglobin, oxygen has significantly different quantum mechanical properties and occasionally, an exceptionally reactive superoxide molecule escapes.30 It is estimated that 2 to 3 percent of total hemoglobin would be oxidized daily in the absence of enzyme systems to protect against escaped superoxide.31,32 Although the hemolysis that occurs when these systems are overwhelmed is dealt with in Chap. 47, a few additional examples are briefly described below. Hemolysis following oxidation is thought to occur via eryptosis (Chap. 33). In addition to oxidative stress, osmotic shock and certain toxic ions, including gold and aluminum, may act through eryptosis.33,34
Hemolytic anemia can occur when ambient oxygen (O2) concentration is increased markedly.35 Hyperbaric oxygenation has been associated with acute hemolysis.36 Ozone (O3), which has been widely used in some countries for a variety of therapeutic purposes, had no apparent effect in vivo on red cell enzymes and intermediates at the 30 mcg/mL concentration commonly infused, but did produce some in vitro hemolysis at that concentration.37
Arsenic exposure is a major cause of anemia in regions with high environmental concentration. Examples include Bangladesh’s tainted water supply, and areas of China where arsenic laden coal is used.38 Arsine gas (arsenic hydride, AsH3) is the most erythrotoxic form of arsenic and inhalation of arsine gas is a well-recognized cause of hemolytic anemia (see Fig. 52–1B).39,40,41 Arsine is formed during many industrial processes, including the reaction of hydrogen with available arsenic compounds in metallurgic processes. The arsenic is usually a contaminant, so contact with arsenic compounds may not be apparent from the patient’s history. The mechanism of erythrocyte damage occurs via oxidation of sulfhydryl groups in the erythrocyte membrane and associated cytoskeleton,42,43 and decreased levels of reduced glutathione in erythrocytes exposed to AsH3 are observed.44
Sodium and potassium chlorate are oxidative drugs that produce methemoglobinemia, Heinz bodies, and hemolytic anemia.45 Although it has been presumed that the mechanism of hemolysis is similar to that resulting from other oxidative drugs, enigmatically, no cases have been observed in patients deficient in glucose-6-phosphate dehydrogenase (G6PD). Hemolytic anemia with Heinz body formation has also occurred in patients undergoing dialysis when the water contained a substantial amount of chloramines. Oxidative damage to the red cells of these patients was demonstrated by the presence of Heinz bodies, a positive ascorbate-cyanide test, and methemoglobinemia.46,47
Leaching of formaldehyde from plastic used in water filters employed for hemodialysis is also a cause of hemolytic anemia. The low level of formaldehyde in the contaminated water does not result in a fixative effect, but instead induces metabolic changes within the red cells.48
CHEMICAL AND PHYSICAL AGENTS NOT DEFINITIVELY DESCRIBED MECHANISTICALLY
A variety of chemical and physical agents cause erythrocyte disorders through unknown or not well-characterized mechanisms. There are isolated reports of hemolytic anemia occurring after the administration of a variety of chemical substances, some of which are listed in Table 52–1. What follows is a collection of miscellaneous erythrocyte damaging agents and processes for which the mechanisms are still largely undefined or disputed.