EPIDURAL ABSCESS
HANS-WALTER PFISTER, MATTHIAS KLEIN, ALLAN R. TUNKEL, AND W. MICHAEL SCHELD
SPINAL EPIDURAL ABSCESS
First described by the Italian anatomist Morgagni in the eighteenth century, a spinal epidural abscess is a suppurative infection in the space around the spinal cord located between the dura mater and the vertebral periosteum (1). It is both a medical and a surgical emergency, requiring prompt and accurate diagnosis and treatment to prevent irreversible spinal cord dysfunction, paralysis, and death.
Epidemiology
Although spinal epidural infections are uncommon, recent studies suggest that the incidence of this infection is increasing (2). Although incidence rates of spinal epidural abscesses have not been calculated from population-based data, estimates from recent case series noted an increase from 0.2 to 1.2 cases per 10,000 admissions in the mid-1970s (3) to significantly higher incidence rates of up to 1.96 (4), 2.8 (5), or 11.31 cases (6) per 10,000 admissions to large tertiary care centers in the 1980s and early 1990s. The reasons for the increasing incidence of epidural spinal infections seem to be an aging population, increased use of spinal procedures, and a rising rate of injection drug use (4–7). However, a change in referral patterns or a greater recognition (more widespread use of magnetic resonance imaging [MRI]) must also be considered.
Approximately 70% of patients with spinal epidural abscess are between 31 and 70 years of age, with no obvious predilection for any given decade (1). The youngest patient in the literature was 10 days of age and the oldest was 87 years of age (1). Almost all large case series observed a preference for the male gender, with an average male-to-female ratio of 1.0:0.56 (1). The reasons for this male predominance are not apparent. However, some risk factors associated with spinal epidural abscesses are more common in males, such as alcohol abuse, injection drug usage, and trauma (1).
Pathogenesis and Pathophysiology
Below the foramen magnum, the epidural space extends the length of the spine. It is composed of two compartments: (a) a true space posterior and lateral to the spinal cord containing a cushioning layer of fat embedded with penetrating arteries and an extensive venous plexus, and (b) a potential anterior space where the dura adheres to the posterior surface of the vertebral body (8,9) (Fig. 32.1). The epidural space is circumferential around the spinal cord distal to the second sacral segment, the terminal point of its anterior attachment. Given these anatomic considerations, it is not surprising that spinal epidural abscesses are located posteriorly in most cases (3,5).
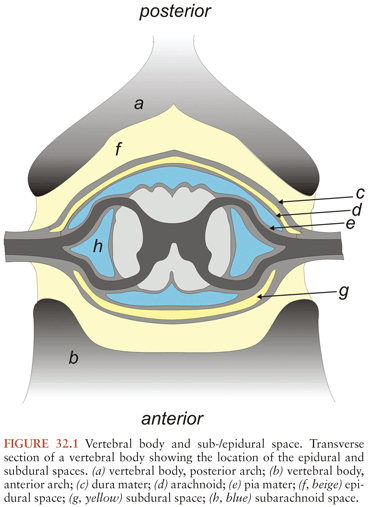
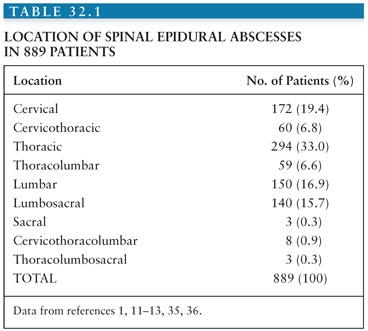
In addition to the boundary imposed by the anterior attachment to the vertebral canal, the dimensions of the epidural space vary from segment to segment. In the cervical region where the epidural space is smallest, epidural abscesses occur less often than in the thoracic or lumbar regions (Table 32.1). In recent years, the increasing use of spinal interventions for pain management has caused a relative increase of lumbar epidural abscesses (2,10). A recent series of 40 cases reported that 11 of 16 patients who were injection drug users had epidural abscesses in the cervical region (6). It was postulated that this may reflect the venous and lymphatic drainage of the upper extremity. Whereas the rich circulation ensures ample blood supply to the spinal cord, it may also act as a conduit along which infection may spread. Infection tracking along these pathways is the likely explanation for the longitudinal extension of spinal epidural abscesses that usually affect multiple adjacent spinal segments. On average, 3.3 ± 2.7 (mean ± standard deviation [SD]) spinal cord segments were reported to be involved in five clinical studies with 133 patients (11,12,14–16); however, infections of the entire length of the spinal epidural space have been reported (panspinal infection) (3,7,17). Of note, spinal epidural abscesses can also be found at noncontiguous sites, such as the cervical and lumbar spinal cord (18).
Source of Infection
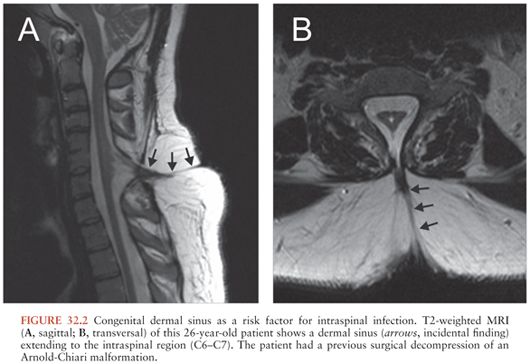
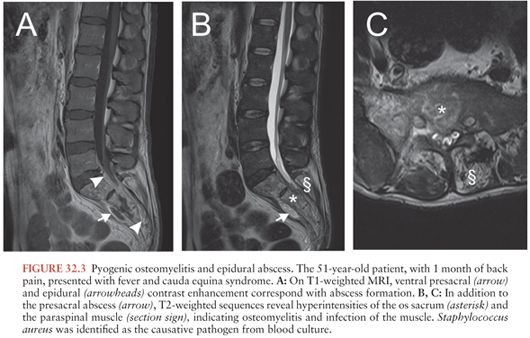
Infection may be introduced into the epidural space by direct extension from a contiguous infection (about 1/3 of cases) or by hematogenous seeding from a remote site (about half of cases); in the remaining cases, the source of infection is not identified (7) (Table 32.2). Contiguous infections include (1) vertebral osteomyelitis/discitis; retropharyngeal, perinephric, paraspinal, or psoas abscesses; decubitus ulcers; and persistent dermal sinus tracts (Fig. 32.2). Local invasion from superficial infections can also occur following penetrating injuries, including prior surgery or spinal procedures including placement of catheters or stimulators; epidural drug injections; paravertebral injections; computed tomography (CT)–guided needle biopsy; and, very rarely, lumbar puncture (1). Metastatic seeding may result from any bacteremic infection. Skin and soft tissue infections are typically found, but sources have also included endocarditis and infected intravascular catheters, respiratory tract infection, urinary tract infection, dental abscesses, abdominal infections, and complications from gastrointestinal surgery (1). In contrast to spinal epidural abscesses in adults (Fig. 32.3), adjacent vertebral osteomyelitis is distinctly uncommon in children with this infection; thus, most spinal epidural abscesses in children have been attributed to hematogenous seeding from a distant focus (21).
One possible explanation for the development of an epidural abscess after a recent or remote trauma including prior spinal surgery is that a small hematoma or area of damaged tissue may provide a fertile area for subsequent hematogenous seeding (22). Whether antecedent trauma is indeed a true risk factor for the subsequent development of a spinal epidural abscess or whether patient recall bias has influenced this history has not been systematically examined. Of course, spinal trauma may also contribute to the development of an infection by creating a site of entry for microorganisms into the epidural space (disruption of anatomic barriers).
The reported risk of an epidural abscess after epidural catheterization varies widely in the literature. Prolonged use of epidural catheters, significant comorbidity, older age, injection drug use, and diabetes mellitus contribute to the rate of infection (2). A retrospective analysis of 505,000 extradural blocks in obstetric practice (84% for relief of pain in labor and 16% for cesarean sections) reported only a single case of an epidural abscess (23). Another mostly retrospective study estimated the risk at approximately 1 per 5,000 catheters (24). A recent prospective study identified nine patients with epidural abscess after 17,372 epidural catheters (1 per 1,930 catheters), with a mean catheterization time of 11 days (25). Immunocompromise and longer duration of catheterization were associated with an increased risk of an epidural abscess in that study: only one of the nine patients had no complicating disease (four had malignancies, two had diabetes mellitus, one had multiple trauma, and one had chronic obstructive airway disease); furthermore, all patients with abscess had epidural catheters in situ for 3 days or longer. This is best illustrated by the relatively high risk of long-term epidural treatment of chronic pain (mostly patients with cancer), which was complicated by an epidural abscess in 3%, 4%, or even 12% of patients in three recent studies (13,26,27). In another study, none of 1,062 patients who had epidural anesthesia for a period of less than 14 days developed a serious infectious spinal complication (28).
Underlying medical conditions that have been associated with spinal epidural abscesses include degenerative joint disease, diabetes mellitus, injection drug use, alcoholism and cirrhosis, malignancy, renal failure and hemodialysis (29–31), pregnancy, ulcerative colitis and Crohn disease (both immunosuppressive treatment and enteroepidural fistulas may be relevant), systemic lupus erythematosus, chronic granulomatous disease, corticosteroid therapy, and acquired immunodeficiency syndrome (1).
Compared with the damage produced by spinal tumors and cysts, the damage produced by bacterial spinal epidural abscesses is often out of proportion to the size of the inflammatory mass, that is, the lesion of the spinal cord can be more extensive than can be accounted for by mechanical effects of compression alone (3). This important feature may be attributed to many factors including thrombosis and thrombophlebitis of veins draining the spinal cord with resultant edema and venous infarction (3); compression of the arterial supply to adjacent cord segments with local ischemia and infarction; focal areas of vasculitis induced by the adjacent inflammatory mass; or bacterial exotoxin production, especially by Staphylococcus aureus.
Still, a rabbit model of spinal epidural abscess has demonstrated that the damage to the spinal cord is more consistent with mechanical compression than with ischemia or infarction (32,33). This model, using a clinical isolate of Staph. aureus, also demonstrated many of the pathologic features seen clinically in spinal epidural abscesses: preservation of gray matter, white matter edema, vacuolization, liquefaction, myelin degeneration, and axonal swelling. This pattern is consistent with compressive damage, as spinal cord ischemia is typically characterized by necrosis of the gray matter and relative preservation of the white matter (33). Furthermore, the posterior spinal arteries and the anterior spinal artery were patent microangiographically in paraplegic animals with minimal and moderate cord compression and even in some cases of large epidural abscesses with severe degrees of compression (32). Dorsal and anterior spinal veins and the anterior epidural venous plexus were also patent in animals with minimal or moderate compression. Only in rabbits with severe cord compression, the dorsal spinal vein and the perforating arterioles were occluded ipsilaterally to the abscess. With profound compression, all blood flow was noted to cease. The authors, therefore, concluded that the initial neurologic deficit results from compression and that vascular compromise (as a result of compression and probably not due to vasculitis or thrombosis) is an important factor in the final pathogenesis of spinal epidural abscess. Although these rabbit experiments (32,33) suggested a primary role for mechanical compression, other animal models favored an additive adverse effect of compression and ischemia on neurologic function (7).
Microbiology
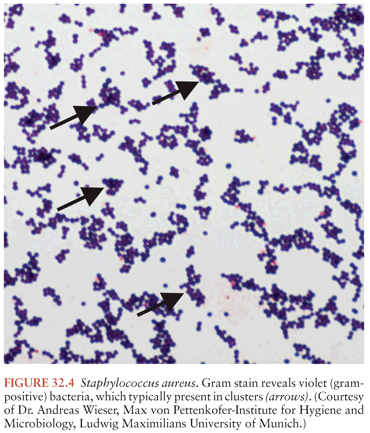
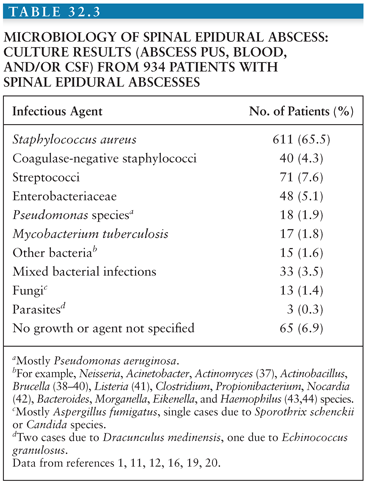
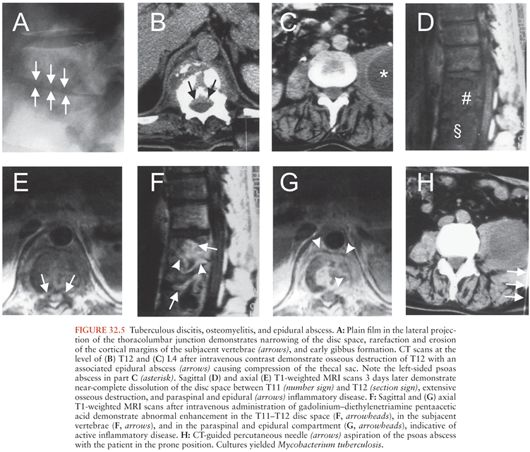
A microbiologic diagnosis is usually made from blood and/or intraoperative cultures. Polymicrobial spinal epidural abscesses are a rarity, blood cultures are positive in about 60% of the patients (7), and pathogens identified by blood culture are almost always identical with the infectious agents cultured from abscess pus. This situation is, therefore, completely different from that in patients with brain abscesses. In the latter, blood cultures are positive in only about 10% of patients, and even if the blood cultures are positive, they do not allow for firm conclusions on the microbial spectrum of the brain abscess because brain abscess pus often grows mixed cultures (see Chapter 31). The microbial spectrum of spinal epidural abscesses also differs greatly from that of brain abscesses, as Staph. aureus (Fig. 32.4) is the most common etiologic agent in children and adults (responsible for about two thirds of spinal epidural abscesses). Coagulase-positive and negative (mostly Staph. epidermidis) staphylococci cause approximately 70% of spinal epidural abscesses (note that in some studies, up to 15% of the isolates were methicillin-resistant Staphylococcus species [34] with a frequency up to 40% in some studies [7,35]). The remaining 30% of cases are caused by a wide spectrum of infectious agents (Table 32.3). Most streptococcal spinal epidural abscesses are due to viridans streptococci and Streptococcus pneumoniae (1,45). Escherichia coli is responsible for most infections due to Enterobacteriaceae. Much less frequently Proteus, Enterobacter, Salmonella, Serratia, Citrobacter, and Klebsiella species have been reported (1). Spinal epidural infections due to Pseudomonas aeruginosa are relatively uncommon but require particular attention because of this pathogen’s resistance to many antibiotics. Anaerobes, such as Bacteroides, Peptostreptococcus, or Fusobacterium species are also an uncommon cause of spinal epidural abscesses. Spinal epidural abscess is rarely caused by agents of actinomycosis or nocardiosis (1,46). The incidence of mycobacterial epidural infections (Fig. 32.5) varies greatly geographically. Although they are very rare, for example, in studies from North America or Western Europe, mycobacteria were responsible for 8 (28%) of 29 cases of spinal epidural abscesses in a series from Taiwan (19) and 19 (53%) of 36 cases in a Turkish investigation (47).
Fungal etiologies of spinal epidural abscesses include Aspergillus species (48,49), Candida species (50), Blastomyces dermatitidis (51), Coccidioides immitis (52), Cryptococcus neoformans (53), mucormycosis (54), Sporothrix schenckii (14), and Pseudallescheria boydii (55). Recently, an outbreak of fungal meningitis after epidural or paraspinal glucocorticoid injection with contaminated methylprednisolone has been described (48,49). The outbreak is ongoing and involves multiple states. Clinical data from 66 patients showed that 47 (71%) had meningitis alone, 11 (17%) suffered from cauda equina syndrome or focal infection nearby the injection site (4 of whom had documented epidural abscess) with or without meningitis, and 8 (12%) had posterior circulation stroke (with or without meningitis). The median time from the last epidural glucocorticoid injection to symptom onset was 18 days. Eight patients (12%) died, and 7 of them had stroke. A total of 22 patients had laboratory confirmation of fungal infection, either Exserohilum rostratum infection (21 patients) or Aspergillus fumigatus infection (1 patient) (49).
Parasitic causes of epidural abscesses have also been reported, including Echinococcus (56), guinea worm (Dracunculus medinensis) (57), and Schistosoma mansoni (58).
The microbiology of spinal epidural abscesses in children does not differ substantially from that in adults. In 2001, a review of the literature summarized 34 cases of childhood spinal epidural abscesses (21): Staph. aureus was cultured in 21 patients (62%), streptococci in 2 (6%, S. agalactiae in one and viridans streptococci in another), Salmonella enteritidis in 2 (6%), E. coli in 1 (3%), Pseudomonas aeruginosa in 1 (3%), Mycobacterium bovis in 1 (3%), Candida tropicalis in 1 (3%), and Aspergillus flavus in 1 (3%). Multiple organisms were isolated in one patient and no organisms were isolated in three children (9%).
Clinical Manifestations
The initial clinical signs and symptoms of a spinal epidural abscess, characteristically including fever, back pain, and malaise, may be subtle. A spinal epidural abscess is often not suspected on admission. The most common initial misdiagnoses are meningitis, intervertebral disc prolapse, vertebral osteomyelitis, lumbar degenerative joint disease, spinal neoplasm, transverse myelitis, spinal ischemia, and urinary tract infection (1). The duration of symptoms is variable; they may be present for just a few days or up to several months before the patient presents for evaluation (4,59).
The four-stage clinical progression of a spinal epidural abscess was first described by Rankin and Flothow (60) more than 50 years ago. In addition to fever and malaise, the first localizing symptoms are backache and focal vertebral pain and tenderness on examination. This is followed by “root pain” manifested by radiculopathy and/or paresthesias, which often may be described as “electric shocks.” Spinal cord dysfunction, the third stage, is characterized by motor and sensory deficits or by bladder or bowel dysfunction. This is followed by the final stage of complete paralysis (Table 32.4).
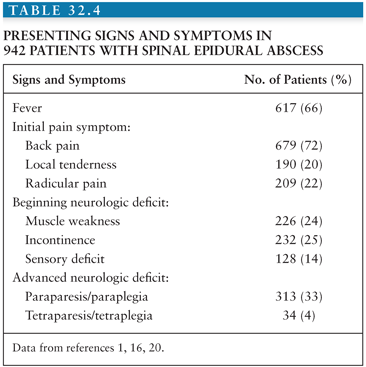
Although early symptoms of backache may be indolent and persist for weeks or months, back pain usually progresses to root pain within 3 to 4 days followed by early signs of spinal cord dysfunction within the subsequent 4 to 5 days. The neurologic deficits at this stage are usually reversible; however, rapid surgical intervention at this point may be crucial because progression to complete paralysis may occur within a few hours regardless of the chronicity of the process up to this point.
Specific neurologic signs depend on the level of spinal cord involvement and is another factor that influences the differential diagnosis at the time of presentation.
The presentation of a spinal epidural abscess has historically been described as “acute” (symptoms persisting for <2 weeks at the time of presentation) or “chronic” (symptoms for >2 weeks at the time of presentation) (3,5). The rate of progression of the initial stages may suggest the route of infection: acute presentations often represent hematogenous seeding and rapid expansion of the inflammatory mass, whereas chronic presentations are more likely the result of a gradually expanding contiguous infection.
Although the prognostic significance of distinguishing acute from chronic spinal epidural abscesses has recently been questioned, patients with acute presentations may have higher peripheral leukocyte concentrations (mean values ranging from 12,300 to 16,700 cells/µL [3–5]) and higher temperatures when compared to those of patients with long-standing symptoms who may have only mild elevations in these two parameters (mean peripheral leukocyte concentrations ranging from 7,100 to 11,900 cells/µL). Other authors, however, reported similar peripheral leukocyte concentrations in acute and chronic cases (6). In both presentations, the erythrocyte sedimentation rate (ESR) is generally elevated (>25 to 30 mm per hour); however, the magnitude of the ESR is not a reliable clinical clue as to the chronicity of the infection (5,6). Serial ESR measurements showed good correlation between resolution of clinical signs and decreases in ESR (34). Regardless of the clinical course, the average peripheral leukocyte concentrations were 15,700 cells/µL in 218 patients from the literature (range, 1,500 to 42,000 cells/µL) and the average ESR was 77 mm (range, 2 to 150 mm) in 117 patients from the literature (1). Recent studies have also reported elevated serum C-reactive protein (CRP) levels in patients with spinal epidural abscesses, 15.0 ± 9.5 mg/dl in one study (20). Serum CRP levels were also shown to respond more quickly to therapeutic interventions than, for example, the ESR (61). Similarly, a persistent strong elevation of the CRP value after 8 days postsurgery was shown to be associated with a poor outcome (20). Blood cultures will be positive on average in 72% of patients (mean, range 64% to 82% in three recent studies [4,16,59]).
Cerebrospinal fluid (CSF) examination is typically that of a parameningeal focus of infection with elevated protein levels and increased leukocytes. The CSF leukocytes may be a mixture of polymorphonuclear leukocytes and mononuclear cells or predominantly polymorphonuclear leukocytes in most patients (3,59). The CSF leukocyte count was 40 cells/µL (median, range 0 to 27,000 cells/µL) in one study (59) and 60 cells/µL (mean, range 0 to 820 cells/µL) in another report (3). Extremely elevated leukocyte counts in the CSF may indicate that the spinal needle has entered a lumbar epidural abscess (3,59). CSF protein values are elevated in approximately 90% of patients (59). Markedly elevated CSF protein levels (>350 mg/dL) are often predictive of a complete block of the spinal canal; however, an elevated protein level of lesser magnitude is found in patients with spinal block in nearly two thirds of cases (5). CSF glucose concentrations were reported to be decreased (<50 mg/dL) in more than half of the cases (59). Except when there is concomitant meningitis, CSF Gram stains rarely demonstrate organisms, and spinal fluid cultures are positive in only 15% of patients (mean, range 7% to 25% [4,5,59,62]), also similar to other parameningeal infections. Most authors recommend against performing a lumbar puncture in patients with an adjacent spinal epidural abscess because of the possibility of inducing meningitis or subdural infection if the needle traverses the epidural space or the potential risk of neurologic deterioration if performed below the spinal block (59). Perhaps the principal reason not to perform lumbar puncture is the meager information that this test provides. The CSF alterations are nonspecific and when CSF cultures are positive, so usually are the blood cultures (59).
At the time of surgery, findings may range from frank pus to granulomatous tissue. Whereas acute cases are more likely to have purulent material, this correlation was not found consistently in other case series (4,63). Overall, intraoperative cultures have the greatest chance of yielding a microbiologic diagnosis; on average 86% of all cases have positive cultures (mean, range 82% to 90% [3–5,16,19,59]). However, when a patient has been receiving antibiotics for more than 1 week before culture, diagnostic cultures are unlikely to be positive (5).
Differential Diagnosis
Signs of fever and back pain should raise suspicion of a spinal epidural abscess, especially when spinal tenderness or radicular signs are demonstrated on physical examination. However, these signs and symptoms may not always be present and lack specificity for this diagnosis. About half of the patients with spinal epidural abscess are initially misdiagnosed (7), particularly when patients present in early clinical stages (3–5,59,62). One review documented that spinal epidural abscesses took significantly longer to diagnose in patients when it was not included among the differential diagnoses at admission: 6.6 days versus 1.9 days (59). The main differential diagnoses include intervertebral disc prolapse, spinal tumors, transverse myelitis, epidural hematoma, vertebral osteomyelitis, spinal subdural or intramedullary abscess, spinal arteriovenous malformation, and spinal arterial ischemia. The clinical picture of spinal epidural abscess can also be mimicked by acute spinal cord dysfunction due to myelitis during bacterial meningitis (64).
Approach to Diagnosis
The imaging method of choice for diagnosis of spinal epidural abscess is contrast-enhanced MRI (65–70). However, availability, contraindications (e.g., heart pacemaker), or insufficient cooperation of the patient sometimes requires the use of the other methods, such as (postmyelography) CT. Plain films of the spine will not directly visualize the spinal epidural abscess but may demonstrate certain findings that can be an indication of the presence of infection in the spinal canal (66,67). These findings include intervertebral disc space narrowing in discitis and loss of definition or destruction of both the inferior cortical margin of one vertebral body and the superior cortical margin of the contiguous vertebral body in osteomyelitis. Rarefaction and loss of bony trabeculae can also be seen. In advanced or rapidly progressive cases, vertebral body collapse (or dissolution) and gibbus formation may be seen on plain films. In general, osteomyelitis most commonly occurs in the lumbar spine, and the bodies of the vertebrae are affected more often than the posterior elements. Helpful ancillary plain film findings include a mass effect or displacement of the larynx in a retropharyngeal abscess or scoliosis and displacement of bowel loops in a lumbar paraspinal abscess. Normal plain films do not exclude the presence of a spinal epidural abscess, particularly in acute presentations. In this setting, additional radiographic examinations are almost always needed for further evaluation. Similar to plain films, bone scans often provide clinically not useful information that may be misleading in this setting.
Myelography, which will not directly visualize a spinal epidural abscess, will demonstrate the associated mass effect on the spinal cord, thecal sac, or nerve roots. A spinal epidural abscess can result in a complete extradural obstructive block to the flow of water-soluble contrast. However, in patients with a prominent ventral epidural space in the lower lumbar spine due to abundant epidural fat, an epidural abscess may not be detected on myelography if it causes indentation or obliteration of the epidural fat without indenting the opacified thecal sac.
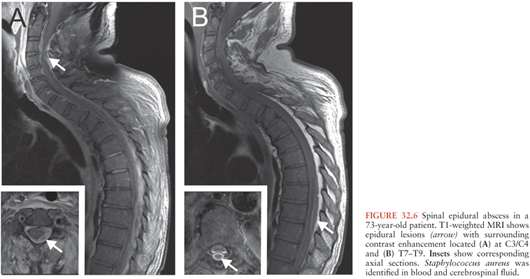
Both CT and MRI can directly visualize an epidural inflammatory mass (65–68,70). As is true in the neuroimaging of many diseases of the brain and spinal canal, MRI has proven superior to CT for evaluation of patients with epidural abscesses. MRI obviates the need for myelography and CT in most cases. The advantages of MRI include its ability to image long segments of the spinal canal in multiple planes (Figs. 32.6 and 32.7), thereby enabling precise delineation of all loculations of inflammatory tissue; its ability to image all compartments around the spinal canal into which an inflammatory process might extend; its ability to directly visualize the neural elements with high-contrast resolution without the need for intrathecal contrast; its noninvasiveness, which is a particular advantage over myelography, because of the desire to avoid inadvertent puncture of the abscess or iatrogenic spread of infection from the epidural to the subarachnoid space following introduction of a needle into the spinal canal. Furthermore, with MRI, patient follow-up is facilitated and assessment of response to therapy is readily performed. The ability of MRI to distinguish active inflammatory tissue from chronic granulation tissue is further enhanced by intravenous administration of gadolinium. MRI demonstrates epidural inflammatory disease to be a soft tissue mass, which compared to the spinal cord is isointense on T1-weighted images and hyperintense on T2-weighted images. Diffusion-weighted imaging may show a signal abnormality within the spinal abscess (70,71). The extradural location of disease and the associated mass effect on the thecal sac, spinal cord, or cauda equina are generally readily appreciated on MRI, as is the extradural location of disease and the associated mass effect on the thecal sac. The presence of concomitant paraspinal abscesses is easily identified (Fig. 32.8). In the presence of discitis, a characteristic finding on MRI is abnormal high signal intensity in the disc on T2-weighted images (Fig. 32.9). This is in contradistinction to degenerative disc disease, which demonstrates low signal intensity. Vertebral osteomyelitis is seen as low signal intensity on T1-weighted images in the involved marrow cavity, with high signal intensity on T2-weighted images. These osseous changes are usually observed in conjunction with inflammatory disease of the subjacent intervertebral disc as described.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree