Jason B. Harris, Edward T. Ryan
Enteric Fever and Other Causes of Fever and Abdominal Symptoms
Most patients with enteric fever present with nonspecific clinical features, and fever without localizing signs may be the sole manifestation of enteric fever. Enteric fever is caused by typhoidal Salmonella, including Salmonella enterica serotype Typhi, and serotypes Paratyphi A, B, and C. Enteric fever is associated with potentially life-threatening complications, including intestinal hemorrhage and perforation, shock, and encephalopathy. Current diagnostic tests for enteric fever lack sensitivity and/or specificity, so the diagnosis and treatment of enteric fever is often empirical.
The term typhoidal fever is used to broadly refer to a syndrome of high-grade and prolonged fevers not caused by Salmonella, often in the absence of localizing symptoms or signs. The differential diagnosis for typhoidal fevers is extensive and includes a number of bacterial, viral, fungal, and parasitic infections, as well as noninfectious entities. The approach to the patient with typhoidal fever and the differential diagnosis of enteric and typhoidal fevers are reviewed in this chapter.
Enteric Fever (Typhoid and Paratyphoid Fever)
History
Historically termed putrid fever or dothienteritis, the name typhoid was coined in 1829 by Pierre Charles Alexander Louis. The name typhoid means “typhus-like” and reflects the difficulty in differentiating the illness from epidemic typhus, another common cause of prolonged fever in Europe during the 19th century. Louis used the term typhoid in a landmark report comparing the intestinal pathology in 50 patients who died of typhoid fever with 83 patients who died of noninfectious causes, and he described the characteristic inflammation of Peyer’s patches, intestinal ulceration, and mesenteric adenitis associated with typhoid fever.1
William Budd is credited with the recognition of the fecal-oral transmission of typhoid fever in 1839, based on his meticulous observations during a large epidemic in the Taw Valley of England, although these findings were not widely recognized until many years later.2
The etiologic agent of typhoid fever was identified in pathologic specimens by Karl Eberth, who referred to it as Bacillus typhosus; the organism was subsequently first cultured in 1884 by Georg Gaffky at the Berlin Institute for Infectious Diseases. The term paratyphoid fever was first used in 1896 by Raoul Bensaude and Emile Achard, who isolated what would subsequently be recognized as S. Paratyphi B. The role of asymptomatic carriers in the transmission of infectious disease was first recognized by Robert Koch, a theory that he derived from his longitudinal observations of patients recovered from typhoid but with continued shedding of Bacillus typhosus.3 An understanding of the role of asymptomatic carriers in disease transmission was soon extended to other organisms and was an established mode of disease transmission at the time of George Soper’s association of Mary Mallon (also known as Typhoid Mary) with repeated outbreaks of typhoid fever in households in New York, ultimately leading to her lifelong quarantine on North Brother Island.4
Widal developed the first serologic test for typhoid fever in 1896, an agglutination assay that detects the presence of antibodies against the O and H antigens of S. Typhi.5 Despite inherent and significant limitations (see “Diagnosis”), the Widal test remains widely used in many resource-limited settings. The first vaccines for typhoid fever were killed whole-cell vaccines developed in 1896, credit for which is shared by Almroth Wright and Richard Pfeiffer.6–9 Despite advances in the development of a vaccine, typhoid fever remained a leading cause of morbidity and mortality in the early 19th century. A prominent example was a typhoid fever epidemic in the Spanish American War, in which 20,000 American Army recruits contracted the disease and 1600 died. This defining event was linked to the first compulsory U.S. military-wide vaccination program, using a killed whole-cell, injectable typhoid vaccine developed by Frederick Russell.10
In the early 20th century, “typhomalaria” or “typhomalarial fever” remained an extremely common diagnosis, indicating that differentiating typhoid from malaria (and other causes of persistent fever) on clinical grounds was difficult. The difficulty distinguishing typhoid fever from typhus and other causes of fever proved clinically fortuitous. In 1948, Theodore Woodward and co-workers11,12 reported the effect of chloromycetin in two patients who were referred for a study of the efficacy of the drug in scrub typhus. Both were subsequently found to have S. Typhi bacteremia, and both patients improved rapidly after antibiotic therapy.11 Typhoid fever mortality has been reduced from 10% to 1% with the use of antibiotics. However, successive pandemics of antibiotic-resistant organisms have occurred, including, most recently, the emergence of fluoroquinolone-resistant strains of S. Typhi. In 2001, the complete sequence of a S. Typhi strain (CT18, isolated from a patient in Vietnam in 1993) was published by Parkhill and co-workers13 at the Wellcome Trust Sanger Institute, ushering in a genomic era in our understanding of typhoid fever.
Etiologic Agents of Enteric Fever
Nomenclature and Classification of Typhoidal Salmonella enterica
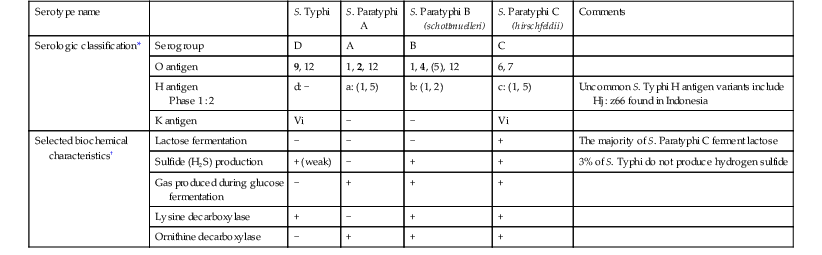
S. Typhi and S. Paratyphi A, B, and C are gram-negative bacilli that belong to the species Salmonella enterica subspecies enterica. All S. enterica are categorized serologically by the Centers for Disease Control and Prevention (CDC) according to a modified Kauffman and White classification scheme, assigned based both on the O (the O polysaccharide) and H (flagellar) antigen.14,15 Although assigning a serogroup based on O antigen agglutination tests is a common procedure in many clinical microbiology laboratories, the approach has limited clinical utility. Complete serotyping based on both the O and H antigen is most commonly performed in reference laboratories. In addition to serogrouping and serotyping, standard microbiologic growth and biochemical parameters are also routinely used in the clinical laboratory for the presumptive identification of S. enterica. Serologic and selected biochemical characteristics that are used in the identification of S. Typhi and S. Paratyphi A, B, and C in microbiology laboratories are summarized in Table 102-1.
TABLE 102-1
Classification and Selected Microbiologic Characteristics of Typhoidal Salmonella
| Serotype name | S. Typhi | S. Paratyphi A | S. Paratyphi B (schottmuelleri) | S. Paratyphi C (hirschfeldii) | Comments | |
| Serologic classification* | Serogroup | D | A | B | C | |
| O antigen | 9, 12 | 1, 2, 12 | 1, 4, (5), 12 | 6, 7 | ||
| H antigen Phase 1 : 2 | d: − | a: (1, 5) | b: (1, 2) | c: (1, 5) | Uncommon S. Typhi H antigen variants include Hj : z66 found in Indonesia | |
| K antigen | Vi | − | − | Vi | ||
| Selected biochemical characteristics† | Lactose fermentation | − | − | − | + | The majority of S. Paratyphi C ferment lactose |
| Sulfide (H2S) production | + (weak) | − | + | + | 3% of S. Typhi do not produce hydrogen sulfide | |
| Gas produced during glucose fermentation | − | + | + | + | ||
| Lysine decarboxylase | + | − | + | + | ||
| Ornithine decarboxylase | − | + | + | + |
* Strong antigenic determinants are listed in bold; weaker or sometimes absent ones are in parentheses.
† Isolates can be identified as Salmonella using traditional methods. A presumptive diagnosis of S. Typhi can often be made for Salmonella isolates based on selected biochemical characteristics, in addition to agglutination with D and Vi antisera.
From World Health Organization (WHO), Department of Vaccines and Biologicals. Background Document: The Diagnosis, Treatment and Prevention of Typhoid Fever. Geneva: WHO; 2003; and WHO, Department of Communicable Disease Surveillance and Response. Salmonella serotype Typhi. In: WHO. Manual for the Laboratory Identification and Antimicrobial Susceptibility Testing of Bacterial Pathogens of Public Health Importance in the Developing World. Geneva: WHO; 2003:103-118.
Clinical Distinction between Typhoidal and Nontyphoidal Salmonella enterica
S. Typhi, and S. Paratyphi A and B are human-restricted pathogens. S. Paratyphi C is pathogenic for animals as well as humans, and S. Typhi and S. Paratyphi are classified clinically as typhoidal Salmonella strains. This distinction separates these strains from all other pathogenic S. enterica serotypes, which are referred to collectively as nontyphoidal Salmonella (often abbreviated as NTS; see Chapter 225). Typhoidal strains cause enteric fever in all human hosts, whereas NTS are classically associated with inflammatory diarrhea in human hosts or preferentially cause invasive disease in immunocompromised individuals. However, the clinical distinction between typhoidal and NTS is not absolute. NTS may cause invasive infection that includes prolonged bacteremia (with or without pyogenic foci) that may mimic the systemic illness caused by typhoidal strains.16 Although host factors influence susceptibility to invasive NTS infection, the likelihood of invasive infection is also dependent on characteristics of the bacterial strain. For example, the recent emergence of a dominant genotype associated with invasive NTS, S. Typhimurium ST313, in sub-Saharan Africa, illustrates the clinical overlap between typhoidal and nontyphoidal S. enterica.17
Genomic Features
Escherichia coli and S. enterica diverged over 100 million years ago, and S. enterica is now a genetically heterogeneous species.18 S. Typhi is genetically homogeneous, meaning there is very limited genetic diversity across S. Typhi isolates throughout the world. Based on standard rates of genetic drift, it is estimated that S. Typhi emerged from a single-point origin within the last 50,000 years, coinciding with the time of human migrations out of Africa and into Asia.19,20 The genetic material of S. Typhi consists of a single chromosome that contains approximately 5 × 106 base pairs encoding approximately 4000 genes. S. Typhi often harbors additional plasmids that may be cryptic (confer an unknown function) or involved in pathogenesis. The latter include IncHI1 plasmids that comprise the majority of resistance plasmids found in S. Typhi and that remain stable in the bacteria without ongoing pressure from antibiotic selection.21,22
The genetic core of S. enterica consists of genes that are conserved across Enterobacteraciae. The genetic core of S. enterica is approximately 90% similar to E. coli. In contrast, the genetic core varies approximately 1% between S. enterica serovars. In addition to the genetic core, S. Typhi contains pathogenicity islands—horizontally acquired genetic elements that are often associated with unique properties of each strain and are essential for specific phenotypes associated with pathogenesis of each serotype.18 In S. enterica, these are specifically identified as Salmonella pathogenicity islands, or SPIs, and are designated with a number. S. Typhi possesses more than 20 SPIs, many of which are found in other S. enterica serotypes. For example, SPI-7 is a 134-kb genetic island that possesses elements encoding the viaB locus, which is involved in production of the Vi capsule and evasion of the host immune response.23 SPI-7 is widely conserved across S. Typhi and is also found in S. Paratyphi C.
One of the most remarkable genetic features of S. Typhi is the accumulation of more than 200 functionally inactive genes known as pseudogenes.13 This adaptation may play a major role in the host restriction of S. Typhi. The accumulation of a large number of pseudogenes is a common feature shared with S. Paratyphi A, B, and C, which emerged independently of S. Typhi (although subsequent recombination events between S. Typhi and S. Paratyphi A may also have occurred),24 yet are also adapted specifically to cause disseminated infection in humans. The same phenomenon has been observed in S. Typhimurium ST313 in sub-Saharan Africa; ST313 has accumulated extensive gene deletions and pseudogene formation (more than half of which are common to S. Typhi and S. Paratyphi A) compared with other less invasive S. Typhimurium strains.25
Epidemiology
Burden and Distribution
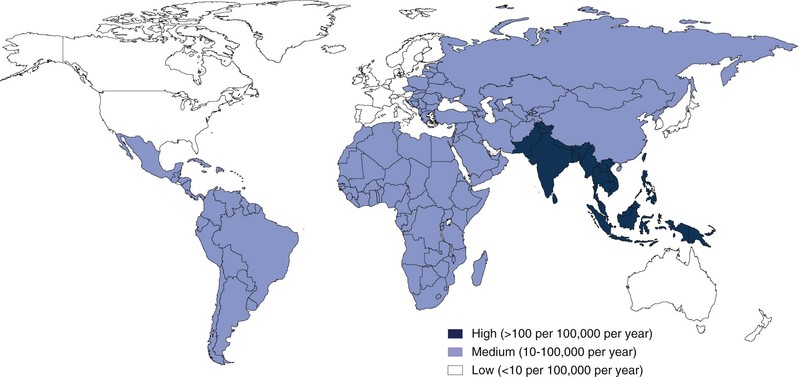
Measures of the burden of enteric fever are limited by the absence of surveillance in many regions and also by the limited sensitivity of current diagnostic tests. Recognizing these limitations, estimates of the number of cases of typhoid fever range from 22 to 27 million annually.26,27 The incidence of typhoid fever and the mortality of the disease vary dramatically by region (Fig. 102-1). In South Asia and parts of sub-Saharan Africa, the incidence approaches 1000 cases per 100,000 person-years.26,28 In parts of Northern Africa, South America, and Southeast Asia, the incidence is much lower and ranges between 10 and 100 cases per 100,000 person-years. In the United States and many other high-resource settings, the disease occurs sporadically, with less than 1 case per 100,000 person-years, and occurs most often in travelers returning from endemic zones (see “Acquisition of Disease in Areas Where Enteric Fever Occurs Sporadically”). It is of particular note that as the global burden attributable to many intestinal infections has fallen over the last 2 decades, the global burden of typhoid has increased significantly.29
S. Paratyphi A is by far the most common cause of paratyphoid fever and is responsible for an estimated 5 million cases of enteric fever annually.27 The proportional burden of enteric fever caused by S. Paratyphi A has increased substantially over the past 2 decades, and in some areas of South Asia, S. Paratyphi A is responsible for more than 20% to 50% of cases of enteric fever.30 In contrast, S. Paratyphi B and C remain less common causes of enteric fever globally, with the proportional incidence of these infections caused by these pathogens varying by location.
Severity
In the preantibiotic era, 10% to 15% of clinically recognized cases of typhoid fever were fatal, whereas other cases resolved over a 3- to 4-week course. Prospective surveillance in the postantibiotic era has revealed that the majority of cases of enteric fever are uncomplicated and do not require hospitalization. Although the current mortality of typhoid fever is estimated at 1%, this estimate is based on few data.26 Reported mortality rates in hospitalized patients with enteric fever in resource-limited settings vary widely by location, ranging from 0% to 15%, with a median of 2%.31 In the United States, the fatality rate of reported cases is 0.2%.32 In addition to fatal cases, in sub-Saharan Africa and other regions where enteric fever is highly endemic, intestinal perforation caused by enteric fever is a leading cause of peritonitis and acute surgical abdomen in children.33
Source of Infection
S. Typhi and S. Paratyphi A and B are human restricted. There are no known animal reservoirs of these pathogens that cause enteric fever, and the source of infection is organisms shed in the stool of infected humans. S. Paratyphi C can be shed by humans and animals. In the preantibiotic era, patients recovering from enteric fever shed organisms in the stool during acute illness and typically for weeks during convalescence. In addition, a small portion of individuals convalescing from typhoid fever develop chronic asymptomatic carriage (defined as shedding of the bacteria in stool or urine for more than a year), which may persist for life.34 In the preantibiotic era, up to 5% of typhoid fever survivors developed chronic asymptomatic bacterial shedding in the stool,35,36 with gallbladder disease being the major risk factor for carriage.37,38 Recent studies from Nepal, where enteric fever is highly endemic, found that 3.5% of individuals undergoing elective cholecystectomy grew S. Typhi or S. Paratyphi A from biliary cultures.39 Urinary carriage may also occur, frequently in conjunction with urinary schistosomiasis.40 Although chronic carriers are a source of transmission where enteric fever occurs sporadically,32,41 their importance in the transmission of enteric fever in highly endemic areas is uncertain.
Mode of Transmission
Infections with typhoidal S. enterica are most often acquired via ingestion of fecally contaminated water or food. S. Typhi may persist for weeks after passage in water and may persist in a variety of contaminated food items, for example, dehydrated formula and iced beverages.41–43 Volunteer studies may not optimally mimic natural exposure; however, the inoculum required to produce disease in 50% of adult volunteers (ID50) is 107 organisms, although fewer than 103 organisms may produce disease.44,45
Molecular epidemiologic studies based on high-resolution genotyping implicate waterborne transmission as the most likely route of transmission in the majority of cases of enteric fever.46 Both waterborne and foodborne transmission may contribute to large epidemics, and in some cases, massive epidemics have been associated with water contamination from a single source.47 In contrast, direct person-to-person transmission of the etiologic agents of enteric fever appears less common, and more than 80% of cases of enteric fever occur in individuals with no known contact with an individual with symptomatic infection.48 Even more remarkably, in households where multiple cases of enteric fever occur, only 20% of those infections shared a common bacterial genotype, suggesting that multiple infections within a household occur through a community-based exposure to multiple circulating genotypes, rather than through direct person-to-person transmission.46 In highly endemic areas, consistent personal risk factors for infection with typhoidal S. enterica include drinking nonboiled water and eating food prepared outside the home.49,50 In Asia, peak transmission occurs during the monsoon season, although disease is present year-round.51
Acquisition of Disease in Areas Where Enteric Fever Occurs Sporadically
In areas where enteric fever occurs sporadically, most cases are imported through travel. In the United States, 80% of cases occur in travelers to endemic areas.32 The rate of typhoid fever is estimated at 10 per 1 million travelers arriving from Asia but increases to 89 per 1 million travelers arriving from India. The risk of travel-related enteric fever is higher among travelers visiting friends and relatives.52 Many of the remaining non–travel-associated cases in the United States are traceable to small, local foodborne outbreaks and/or a chronic carrier.32 From approximately 1960 to 2000, more than half of foodborne outbreaks in the United States were linked to an asymptomatic carrier.53 In 2010, an outbreak of S. Typhi occurred that involved at least 12 cases across three western U.S. states and was attributed to imported fruit pulp from Guatemala.54 Also, in areas where enteric fever occurs sporadically, small clusters of cases of sexual transmission of S. Typhi have been documented in men who have sex with men.55
Antibiotic Resistance and Emergence of Pandemic S. Typhi
Chloramphenicol-resistant S. Typhi was first reported in 1950, 2 years after the antibiotic was first used to treat patients with typhoid fever.56 However, widespread dissemination of antibiotic-resistant strains did not occur until the 1970s. Multidrug-resistant S. Typhi carrying IncHI1 plasmid-mediated resistance to chloramphenicol, ampicillin, trimethoprim, and sulfonamides became common in the 1980s. Nalidixic acid–resistant strains of S. Typhi and S. Paratyphi A, with decreased susceptibility to fluoroquinolone antibiotics, emerged in the 1990s, and are now common.57
Although the lack of genetic diversity of S. Typhi previously hindered efforts to characterize the global epidemiology of disease, the increasing use of high-density genotyping methods, including whole-genome sequencing, has circumvented these challenges and resulted in a better understanding of the forces driving the evolution and transmission of S. Typhi. Although a general lack of diversity among S. Typhi suggests a lack of strong selection pressure over millennia, the recent expansion of a single fluoroquinolone-resistant haplotype known as H58 into a pandemic strain found throughout Asia and Africa, and the emergence of several other lineages with diverse gyrA mutations, suggest that fluoroquinolones may be exerting a uniquely strong selection pressure that is shaping the global evolution of S. Typhi.20,58
Host Factors and Susceptibility to Infection
Historically, enteric fever was considered a disease that predominantly affected school-aged children and young adults. However, prospective community-based surveys in highly endemic areas have demonstrated the incidence of S. Typhi bacteremia is in fact highest in young children (<5 years old), and may be responsible for more than 75% of cases of occult bacteremia in these settings.59 The higher incidence of enteric fever in older children among inpatients may reflect a predisposition to more severe disease in this older age group. In highly endemic areas, S. Typhi bactericidal antibodies and anticapsular (Vi) antibodies increase significantly over the first decade of life.60 In less endemic areas, the median age of patients with typhoid fever increases, suggesting that the age distribution of patients may be influenced by acquired immunity. However, protection from a single episode of infection is limited, as demonstrated by frequent relapse and recurrent infections among patients who have recovered from typhoid fever.61
Although invasive NTS infections classically occur in persons who are compromised by extremes of age, malnutrition, immunodeficiency, or genetic risk factors, there is not an overt association between host risk factors and susceptibility to enteric fever. Various candidate gene analyses have drawn associations between single-nucleotide variations in Toll-like receptor 4 (TLR4), cystic fibrosis transmembrane conductance regulator (CFTR), and specific human leukocyte antigen (HLA) types with susceptibility to typhoid fever in Vietnam62,63; however, no detailed genome-wide study of typhoid fever associations has yet been reported.
Pathogenesis
Overview
Enteric fever and NTS infections differ in fundamental aspects. With NTS, infections are typically self-limited in immunocompetent hosts and manifest largely as an inflammatory gastroenteritis. In comparison, typhoidal serotypes are able to evade the normal host inflammatory response and cause prolonged bacteremia even in immunocompetent individuals, typically without overwhelming sepsis or pyogenic foci of infection. Specific bacterial adaptations used by S. Typhi to produce persistent bacteremia in the absence of an immediately overwhelming inflammatory response include strategies to breach the intestinal epithelium in the absence of inflammatory diarrhea, to evade detection by host pattern-recognition receptors,64 to produce an AB5 bacterial toxin that acts through cell-to-cell paracrine signaling pathways,65 and to persist and disseminate throughout the human host within phagocytic cells.66 Much of our understanding of host-pathogen interactions in enteric fever is extrapolated from mouse models of enteric fever, which use Nramp1-deficient mice that develop disseminated infection from S. Typhimurium. Newer mouse models of infection include both humanized mice, as well as genetically altered mice that lack TLR11, a pattern-recognition receptor for flagellin that is absent in humans.67,68 These mice are susceptible to disseminated infection with S. Typhi, whereas wild-type mice are not.
Invasion
In contrast to NTS and other enteroinvasive pathogens, it is thought that typhoidal Salmonella pass though the intestinal epithelium through specialized microfold cells (M cells) that transport the bacteria across the basolateral membrane, where they are then phagocytosed by macrophages in the intestinal lymphoid tissue.69 Such a stealth entry mechanism may explain why the invasion phase of S. Typhi infection is usually asymptomatic and is only accompanied by transient or mild diarrhea in 10% to 20% of patients.
Latency and Dissemination
Typhoidal Salmonella deploy an array of virulence factors that enable them to persist and replicate in an intracellular compartment.70,71 Important cloaking mechanisms of S. Typhi include suppression of flagellar protein synthesis, thus masking a potent inducer of TLR5 responses,72 and synthesis of the Vi capsule that presumably masks the detection of lipopolysaccharide (LPS) and other outer membrane components.73 The genes that repress flagellin biosynthesis and are responsible for Vi biosynthesis are encoded on the viaB locus located on SPI7, which is absent in most NTS.23 Induction of other bacterial defenses that are required for survival in the intracellular compartment are regulated by a two-component bacterial sensory-regulatory system, PhoP/PhoQ.74,75 The sensor PhoQ detects signals present inside the host phagosome and activates the transcriptional regulator PhoP, which in turn controls the expression of a large number of downstream genes involved in modification of lipid A, resistance to antimicrobial peptides, and acidification of the phagosome.76,77 Consistent with a role in pathogenesis, phoPQ mutants are nonvirulent in humans and have been evaluated as candidate live-attenuated vaccines.78,79
Quantitative blood cultures demonstrate that more than 60% of culturable circulating S. Typhi organisms reside in the intracellular compartment.80 Presumably, this allows for dissemination throughout reticuloendothelial tissues via the blood and lymphatic systems. The heaviest burden of infection is established in the intestinal lymphoid tissue, liver, spleen, gallbladder, and bone marrow. Patients with typhoid fever most often have very low-grade bacteremia, a feature that presents a formidable diagnostic challenge. The kinetics of quantitative bacterial cultures has been studied in Vietnam.80 The median number of culturable bacteria is less than 1 colony-forming unit (CFU) per mL of whole blood in adults, and slightly higher (1.5 CFU/mL) in children.80 Slightly more culturable organisms are present in the bone marrow, with a median of 10 per mL of bone marrow.81 However, over the course of illness, the number of culturable S. Typhi in the blood decreases, and the proportion of bacteria in the bone marrow increases from 5 : 1 (marrow : blood) in the first week of illness to more than 150 : 1 in the third week of illness, reflecting relative clearance of bacteria from peripheral blood but persistence in the marrow compartment.81 Pathologic examination of bone marrow may demonstrate monocytic infiltrates typical of typhoid fever (see “Intestinal and Other Local Pathology”).
The initial period of replication and dissemination likely represents a prepatent phase of infection. Prepatent infection typically lasts between 1 to 2 weeks but can range widely (3 to 60 days), depending on the number of organisms ingested.82 Ultimately, infection results in the sufficient secretion of inflammatory cytokines, including pyrogenic cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), which result in fever. However, unlike what occurs during prototypical gram-negative bacteremia, septicemia with hypotension, neutrophilia, and disseminated intravascular coagulopathy (DIC) are extremely uncommon in patients with typhoid or enteric fever.
Intestinal and Other Local Pathology
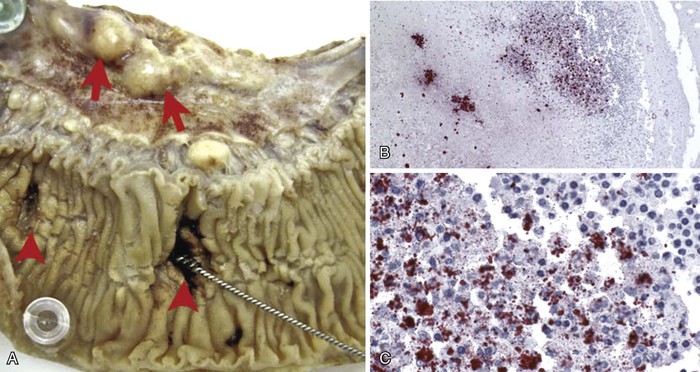
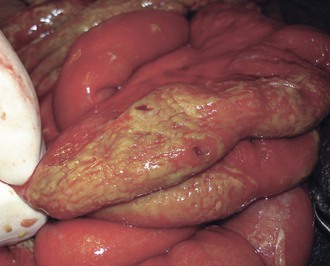
Intestinal lymphoid tissue is a predominant site of localized inflammation and persistent bacterial replication in cases of severe enteric fever (Fig. 102-2).83,84,85,86 Early in the course of illness, Peyer’s patches in the terminal ileum and draining mesenteric nodes are enlarged and contain infiltrates consisting of mononuclear cells, including macrophages and lymphocytes.83 As disease progresses, necrosis of intestinal lymphoid tissue occurs, with mixed inflammatory infiltrates, including neutrophils and ulceration and sloughing of overlying intestinal mucosa.83 Immunohistochemical staining for the S. Typhi O antigen reveals abundant organisms in necrotic areas.84 The clinical manifestations of these changes include hemorrhage and/or intestinal perforation, two of the major life-threatening complications of enteric fever (Fig. 102-3). Other affected organs include the liver, with monocytic infiltrates and foci of parenchymal necrosis, and the spleen, with nodular monocytic infiltrates in the red pulp.87 Similar monocytic infiltrates may occur in the gallbladder, and gallbladder perforation, which may mimic intestinal perforation, is a rare complication of typhoid fever.88
Relapse and Chronic Carriage
Untreated, typhoid fever may persist for up to 4 weeks, and relapse occurs in up to 10% of untreated cases, usually within 2 weeks after initial resolution of fever.89 Relapse is usually due to the same strain as that associated with the original infection.90 The gallbladder is the primary site of chronic carriage, although some asymptomatic carriers continue to shed the organism in stool even after cholecystectomy. The in vitro and clinical observations that S. Typhi forms biofilms on cholesterol gallstones may explain the strong epidemiologic association between gallstones and carriage.91,92 An association has been reproducibly observed between chronic carriage of S. Typhi and risk of gallbladder carcinoma, but it is unknown whether carriage causes cancer or reflects underlying biliary epithelial surface abnormalities.93
Clinical Manifestations
The clinical manifestations of enteric fever are nonspecific. Although paratyphoid fever was considered less severe than typhoid fever, recent comparisons suggest that typhoid fever and paratyphoid fever caused by S. Paratyphi A are generally indistinguishable on clinical grounds.94 Most patients with enteric fever are diagnosed in the ambulatory setting, and up to 90% are treated as outpatients.89 In contrast, classic descriptions of the features of enteric fever are derived from series of hospitalized patients with more severe disease.95 Complications of enteric fever were generally thought to occur late in the course of disease, usually after 2 weeks of fever. However, recent experience demonstrates that major complications of enteric fever, including intestinal perforation and encephalopathy, may occur within days of onset of fever.84,85,96
Uncomplicated Typhoid Fever
Clinical features of enteric fever diagnosed in the ambulatory setting are listed in Table 102-2. Fever without localizing signs or symptoms may be the sole manifestation of enteric fever. The onset of fever may be insidious, and fevers typically increase over the first week of illness.97 A variety of other nonspecific flulike symptoms are common early in the course of enteric fever, and headache, anorexia, myalgias, and malaise may precede the onset of fever.94,98 Mild confusion may be seen even in ambulatory patients, and a nonproductive cough is also a common feature of uncomplicated enteric fever. Abdominal complaints may include diarrhea, constipation, and abdominal pain. Invasive diarrhea, such as seen in NTS gastroenteritis, does not typically occur in enteric fever.
TABLE 102-2
Clinical Features of Typhoid and Paratyphoid Fever
| CLINICAL FEATURE | APPROX. FREQUENCY* | |
| Flulike symptoms | Fever | >95% |
| Headache | 80% | |
| Chills | 40% | |
| Cough | 30% | |
| Myalgia | 20% | |
| Arthralgia | <5% | |
| Abdominal symptoms | Anorexia | 50% |
| Abdominal pain | 30% | |
| Diarrhea | 20% | |
| Constipation | 20% | |
| Physical findings | Coated tongue | 50% |
| Hepatomegaly | 10% | |
| Splenomegaly | 10% | |
| Abdominal tenderness | 5% | |
| Rash | <5% | |
| Generalized adenopathy | <5% |
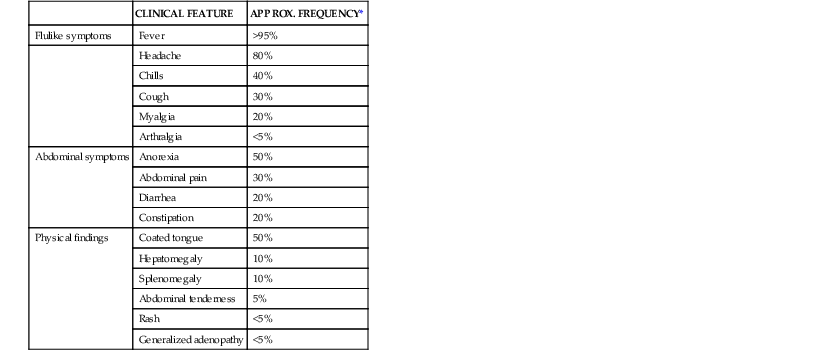
* The proportion of patients demonstrating these clinical features of enteric fever varies, depending on the time, region, and the type of clinical population (hospitalized or ambulatory) assessed. Estimates are drawn from recent case series in an endemic area, with patients presenting for ambulatory or inpatient care.94,98
Modified from Magill AJ, Ryan ET, Hill DR, Solomon T, eds. Hunter’s Tropical Medicine and Emerging Infectious Diseases. 9th ed. Philadelphia: Saunders; 2013:568-576.
The physical findings of uncomplicated enteric fever are also nonspecific. Although relative bradycardia, or pulse-temperature dissociation, is a classic sign of enteric fever, it may not be a clinically useful predictor of enteric fever for individual patients.99 Rose spots, the classic cutaneous manifestation of enteric fever, are 1- to 4-mm blanching pink macules, and are most often seen on the chest, back, and abdomen during the second week of fever. However, rose spots are uncommon in uncomplicated typhoid fever. Mild, nonlocalizing, abdominal tenderness may be present. Hepatomegaly and splenomegaly, if present, are usually modest. A white or yellowish/brown coating of the tongue that spares the tongue’s edges is a common physical finding.94
Laboratory Findings in Uncomplicated Infection
Although abnormal white blood cell counts in peripheral blood are common in patients with bacteremia, the majority of patients with uncomplicated enteric fever do not have leukocytosis, neutrophilia, or increased immature neutrophils.94 However, either leukocytosis or leukopenia may be present. Both the hematocrit and platelet counts are typically normal or slightly low. Elevated serum aspartate transaminase and alanine transaminase are very common in enteric fever; values two to three times above the upper limit of the normal range are typical.100 On occasion, more severe hepatitis is observed, but very high levels of blood transaminases (>500 IU/L) should prompt concern for other etiologies, including viral hepatitis or drug toxicity.100
Severe Illness
Classic descriptions of the features of enteric fever are drawn from observations of series of hospitalized patients in the preantibiotic era, in which mortality rates were consistently in the 10% to 15% range. The natural history of untreated disease included progressively increasing fevers over the first week of illness, followed by increasing abdominal complaints and rash over the second week of illness, followed by complications, including intestinal hemorrhage and perforation, or gradual resolution in the third and fourth weeks of illness.95
In the present antibiotic era, it remains true that because enteric fever progresses gradually, patients who are severely ill with enteric fever may have been febrile for greater than a week when they first come to clinical attention. Patients with severe enteric fever may appear toxic, and characteristically would have moderate abdominal pain or tenderness, as well as constipation or diarrhea. Physical findings in severe enteric fever include hepatomegaly and splenomegaly. Patients with severe enteric fever are more likely to suffer major complications listed in Table 102-3. Complications associated with increased mortality in severe typhoid fever include intestinal hemorrhage and perforation, severe encephalopathy, seizures, and pneumonia.101
TABLE 102-3
Complications of Typhoid and Paratyphoid Fever
| SYSTEM | COMPLICATION | NOTES |
| Gastrointestinal | Hemorrhage | 10%-15% in hospitalized patients |
| Perforation | 3% in hospitalized patients | |
| Hepatobiliary | Jaundice | 1%-3% in hospitalized patients |
| Hepatitis | Usually subclinical (↑ ALT/AST) | |
| Acute cholecystitis | Rare; gallbladder may perforate | |
| Neurologic | Mild encephalopathy | Confusion or apathy common |
| Severe encephalopathy | Delirium, stupor, or coma | |
| Seizures | Common in children ≤5 yr | |
| Meningitis | Rare, primarily infants | |
| Guillain-Barré syndrome | Reported | |
| Respiratory | Bronchitis | Dry cough is common |
| Pneumonia | May be other concomitant bacterial infection (e.g., Streptococcus pneumoniae) | |
| Empyema | Rare reports | |
| Cardiovascular | Myocarditis | Usually subclinical (ECG changes) |
| Endocarditis | Rare reports | |
| Hematologic | Anemia | Usually subclinical |
| Disseminated intravascular coagulation | Usually subclinical (↑ PT/PTT) | |
| Other | Musculoskeletal pyogenic infections | Osteomyelitis (particularly vertebral), psoas abscess and others reported |
| Hemolytic-uremic syndrome | Reported | |
| Miscarriage | Reported |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree