William A. Petri Jr., Rashidul Haque
Entamoeba Species, Including Amebic Colitis and Liver Abscess
Entamoeba histolytica is an invasive enteric protozoan parasite that is the cause of amebiasis.1 Entamoeba dispar, Entamoeba moshkovskii, and Entamoeba bangladeshi are parasites that are identical morphologically to E. histolytica, of which E. dispar is nonpathogenic, E. moshkovskii causes noninvasive diarrhea, and E. bangladeshi is of unknown virulence.2–4,5,6 E. histolytica, E. dispar, and E. moshkovskii therefore cannot be distinguished by a stool ova and parasite (O&P) test, which has been the traditional diagnostic method. Because E. dispar and E. moshkovskii are often as prevalent as E. histolytica, it is important clinically to use E. histolytica–specific diagnostic tests (stool antigen detection or polymerase chain reaction [PCR]).7–10
Entamoeba spp. are taxonomically within the subphylum Sarcodina, class Lobosea, and family Entamoebidae.11 At least seven additional species of amebae (Entamoeba coli, Entamoeba hartmanni, Entamoeba polecki, Entamoeba chattoni, Dientamoeba fragilis, Iodamoeba bütschlii, and Endolimax nana) infect the human intestine.11–19 However, these are generally accepted as commensal organisms, although E. polecki, Dientamoeba fragilis, and I. bütschlii have occasionally been implicated as causes of diarrhea.14–16
Hippocrates (460-377 bc) wrote that “Dysentery, if it commence with black bile, is mortal,” and the Old Testament and Huang Ti’s Classic in Internal Medicine (140-87 bc) also made reference to dysentery. In 1828, James Annesley may have made the first association of dysentery to liver abscess when he wrote in Prevalent Diseases of India that “… hepatic disease seems to be induced by the disorder of the bowels, more particularly when this disorder is of a subacute or chronic kind.” Approximately 3 decades later, in 1855, Vilém Lambl described amebae in the stool of a child who had diarrhea.20 In 1875, Fedor Lösch described ameba in the stool of a young farmer with chronic dysentery that resulted in death. He described the amebae as “round, pear shaped or irregular form and which are in a state of almost continuous motion.” Autopsy studies revealed ulcerations of the colon, and Robert Koch’s postulates were met when the patient’s stool inoculated orally and rectally into a dog caused dysentery with amebic ulcers.21 Stephen Kartulis is credited with first demonstrating amebae in liver and brain abscess in the 1880s.22,23
The first North American case of amebiasis was reported in 1890 by Sir William Osler: “Dr. B., age 29, resident in Panama for nearly six years, where he had had several attacks of dysentery, or more correctly speaking a chronic dysentery, came north in May, 1889. …” Subsequently in 1890 the patient developed tenderness and hepatosplenomegaly, and amebae were observed in the stool and abscess fluid: “The general character of the amoeba [found in the stool] correspond in every particular with those found in the liver.” A year later, Osler’ s colleagues William Councilman and Henri Lafleur proceeded through a classic investigation of 14 cases of amebic dysentery to distinguish amebiasis from bacterial dysentery, and they coined the terms amebic dysentery and amebic liver abscess.23
Ipecac bark was used in the treatment of dysentery for centuries in Peru. Piso introduced ipecac bark to Europe in 1658. Helvetius used ipecac to successfully treat the dysentery of King Louis XIV and subsequently sold it as a secret remedy to the French government. Not until 1858 was the use of large doses of ipecac for the treatment of dysentery promoted by the surgeon E. S. Docker in Mauritius. He demonstrated that ipecac (60 grains two to three times a day) decreased mortality from as much as 18% to only 2%. However, large doses of ipecac by mouth were complicated by severe nausea and vomiting and necessitated the coadministration of opium, chloral hydrate, or tannic acid. An alternative therapy was discovered by Leonard Rogers in India, who found that emetine, the principal alkaloid in ipecac, killed amebae in the mucus of stools from patients with dysentery at dilutions as high as 1/100,000. In 1912 he reported successfully treating three patients in Calcutta, who had been unable to tolerate oral ipecac, by injection of emetine.24
The life cycle of E. histolytica was described by Dobell.25 The cyst form of E. histolytica was implicated as the infective form of the parasite by Walker and Sellards26 in the Philippines in 1913, and the parasite’s life cycle was outlined by Dobell in 1925. Brumpt27 proposed that E. histolytica and E. dispar were identical morphologically, but only E. histolytica was pathogenic for humans. Axenic culture of E. histolytica (free of any associated microorganisms) was accomplished by Diamond28 at the National Institutes of Health in 1961. In 1978, Sargeaunt and colleagues2 wrote that E. histolytica and E. dispar species could be differentiated using zymodeme analysis, and in 1989, Tannich and associates3 demonstrated that their DNA genomes were distinct.
Organism
Species of Entamoeba
Many Entamoeba species infect humans, but only E. histolytica is a cause of invasive amebiasis, whereas E. moshkovskii is associated with a noninvasive diarrhea.1,4,5,6 Of greatest significance because of their prevalence are the three morphologically identical amebae E. histolytica, E. dispar, and E. moshkovskii. All three species are in the quadrinucleated cyst clade of Entamoeba. The E. histolytica and E. dispar genomes share 90% identity in genic regions, and E. moshkovskii is also closely genetically related.29 In most industrialized countries, E. dispar is 10 times more common than E. histolytica,1,30–34 and E. histolytica and E. dispar can be equally prevalent even in a developing country.18 The presence of ingested erythrocytes was the sole morphologic characteristic of some usefulness in identifying E. histolytica, but in one study, this characteristic was present in only 68% of cases of E. histolytica but also in 16% of cases of E. dispar.18 E. moshkovskii is also prevalent and geographically widely distributed.5,6,29,35–37 In preschool children from an urban slum, E. moshkovskii was present in 21%, E. histolytica in 16%, and E. dispar in 36%.6,30 In another study in Tanzania, among approximately 100 human immunodeficiency virus (HIV)-infected individuals with diarrhea, E. histolytica was present in 4%, E. moshkovskii in 13%, and E. dispar in 5%.36 In Sydney, Australia, 50% of cases of Entamoeba identified by stool O&P examination were E. moshkovskii.37 E. moshkovskii has recently been shown to be a cause of diarrhea.5
E. hartmanni is also in the quadrinucleated cyst clade, but it is smaller than E. histolytica, having trophozoites of 3 to 12 µm in diameter and cysts of 10 µm in diameter, whereas E. histolytica trophozoites are 12 to 60 µm in diameter, and cysts are 10 to 20 µm in diameter. E. coli cysts have up to eight nuclei, with trophozoites of size similar to those of E. histolytica. Entamoeba gingivalis does not form a cyst and is not an inhabitant of the intestine; instead, it is observed in the mouth in gingival scrapings. Human infection with the uninucleated cyst amebae E. polecki, E. chattoni, and Entamoeba suis is generally rare. E. polecki and E. suis infections are linked to contact with pigs, and E. chattoni infection is linked to contact with monkeys.38–40 Other nonpathogenic amebae include I. bütschlii, which has characteristic glycogen vacuoles in the cysts; E. nana, which has a characteristic nuclear structure that lacks peripheral chromatin; and D. fragilis, which is more closely related to the flagellates than to the ameba with binucleate trophozoites.12,13
Genotypes of Entamoeba histolytica
In addition to the genetic differences between the three morphologically identical amebae E. histolytica, E. dispar, and E. moshkovskii,4,5,11 genetically distinct strains or genotypes also exist within E. histolytica. Genotypes have been distinguished by the use of isoenzymes, polymorphisms in protein-coding DNA, and polymorphisms in short tandem repeat loci linked to transfer RNA genes (reviewed by Ali and colleagues30). Important findings from genotyping include the following: E. histolytica contains many genotypes41–47; patients may be infected with more than one genotype at a time47; and certain genotypes are associated with diarrhea, others with colonization, and others with amebic liver abscess formation.46,47 In contrast to this high level of diversity in repetitive noncoding DNA, individual protein-encoding genes such as the galactose and N-acetyl-d-galactosamine (Gal/GalNAc) lectin are conserved between genotypes.45 An additional nuance to genotypes is that in a study of patients with amebic liver abscess, comparison of the amebae in the intestine and liver demonstrated in every case that each patient had different genotypes in the two locations. This surprising result suggested that there is a genetic bottleneck between the intestine and liver, and only a subset of intestinal isolates is capable of causing extraintestinal disease.47
Life Cycle
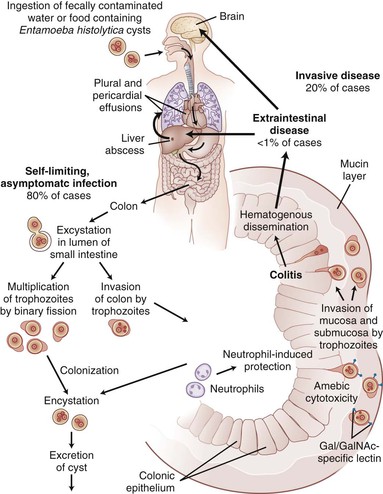
The life cycle of E. histolytica begins with an infectious quadrinucleated cyst and continues with an invasive uninucleated trophozoite. The cyst is ingested from fecally contaminated food or water or through oral-anal sexual practices and, in the intestine, excysts to eight trophozoites (Fig. 274-1). The environmental stability of the cyst and relative resistance to chlorine has resulted in waterborne outbreaks caused by contamination of municipal water supplies.48 In most laboratory-based studies of the cyst, the reptilian parasite Entemoeba invadens has been used because E. histolytica does not encyst in culture. Studies of the process of encystation in E. invadens have demonstrated the role of quorum sensing through a surface Gal/GalNAc lectin to initiate encystation49 after an initial environmental signal such as osmotic shock, low glucose level, or interaction with colonic mucins. Later steps in formation of the cyst require signaling through β-adrenergic receptors and autophagy.50,51 The cyst wall of E. invadens contains a chitin-binding lectin.52 Transcriptional networks associated with encystation were identified in cultures of clinical isolates of E. histolytica that contain encysting organisms.53 A total of 672 cyst-specific transcripts were identified, including chitin synthetase, some of the transmembrane kinases, and cysteine proteinases, as well as genes involved in transcription, such as chromodomain proteins, and an myb-like protein, EhMyb.53
Metabolism
No tricarboxylic acid cycle or oxidative phosphorylation exists for E. histolytica.54 Many metabolic enzymes appear to have been acquired through lateral gene transfer from bacteria.55 Functional mitochondria, or any other compartmentalized energy generation system, are lacking, although there is a remnant of the mitochondria called the mitosome.56 Glycolysis is the major pathway for adenosine triphosphate (ATP) generation and occurs in the cytosol.57 Catabolism of amino acids is a second energy source.58 Pyruvate ferredoxin oxidoreductase is essential in both glycolysis and amino acid catabolism and also serves to activate metronidazole through its reduction of ferredoxin, which suggests that metronidazole resistance would probably not develop.54 Energy stores are predominantly in glycogen that occurs in cytoplasmic granules.54
Pathways for the biosynthesis of amino acids, with the exception of serine and cysteine, are lacking.59,60 Cysteine is of special importance because it is the major thiol. Synthetic enzymes for cholesterol fatty acid and phospholipids also have been identified. In contrast, de novo purine synthesis is lacking.61 More than 100 transporters have been identified in the genome, but their characterization is incomplete at this time.54
Cell Biology
Vesicular trafficking is of paramount importance to the parasite, inasmuch as endocytosis and phagocytosis serve as mechanisms of nutritional uptake. Exocytosis of the cysteine proteinases and ameba pores implicated in virulence and cyst wall components are also important functions of vesicular trafficking, in addition to the more typical roles in transport to and from the endoplasmic reticulum to the Golgi complex and cell surfaces. This complexity of function is reflected in the presence of 91 Rab genes (in comparison with 11 in Saccharomyces cerevisiae) involved in vesicle fusion.62–64 N-linked glycosylation of proteins is unusual in that Man 5 GlcNAc 2 is the most abundant N-linked glycan, whereas in other eukaryotes this would typically be processed by the addition of branching sugars.65
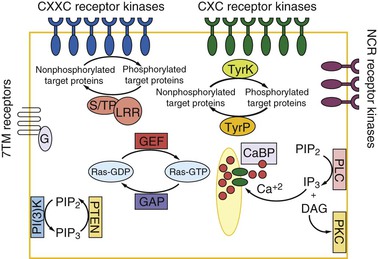
Transmembrane kinases are present and extraordinarily diverse (Fig. 274-2).66–69 These kinases number more than 100 and are part of the family of Gal/GalNAc lectin-related proteins that share the extracellular CXXC and CXC motifs of the lectin intermediate (igl) subunit. The kinase activity (ser/thr vs. tyr) of the transmembrane kinases, their substrates, ligands, and biologic functions are all yet to be determined. The immediate downstream effectors of the transmembrane kinases are also yet to be identified, although more than 100 protein phosphatases are known to be present in the genome.69 An unusual feature of these phosphatases is the presence of leucine-rich repeat domains implicated in protein-protein interactions. There are numerous seven-transmembrane G-coupled receptors and trimeric G proteins. A G protein–regulated adenylyl cyclase that functions downstream of an adrenergic ligand receptor has been biochemically identified. Cytosolic proteins involved in signal transduction include Ras, rac, rab, rho, and arf and their exchange factors: EF-hand calcium-binding proteins, phosphatidylinositol-3-OH kinase, and protein kinase C (PKC) and mitogen-activated protein (MAP) kinases.69 It seems likely that this complex signaling system is required for the adaptation of the parasite to its host.
Some of the unique aspects of the cytoskeleton include the lack of dependence on microtubules for motility, which is instead mediated by actin-myosin motors, and the lack of intermediate filament proteins such as keratins, desmin, and vimentin. Polymerization of actin into polymers of F-actin leads to microfilament assembly, which, through the myosin family of molecular motors, provides vesicular transport and motility by means of pseudopods.69,70
Genome Structure
The original genome sequence was from the HM-1:IMSS strain originally isolated from the rectal biopsy sample of a Mexican man with amebic dysentery.69 Because of a high content of repetitive DNA, the genome has not been completely assembled but instead exists in approximately 1800 fragments, with the average of 12 sequencing reads per fragment. The incomplete nature of the genome ensures that some genes will be missing and that some misassembly will occur. The E. histolytica genome is estimated to be 14 chromosomes, 8000 genes, and 24 million base pairs of DNA, a size that is approximately comparable with those of Plasmodium and Trypanosoma spp. The average gene length of 389 amino acids, however, is approximately half that of Plasmodium.54,69 Approximately 50% of the genome is noncoding DNA, including 20% of the genome that is dedicated to ribosomal RNA genes that are encoded in extrachromosomal circles, and another 10% that encodes transfer RNA genes organized in repetitive linear arrays (probably at chromosome ends). Additional repetitive DNA in the genome includes long interspersed repeated sequence (LINE) and short interspersed repeated sequence (SINE) transposable elements.69
The DNA content of Entamoeba appears to vary under different growth conditions. The nuclear DNA content of E. histolytica was shown to be 10-fold higher in axenic (bacteria-free) than xenic culture. In addition, 40-fold increases in DNA content were observed as trophozoites emerged from E. invadens cysts. The most plausible explanation for these observations is that the ploidy of E. histolytica varies through a growth-dependent process of DNA replication without nuclear division.71
Control of messenger RNA (mRNA) expression in E. histolytica shares similarities with later branching eukaryotes: The parasite transcribes mRNA monocistronically by using RNA polymerase II under the control of upstream regulatory elements.72 Thirty percent of genes are predicted to contain introns, and the pre-mRNA splicing machinery includes conserved U2, U4, and U5 small nuclear RNAs.73 Most of the protein subunits of RNA polymerase II are also conserved, but not all general transcription factors are identified yet. Other differences from yeast and metazoans include the presence of a third core promoter regulatory element for RNA polymerase II,74 altered histone code,75,76 and unique aspects of mRNA silencing through small RNAs.53,77
Pathogenesis
The pathogenesis of amebiasis centers on the unique tissue-destructive properties for which the organism was named histolytica. Tissue invasion involves a contact-dependent process of adherence followed by cell killing that is called “trogocytosis-like” because the host cell is killed by its partial ingestion by the parasite (Fig. 274-3).78–80
Adherence
The initial contact of parasite to host is mediated by the parasite’s Gal/GalNAc lectin, which binds to carbohydrate determinants on the host.79–83 Adherence to human colonic mucin glycoproteins,84 human neutrophils and erythrocytes,85 certain bacteria,86 and a variety of cell culture lines87,88 is inhibited by up to 90% by Gal or GalNAc.84 Blockade of lectin activity with Gal or GalNAc prevents contact-dependent cytolysis,79 and glycosylation-deficient mutant cell lines lacking terminal Gal/GalNAc residues on N- and O-linked sugars are nearly totally resistant to amebic adherence and cytolytic activity.88
The colonic mucin layer of the large intestine is the first receptor encountered by the trophozoite lectin. Binding of the lectin to colonic mucins inhibits Gal/GalNAc and is of very high affinity (dissociation constant of 8.2 × 10−11 M−1).84 The mucin layer may protect the host from the parasite’s contact-dependent cytolysis by binding to and neutralizing the lectin, while at the same time serving as a site of attachment for the parasite to colonize the large bowel. Interaction of trophozoites with colonic mucins appears to be a dynamic process, whereby trophozoites both induce the secretion of colonic mucins and degrade them.89
The Gal/GalNAc lectin is composed of a 260-kDa heterodimer of disulfide-linked heavy (170-kDa) and light (35- to 31-kDa) subunits that is noncovalently associated with an intermediate subunit of 150 kDa.80–83 The 170-kDa subunit contains a carboxyl-terminal cytoplasmic and transmembrane domain adjacent to a cysteine-rich extracellular domain.90,91 Five distinct genes (termed hgl1 to hgl5) encoding the lectin’s heavy subunit have been identified, sequenced, and shown to be expressed in trophozoites.92 The sequence of the hgl genes is nearly completely conserved in isolates of E. histolytica from different continents, an important consideration for vaccine design.45 The carbohydrate recognition domain is located within the cysteine-rich domain of the heavy subunit.93 The lectin localizes to lipid rafts in the plasma membrane.94 The lectin can be specifically released from the cell surface through the action of an amebic rhomboid protease, which probably explains earlier observations of both membrane-bound and soluble forms of the lectin.80,81,95
The Gal/GalNAc lectin appears to have other biologic functions in addition to adherence. Interference with lectin activity blocks chemotaxis in response to tumor necrosis factor-α (TNF-α).96 The function of the light subunits may include lateral mobility of the Gal/GalNAc lectin, as evidenced by defects in capping of the lectin in ameba silenced for one of the genes that encodes the light subunits (lgl genes).97 In an animal model, disruption of lectin function by expression of a dominant negative mutant blocked the ability of the parasite to cause liver abscess.98,99 The lectin may serve as an organizing site for cytoplasmic proteins, with cytoplasmic proteins of the amebae that bind directly or indirectly to the lectin, including a thiol-specific antioxidant, spectrin, actin, myosin, talin, calreticulin, and cysteine proteinase 2.100–102 The lectin has a role in evasion of serum lytic activity by inhibiting the formation of the complement membrane attack complex through blockade of C5b-9 assembly.103 As mentioned previously, the lectin also appears to play an initiating role in cyst production.49
Cytolysis
Cytolysis occurs after adherence in a process that involves phagocytic ingestion of the host cells in bites, a process called “trogocytosis-like” (see Fig. 274-3). Apposition of amebic and target cell plasma membranes, as can be achieved by centrifugation of target cells and amebae into a pellet, does not lead to cytolysis if the amebic lectin is inhibited with Gal/GalNAc78 or if the target cell lacks Gal and GalNAc on its surface.86–88 This is consistent with the fact that lectin not only mediates adherence but also participates in the trogocytosis-like cytolytic event. Anti–lectin monoclonal antibody directed against epitope 1 of the lectin heavy subunit blocked cytotoxicity but not adherence, which implicates the lectin directly in the cytotoxic event. Killing occurs after the lectin engages GalNAc on O-linked target cell surface oligosaccharides: lectin-mediated capping of the O-linked structures (sialic acid-Gal–[sialic acid]–Gal-NAc) could be deduced to mediate killing.
Killing of host cells is not caused by an isolated toxin, inasmuch as parasite extracts have no cytotoxic activity. Cytolysis does require an intact parasite cytoskeleton, as demonstrated by inhibition of Rho,105 by cytochalasin disruption of the cytoskeleton,79 and by expression of dominant-negative myosin II.106 The earliest observed event in a dying cell is a rise in intracellular calcium within seconds of direct contact by an amebic trophozoite; this event is associated with membrane blebbing.107 Extracellular ethylenediaminetetraacetic acid (EDTA) and treatment of the target cells with the slow sodium-calcium channel blockers verapamil and bepridil108 significantly reduce amebic killing of target cells in suspension. Isolation of amebic pore-forming proteins similar in function to pore-forming proteins of the immune system has been reported by a number of investigators. A purified 5-kDa amebapore and a synthetic peptide based on the sequence of its third amphipathic α-helix have cytolytic activity for nucleated cells at high concentrations (10 to 100 µM).109,110 Silencing of amebapore A blocked the ability of amebae to release monolayer tissue culture cells from a plastic well, although cell death was not specifically measured.111 The optimal pH of amebapore is 5.3, and amebapore is inactive at a pH of 7, which may be of some significance in view of the inhibition of cytotoxicity with weak base treatment of amebae.112 Interestingly, no DNA degradation was observed in cells lysed in vitro by the purified amebapore, which is suggestive of a different mechanism of cell killing by the purified amebapore than by the intact parasite.113
Cells killed by the parasite undergo nuclear chromatin condensation, membrane blebbing, and internucleosomal DNA fragmentation.113 There is evidence of a nonclassical mechanism of apoptotic killing by E. histolytica. Overexpression of the Bcl-2 protein that inhibits apoptosis caused by a variety of cellular stresses (e.g., serum starvation and ultraviolet radiation) did not prevent murine cell DNA fragmentation after exposure to E. histolytica.113 Furthermore, E. histolytica caused hepatocyte apoptosis in mice deficient in the Fas/Fas ligand and TNF receptor 1 signaling pathways.114 Caspase 8–deficient cells, resistant to killing by Fas ligand, were readily killed by E. histolytica. Caspase 8–deficient cells treated with a caspase 9 inhibitor (Ac-LEHD-fmk) (at a level sufficient to inhibit apoptosis through etoposide) were readily killed as well. Together, these data suggest that E. histolytica initiates host cell apoptosis by directly activating the host cell’s distal apoptotic machinery. Caspase 3 was activated within minutes of E. histolytica adherence, and the caspase 3 inhibitor Ac-DEVD-CHO at 100 µmol (sufficient to block killing through actinomycin D) blocked E. histolytica killing, as measured both by DNA fragmentation and by Cr51 release; this outcome indicates that both apoptotic death phenotype and necrosis were necessary.115 In conclusion, amebic killing of the host is a result of parasite activation of apoptosis in the host cell, at the level of caspase 3 activation.
Phagocytosis
In multicellular organisms, phagocytosis is the final step in the apoptotic pathway and serves to limit inflammation by preventing spillage of toxic intracellular contents of dead cells. Although amebic killing of cells by contrast involves phagocytosis followed by death, phagocytosis could similarly limit the host inflammatory response and enable E. histolytica to establish a persistent infection.68,116,117 Inhibition of endocytosis by galactose and phosphatidylserine is additive, consistent with the Gal/GalNAc lectin and an as yet unidentified phosphatidylserine receptor acting as co-receptors for ingestion.118,119 Understanding the molecular mechanisms of engulfment of and subsequent death of the host promises to reveal much about pathogenesis, inasmuch as amebae that are defective in phagocytosis are also defective in virulence.68,120
Role of Bacteria
The effect of the gut bacteria on the biologic properties of E. histolytica may be profound. As mentioned previously, the genome content of Entamoeba is lower when the parasite is grown in the presence of bacteria.54 In addition, amebae cultured with bacteria are better able to destroy monolayers of tissue culture cells and resist oxidative stress.121–125
Cysteine Proteinases
E. histolytica encodes at least 44 genes that are cysteine proteinases, some of which are membrane bound and others predicted to be soluble.54 These activities are implicated in a number of potentially important activities and include degradation of colonic mucin glycoproteins,126 digestion of hemoglobin and villin,127,128 inactivation of interleukin(IL)-18,129 and digestion of extracellular matrix.130
Role of Leptin in Host Resistance
Amebiasis is more common in malnourished children (a physiologic state of leptin deficiency). This suggests that the nutritional hormone leptin could play a protective role in amebiasis. In fact, a mutation in the leptin receptor was discovered that was associated with amebiasis susceptibility in children. The mutation is a nonconservative substitution (Q223R) in the extracellular cytokine receptor homology domain 1. The mutation accounted for the majority of susceptibility to amebiasis in children (because it conferred a 3.9-fold greater risk of intestinal amebiasis and was present in almost half the children). The purely nutritional role of leptin did not explain protection because the Q223R mutation was not associated with children’s nutritional status. Experiments in mice validated the human study, with leptin-deficient (ob/ob), leptin receptor-deficient (db/db), and 223R leptin receptor knock-in mice highly susceptible to intestinal E. histolytica infection. The site of leptin action was localized to the gut, as an intestinal epithelial cell–specific deletion of the leptin receptor rendered mice susceptible, whereas lack of the leptin receptor in the CNS or bone marrow–derived cells did not. Leptin receptor STAT3 and SHP2 signaling were required for protection in vivo as susceptibility was conferred by mutation of tyrosine 985 or 1138, which mediate leptin signaling through the SHP2/ERK and STAT3 pathways, respectively. The importance of leptin signaling in prevention of amebic killing of the host could even be seen in single cell studies, where transfection of HEK cells with the leptin receptor rendered them resistant to amebic killing.131,132
Immune Response and Immunity
Innate Immunity
Neutrophils
Neutrophils are the earliest innate cellular immune response for both intestinal and hepatic amebiasis. They occur as a dominant infiltration of polymorphonuclear leukocytes surrounding trophozoites. Lymphocytes, macrophages, and epithelioid cells are recruited to infected tissue by day 3, in association with the formation of granulomas, which contribute to the confinement of invading trophozoites.133–137 Neutrophils may be recruited by the chemotactic activity of an amebic membrane-bound peptide and chemokines secreted by epithelial cells exposed to E. histolytica.138–141 As the consequence of interaction with trophozoites, neutrophils become activated and release reactive oxygen species and antimicrobial peptides. Many in vitro studies have reported neutrophil amebicidal activity after stimulation by interferon-γ (IFN-γ), tumor necrosis factor-γ (TNF)-γ, lipopolysaccharide, or amebic antigens.142,143 In accordance with a protective role for neutrophils, depletion of neutrophils with anti–Gr-1 neutralizing antibodies resulted in exacerbated amebic hepatic and intestinal disease.144–146 It is worth noting that the GR-1 antibodies also deplete other granulocytes such as eosinophils, which are also observed as part of the innate immune response.147 However, neutrophils can also be lysed by virulent ameba.147,148 This can occur through disruption of the oxidase activities of the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH)149,150; through protection of the amebae from oxidative damage by amebic peroxiredoxin, a 29-kDa surface protein conferring resistance to host reactive oxygen defenses100,151,152; or by inducing neutrophil apoptosis.150 Neutrophil destruction in turn could result in tissue damage through the release of cytotoxic oxidase and lytic peptidases.147
Macrophages
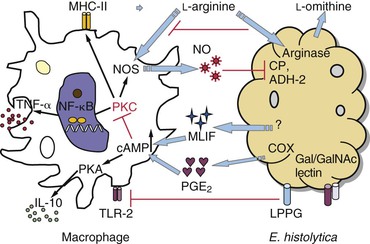
Macrophages acquire amebicidal activity after in vitro stimulation with IFN-γ, TNF-γ, or colony-stimulating factor-1 (Fig. 274-4).153–155 The Gal/GalNAc lectin of E. histolytica upregulates Toll-like receptor (TLR)-2 expression in macrophages, resulting in activation of nuclear factor kappa B (NF-κB) and production of proinflammatory cytokine.156 Macrophages lacking TLR-2 and TLR-4 showed impaired response to E. histolytica lipopeptidophosphoglycan (LPPG), which suggests that pattern recognition is essential in the macrophage response.157 Inducible nitric oxide synthase (iNOS)–deficient mice were more susceptible to amoebic liver abscess and to E. histolytica–induced hepatocytic apoptosis,29 which suggests that nitric oxide plays a critical role in host defense against amebiasis.
Despite the sensitivity of E. histolytica to nitric oxide–mediated cytotoxicity,157,158 impaired macrophage function has been observed in human and experimental amebiasis, which suggests that amebae have developed strategies to modulate macrophage responses (see Fig. 274-4). Macrophage exposure to E. histolytica trophozoites or amebic components suppresses the respiratory burst and nitric oxide production.159,160 A decrease in TNF-α secretion and IFN-γ–induced expression of major histocompatibility complex class II (MHC-II) has also been observed.161,162 Macrophage suppression may depend at least partially on prostaglandin E2 (PGE2), an immunoregulator produced by E. histolytica, or on macrophages exposed to amebic proteins.163,164 PGE2 elevates cyclic adenosine monophosphate (cAMP) levels in macrophages, triggering the protein kinase A (PKA) pathway, which in turn inhibits the expression of MHC-Ia molecules, the release of helper T cell type 1 (Th1) cytokines, NADPH-mediated oxidative burst, and nitric oxide synthesis through the PKC pathway (see Fig. 274-4). In addition, an immunosuppressor synthesized by ameba, monocyte locomotion inhibitory factor (MLIF), also contributes to the modulation of host immune responses. MLIF is a soluble pentapeptide with anti-inflammatory properties.164,165
Natural Killer Cells and Natural Killer T Cells
Natural killer cells and natural killer target cells have an innate role in host defense by production of IFN-γ and cytolytic peptides. Elevated cytotoxic activity of natural killer cells was found in mice infected with pathogenic amebae, and this may explain gender-dependent differences in the control of amebic liver abscess in C57BL/6 mice.166,167
Activated Mast Cells
Activated mast cells produce IL-6 and TNF-γ, can recruit phagocytes, and can influence lymphocytic development and functions. Increased mast cell infiltration and upregulated mast cell protease expression has been observed in infected mouse ceca, but whether mast cells contribute to parasite clearance or play a pathologic role in tissue damage remains unanswered.168
Complement-Mediated Lysis of Entamoeba histolytica
After trophozoites penetrate the epithelial layer, the alternative complement pathway is initiated at least in part through cleavage of C3 and C5 by the amebic cysteine proteinase.169,170 C3a and C5a act to chemoattract neutrophils to the site of infection. The Gal/GalNAc lectin heavy subunit of E. histolytica inhibits the assembly of C8 and C9 into the C5b-9 membrane attack complex, thereby preventing complement-mediated lysis of the parasite.103
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree