CAROL GLASER AND ARUN VENKATESAN
Encephalitis is one of the most challenging syndromes for clinicians to manage. Patients are often critically ill, and there are many potential etiologies. Despite exhaustive testing, an etiology is only identified in 40% to 70% of cases. Even when a cause is identified, there may be no effective treatment (1–3). Mortality rates vary substantially across studies and range from 3% to 15% (4,5). The frequency of sequelae, including cognitive and motor impairment as well as seizures, is also variable; some case series report severe disability in 20% to 40% of patients (4,6,7). Not surprisingly, given the severity of the disease as well as the complexity of diagnosis and clinical management, substantial health care costs are associated with encephalitis.
The term encephalitis generally refers to inflammation of the brain parenchyma. However, without the identification of a neurotropic agent or confirmation of inflammation in brain tissue, the diagnosis of encephalitis is presumptive and based on clinical features. Clinically, patients with encephalitis often present with fever, headache, and altered mental status. Seizures or focal neurologic deficits may also be present. In principle, alteration in mental status distinguishes encephalitis from uncomplicated meningitis as meningitis symptoms typically include fever, headache, and nuchal rigidity but lack global or focal neurologic dysfunction. In practice, however, the distinction between these two entities is not always readily apparent, and in descriptions of central nervous system (CNS) infections with mental status changes due to agents such as enteroviruses, rabies, West Nile virus or herpesviruses, the terms encephalitis or meningoencephalitis are often broadly applied.
In contrast to encephalitis, encephalopathy refers to any diffuse disease of the brain that results in changes in function; the clinical hallmark of encephalopathy is an altered mental state. Many entities including metabolic or mitochondrial dysfunction, toxins, trauma, poor nutrition, or lack of oxygen or blood flow to the brain can lead to encephalopathy.
This chapter focuses predominantly on the immunocompetent host and pathogens in North America that either cause encephalitis or an encephalitis-like syndrome. Moreover, many patients with encephalitis also have meningeal inflammation, thus demonstrating the overlap of encephalitis and meningoencephalitis. For the purposes of this chapter, the terms encephalitis and meningoencephalitis are used interchangeably. Other regions of the CNS may be variably affected, including the spinal cord (myelitis), nerve roots (radiculitis), and nerves (neuritis).
GENERAL: ETIOLOGIC AGENTS AND EPIDEMIOLOGY
Although the term encephalitis is often used in conjunction with a viral etiology, many other infections and noninfectious entities can cause encephalitis or encephalitis-like symptomatology. The incidence of encephalitis varies throughout the world and is contingent upon the population under study, the geographic region, the availability of vaccines for some causes of encephalitis, and differences in case definitions and surveillance activities. In tropical regions of the world, the minimum estimated incidence of encephalitis is 6.3 per 100,000 (8). In the Western world, the incidence ranges between 0.7 and 13.8 per 100,000 (8–10). Most reports find the incidence of encephalitis higher in the pediatric age-group than in adults. For example, a study in England of hospitalized patients with encephalitis over a 10-year period demonstrated an overall incidence of 1.5 per 100,000 population, a rate of 2.8 per 100,000 in children, and a rate of 8.7 per 100,000 in infants (11). Somewhat higher rates in children were reported from Finland (8.8 per 100,000 from 1973 to 1987) (12) and in Slovenia (6.7 per 100,000 from 1979 to 1991) (13).
The epidemiology of encephalitis is a dynamic process. In countries where vaccines are widely used for measles, mumps, rubella, and varicella infections, the incidence of encephalitis due to these viruses has decreased (14,15). However, there is a growing list of emerging and reemerging pathogens such as Nipah virus, enterovirus 71, Balamuthia mandrillaris, European tick-borne encephalitis virus, Hendra virus, Baylisascaris procyonis, and Chandipura virus that can cause encephalitis. Moreover, some agents are now identified in previously nonendemic regions of the world. Notable is West Nile virus, which has expanded its geographic region from Africa to North and South America, Europe, the Middle East, Western Asia, and Australia (16). Chikungunya virus is yet another striking example of a virus that has spread from its origin in Africa to nearly 40 countries including a number of countries adjacent to the Indian Ocean: La Reunion Island, Madagascar, the Maldives, the Seychelles, and India. (17).
The increasing recognition of specific autoimmune causes, as discussed later in this chapter, has also had a tremendous impact on our understanding of the epidemiology of encephalitis.
INFECTIOUS CAUSES: SPECIFIC EPIDEMIOLOGIC AND CLINICAL FEATURES
Viruses
Many cases of viral encephalitis are either an uncommon complication of a common infection, such as a herpesvirus or enterovirus infection, or a predictable presentation of a rare pathogen such as rabies or lymphocytic choriomeningitis virus. The clinical manifestations of encephalitis are variable and reflect the degree of brain involvement, host factors, and the inherent pathogenicity of the offending agent. Most patients with encephalitis have headache and fever, followed by altered mental status. Seizures, behavioral changes, impaired cognition, aphasia, hemiparesis, and other focal neurologic signs may be seen. Arboviruses are often associated with global CNS dysfunction, whereas agents such as herpes simplex encephalitis typically result in focal manifestations. Although there is significant overlap in the clinical presentation of various agents and diagnosis can rarely be made on clinical grounds alone, the most typical and/or characteristic features of some of the causes are highlighted in the following sections.
Herpes Simplex Virus
Herpesviruses are enveloped DNA viruses that are among the most common causes of infections in humans. At least eight herpesvirus types are known to infect humans. Herpes simplex virus type 1 (HSV-1) is one the most common causes of sporadic encephalitis in the world (see Chapters 9 and 10). The epidemiology and clinical features of neonatal herpes CNS infections differ from children and adults and are not covered in this chapter. The incidence of herpes simplex encephalitis (HSE) caused by HSV-1 is estimated to be 4 per 1,000,000 (11,18). HSE is responsible for 10% to 20% of adult encephalitis cases (3,19). HSE is less common in children than in adults; in a large cohort of over 300 pediatric encephalitis patients over a 12-year period, only 5% were due to HSE. In the pediatric age-group, HSE is often a result of a primary infection, whereas most HSE infections in adults are the result of HSV reactivation. Importantly, the presence of herpes labialis has no diagnostic specificity for encephalitis causality but does serve as a marker of HSV infection.
The characteristic clinical presentation for HSE includes altered mental status (97%), fever (90%), and headache (81%) (20). Other common neurologic findings include personality change (85%), aphasia (40%), ataxia (40%), hemiparesis (38%), cranial nerve deficits (32%), and seizures (31%) (4,21). Children are more likely to have extratemporal involvement as manifested by clinical symptoms as well as neuroimaging (22).
Unlike HSV-1, HSV-2 is more likely to cause disseminated encephalitis and does not generally localize to the temporal and inferior frontal regions of the brain (23). Most neurologic CNS HSV-2 infections present with lymphocytic meningitis. Relapsing meningitis, encephalitis, and myelitis can also occur.
HSV-1 and HSV-2 can also cause brainstem encephalitis. A recent comprehensive literature review of HSV brainstem encephalitis identified 24 cases: 79% due to HSV-1 and 29% due to HSV-2 (24). The most prominent features were neuro-ophthalmologic manifestations; these were seen in over 80% of patients and included nystagmus, impaired ocular movements, anisocoria, ptosis, oscillopsia, or spasmodic movements (24). Other cranial deficits, altered mentation, ataxia, and corticospinal tract findings (e.g., hyperreflexia) were also described. Although not common, quadriplegia was also present in some (19%) of the patients (24).
Varicella-Zoster Virus
Both primary infections with varicella-zoster virus (VZV) and endogenous reactivation (herpes zoster) can lead to encephalitis (25) (see Chapter 10). The most characteristic manifestation of VZV encephalitis in children is acute cerebellar ataxia (e.g., nystagmus, dysarthria, and ataxia), which usually occurs 1 week after rash onset (26). VZV encephalitis, once a leading cause of encephalitis in children, is much less common due to the widespread use of VZV vaccine (27).
However, VZV encephalitis is relatively common in adults, and its incidence rivals that of HSE (28–31). The clinical presentation in adults is different than in children and includes diffuse brain dysfunction, seizures, cranial nerve palsies, and other focal neurologic signs (4,32). The presence of a diffuse varicella rash or a vesicular rash in a dermatome distribution can be an important clue to diagnosing VZV encephalitis. Notably, however, as many as 44% of patients lack cutaneous findings, a condition termed herpes sine zoster or preeruptive varicella (32–36). Although the pathophysiology of VZV-associated encephalitis remains unclear, some cases appear to be due to a medium and large vessel vasculopathy (25).
Epstein-Barr Virus
Epstein-Barr virus (EBV), another herpesvirus, is most often associated with “mononucleosis” but can also cause several distinct neurologic syndromes including aseptic meningitis, Guillain-Barré syndrome, Bell palsy, transverse myelitis, cerebellitis, and encephalitis (37,38) (see Chapter 12). Most neurologic complications due to EBV occur during primary infection, typically in childhood. Importantly, many patients with EBV-associated encephalitis do not have classic mononucleosis symptoms (39,40). In a case series of 216 pediatric encephalitis patients in Canada, 21 (9.7%) were identified with EBV-associated encephalitis (40). Of these, only one patient had classic mononucleosis symptoms; most others had a nonspecific prodrome of fever (81%) and headache (66%) (40). Seizures occurred in almost half (48%) (40). Some individuals with EBV-associated encephalitis experience micropsia, macropsia, and/or size distortion. This pattern of unusual images of body and objects is referred to as the “Alice in Wonderland syndrome” (41). There are sporadic reports in the literature that reactivation of chronic EBV infection in adults may cause neurologic manifestations, including encephalitis (42).
Human Herpesviruses 6 and 7
Human herpesvirus-6 (HHV-6), the primary cause of roseola infantum in young children, has been identified as the causal agent of 10% to 20% of febrile seizures and is also occasionally associated with encephalitis (43,44) (see Chapter 13). Several studies of encephalitis in children have identified HHV-6 as a causative agent, with the incidence of HHV-6 encephalitis ranging from 1% to 11% of cases (45–49). In one study of nine children with HHV-6 CNS infections, characteristic clinical features included fever, gastroenteritis, rash, seizures, and ataxia (50). Another case series reported three pediatric patients with HHV-6–associated rhombencephalitis; clinical manifestations included encephalopathy, seizures, ataxia, and myoclonus (51). HHV-7, a recently described herpesvirus, is occasionally associated with roseola and is typically acquired in the first few years of life. Recent studies from the United Kingdom suggest HHV-7 may be an important cause of febrile seizures and encephalitis in young children (52).
Enteroviruses and Parechoviruses
Enteroviruses (EVs) are small, nonenveloped, single-stranded RNA picornaviruses. Similar to herpesvirus infections, EV infections are very common, and neurologic complications, including encephalitis, represent a rare complication of EV infection. Because EV infections occur frequently in children, they are a leading cause of encephalitis in children and are responsible for 10% to 15% of encephalitis cases for which an etiology is identified (53). In general, EVs cause a milder clinical illness than many other etiologies of encephalitis. In the California Encephalitis Project, EV encephalitis patients had less severe manifestations, including lower frequencies of coma and shorter hospitalization stays than those due to other agents (54). CNS infections with EV-71 are an important exception to the decreased severity of EVs (55,56). In addition to causing acute flaccid paralysis (aka polio-like syndrome), EV-71 has also been associated with a distinctive form of encephalitis initially described in Taiwan and Malaysia (55,57). Most cases were in young children (younger than 5 years of age), with a characteristic hand, foot, and mouth rash along with ataxia, nystagmus, myoclonus, and oculomotor palsies (55). The predominant neurologic presentations included myoclonus (68%), vomiting (53%), and ataxia (35%) (57). Many of the fatalities associated with EV-71 are due to pulmonary edema and hemorrhage, which are thought to be a consequence of pronounced autonomic instability due to lesions in the medulla and spinal cord (58,59). Sporadic outbreaks involving substantial numbers of EV-71 encephalitis cases have been observed among young children in Europe and Asia over the past several decades (60–62).
The reclassification of former EVs, echovirus 21 and echovirus 22, resulted in the human parechovirus (HPeV) genus. Echoviruses 21 and 22 are currently classified as HPeV-1 and HPeV-2, respectively. At least 12 HPeV serotypes have been described to date; nearly all have been associated with encephalitis, typically in children younger than 2 years of age (63,64). Clusters of HPeV-3 CNS infections have been reported (65). Young children and infants with HPeV encephalitis develop fever, seizures, irritability, feeding problems, and rash (66). The relative frequency of HPeV encephalitis is unknown, particularly because HPeV testing has only recently become available.
Arboviruses
Arboviruses, viruses transmitted by an arthropod vector, are well-recognized causes of encephalitis. The vast majority of neurologic illnesses seen in humans are caused by three arbovirus families: Togaviridae, Flaviviridae, and Bunyaviridae (see Chapter 15).
West Nile virus (WNV), a flavivirus, was first detected in the Western Hemisphere in 1999 in New York City and rapidly spread across North America from the Atlantic to the Pacific coasts and into Mexico and Canada. It is the now the most common cause of arboviral encephalitis in the United States (67). Most individuals infected with WNV will experience subclinical infection (70% to 80%) or febrile illness (20% to 30%). Less than 1% of infected individuals develop West Nile neuroinvasive disease (WNND), which includes meningitis, encephalitis, and/or acute flaccid paralysis.
WNND is more common in older individuals, with an incidence of 1.35 per 100,000 in persons 70 years of age or older compared with 0.05 per 100,000 in persons younger than 10 years of age (67). Other risk factors for WNND include male gender, hypertension, diabetes, renal disease, and immunosuppression (68,69). Characteristic presentations of WNND include altered mental status or lethargy with or without movement disorders (tremors, Parkinsonism, or myoclonus). Acute flaccid paralysis is also a feature of WNV infection and can be seen along with encephalitis or may occur in isolation (70,71).
Although the number of WNND cases has far surpassed the number of cases due to other arboviruses in recent years, other arboviruses in the United States cause seasonal outbreaks and sporadic cases of neurologic disease. These include La Crosse virus (LACV), eastern equine encephalitis virus (EEEV), Powassan virus (POWV), and St. Louis encephalitis virus (SLEV). In 2012, over 2,500 WNND cases were recognized in the United States compared with 78 LAC cases (71 neuroinvasive), 15 EEE cases (all neuroinvasive), 7 POW cases (all neuroinvasive), and 2 SLE cases (1 neuroinvasive) (72).
LACV, primarily found in the upper Midwestern, mid-Atlantic, and Southeastern regions of the United States, is the second most common cause of arbovirus-associated CNS infections in the United States. Unlike WNV, most neuroinvasive LACV cases occur in the pediatric population rather than in adults; in 2012, 86% of cases were younger than 20 years of age (73).
Outside the United States, other arboviruses predominate. Japanese encephalitis virus (JEV) is the most common cause of mosquito-borne encephalitis worldwide. An estimated 50,000 cases of JEV clinical disease occur annually, primarily in children younger than 10 years of age in Asia, South Asia (east of Pakistan), and Southeast Asia (74–76).
Similar to many other arboviruses, most JEV infections are asymptomatic, with less than 1% of infections leading to clinical disease. When symptoms occur, encephalitis is the most common presentation. After a characteristic febrile prodrome including headache and vomiting, mental status changes, seizures, focal neurologic deficits, and movement disorders develop. Similar to patients with WNV, those affected by JEV can also develop acute flaccid paralysis (77).
In Europe, tick-borne encephalitis virus (TBEV), another flavivirus, is the most common cause of arboviral encephalitis (78). It is also found in China and Japan. Analogous to WNV, neuroinvasive disease is more common in older populations (79). Growing numbers of cases have been recognized in recent years as a result of improvements in diagnostics and case reporting as well as increased recreational activities in tick-infested areas (80). In Europe and Russia, there was an average of 8,755 reported cases per year from 1990 to 2007 compared to an average of 2,755 cases per year from 1976 to 1989 (81). TBEV is characterized by three different subtypes: European (TBEV-Eu), Siberian (TBEV-Sib), and Far Eastern (TBEV-Fe). The TBEV-Eu subtype circulates predominantly in Western, Central, Northern, and Eastern Europe; the TBEV-Sib circulates predominantly in Asian parts of Russia; and TBEV-Fe circulates predominantly in China, Japan, and Eastern Russia. The clinical spectrum of disease ranges from mild meningitis to severe meningoencephalitis with or without paralysis (82). In individuals affected with the European virus subtype, the illness is often biphasic, with the first stage characterized by fever, fatigue, general malaise, headache, and body pain. During the second phase of the illness, clinical manifestations range from mild meningitis to severe encephalitis, with or without myelitis and paralysis. Seizures are uncommon. The disease associated with TBEV-Fe subtype is the most severe, with a case fatality of 20% to 40% and higher rates of neurologic sequelae compared with other subtypes (82).
Although JEV is recognized to cause more cases of encephalitis than any other mosquito-borne virus worldwide, dengue viruses are the most prevalent arboviruses that infect humans and result in an estimated 390 million infections every year (83). Infections with dengue typically result in dengue fever, dengue hemorrhagic fever, and dengue shock syndrome. Unlike many of the aforementioned arboviruses, neurologic manifestations of dengue have traditionally been considered to be the result of an encephalopathy rather than encephalitis. However, detection of dengue viral RNA in brain tissue, virus isolation in CSF, and the presence of dengue-specific CSF antibody suggesting intrathecal synthesis have been described in recent studies and strongly suggest the neuroinvasive potential of dengue (84–86).
Rabies
Rabies virus is one of the oldest known infectious diseases and is considered to be the most deadly of all infectious diseases (see Chapter 17). The number of rabies encephalitis cases in the United States has declined dramatically from an average of 100 or more cases per year before 1940 to only 2 to 3 cases per year (87). Although rabies is rare in the United States, an estimated 50,000 rabies cases occur annually worldwide; most are acquired via rabid dog contact (88). In Asia, Africa, and Latin America, animal rabies control programs and postexposure prophylaxis are limited. A recent report of 49 rabies cases in the United States, from 1995 to 2011, identified 10 imported and 39 cases acquired in the United States; of the cases acquired in the United States, one was associated with a raccoon strain of rabies, and the rest were due to bat exposures. The incubation period is generally between 20 and 60 days but can range from a few days to several years (89). Paresthesia at the site of the bite is unique to rabies and can be an important clue to the diagnosis. Approximately 80% of human rabies cases develop the encephalitic (“furious”) form characterized by unusual behavior, extreme agitation, hydrophobia, delirium, and seizures. The remainder of cases develops the paralytic (“dumb”) form which is characterized by ascending paralysis followed by confusion and coma. Patients generally have a predominance of one form, but many affected individuals have components of both forms.
Lymphocytic Choriomeningitis Virus
Lymphocytic choriomeningitis (LCM) virus is an Old World arenavirus that can be acquired from infected house mice, hamsters, and guinea pigs. Humans become infected with LCM virus when aerosolized saliva, respiratory secretions, or urine from rodents or virus-contaminated dust are inhaled or possibly ingested. Infections occur more frequently in the winter months when rodents migrate indoors (90). The incidence of LCM is unknown but appears to have decreased substantially in the last several decades due to improvements in housing, which have resulted in less contact between house mice and humans. However, it is likely that cases continue to occur but are not recognized (91). LCM often results in a biphasic illness with an initial phase of fever, anorexia, headache, muscle aches, nausea, and vomiting. Several days later, CNS symptoms can occur with either meningitis or encephalitis. Extra-CNS manifestations may also be present, including orchitis, parotitis, arthritis, or alopecia (92,93).
Hendra Virus
Hendra virus is a paramyxovirus first recognized in Hendra, Australia where it was associated with an outbreak of respiratory and neurologic disease in horses and humans in 1994. The natural reservoir of the virus is thought to be flying foxes (bats of the genus Pteropus). The virus is transmitted from bats to horses and then transmitted to humans as a result of direct contact with infected horses. More than 60 equine and 4 human fatalities have been reported (94). The high fatality rate of this infection in horses and people, as well as the large reservoir species, underscores the potential of this virus, and other similar viruses, to emerge and cause outbreaks of severe illness. Human illness due to Hendra virus is characterized by influenza-like symptoms often followed by acute encephalitis. A relapsing neurologic syndrome has also been described in a few individuals (94).
Nipah Virus
Nipah virus, another emerging paramyxovirus, was first recognized in 1999 and associated with an encephalitis outbreak among pig farmers in Malaysia. This virus has also caused outbreaks in Singapore, Australia, Bangladesh, and India (95–97). Human infections can range from asymptomatic infection to fatal encephalitis. When neurologic illness occurs, individuals often experience influenza-like illness followed by dizziness, excess drowsiness, and altered consciousness.
Measles Virus
Measles virus infection causes acute encephalitis in approximately 1 in 1,000 cases, often resulting in permanent brain injury (see Chapter 8). Although indigenous transmission of measles was eliminated in the United States in 2000, it is still a common infection in much of the world. In addition to acute encephalitis, measles is associated with subacute sclerosing panencephalitis (SSPE), an indolent, progressive, and often fatal form of encephalitis that typically occurs 7 to 12 years after the initial infection and usually affects children between 10 and 14 years of age (98,99). A history of prior measles vaccination does not preclude the diagnosis of SSPE because an unrecognized measles infection may occur at an early age prior to immunization. This is supported by molecular studies that have identified wild type measles virus (rather than vaccine-type virus) from brain specimens of SSPE cases (100). Early clinical features of SSPE include personality and behavior changes, lethargy, decline in school performance, and hyperactivity. More pronounced neurologic manifestations such as aphasia, difficulty walking, and involuntary movements (e.g., tremors, myoclonic jerks, and choreoathetosis) later ensue. In the final stages, neurologic deterioration resulting in a vegetative state occurs in most affected patients (99).
Bacteria
While many infectious encephalitis cases have a viral etiology, bacterial causes are important to consider in the diagnosis either as a “mimicker” of encephalitis or as an actual cause of encephalitis. For example, Neisseria meningitidis and Streptococcus pneumoniae do not cause encephalitis per se but can cause clinical manifestations that are indistinguishable from encephalitis. Mental confusion, drowsiness, convulsions, and coma are not uncommon manifestations of N. meningitidis and S. pneumoniae bacterial meningitides (see Chapter 24).
Mycobacterium tuberculosis
Although meningitis is the most common form of neurotuberculosis, Mycobacterium tuberculosis was the third leading cause of encephalitis in a French study where it was identified in 15% of cases (101). In England, from 2005 to 2006, M. tuberculosis was the causative agent in 12% of encephalitis cases with an identified cause (29). Analogously, in a multicenter study of encephalitis in Taiwan, M. tuberculosis was the third most common cause of encephalitis in both pediatric and adult patients (31). Of 20 patients with M. tuberculosis encephalitis referred to California Encephalitis Project (CEP) between 1998 and 2005, many had features in common with patients with viral encephalitis, including fever (75%), altered consciousness (65%), personality change (45%), and hallucinations (16%) (102). Because the base of the brain is often affected by M. tuberculosis, signs referable to cranial nerves are often seen along with fever, headache, irritability, and drowsiness. Diffuse meningeal irritation may also result in impairment of CSF resorption with accompanying hydrocephalus (see Chapter 29).
Listeria monocytogenes
Unlike S. pneumoniae and N. meningitidis that rarely cause parenchymal brain infections, Listeria monocytogenes has tropism for the brain parenchyma itself as well as the meninges (103). The most common CNS manifestation of listeriosis is of an isolated meningitis, but approximately 10% of patients present with brainstem encephalitis, encephalitis, diffuse cerebritis, or abscess in cerebral cortex or spinal cord (104). In the French study cited earlier, L. monocytogenes was the fourth most common etiology identified (101). Reported risk factors for CNS Listeria include male gender, immunosuppression, chronic illness, and advanced age (35,105–108). Conversely, an in-depth review of Listeria rhombencephalitis found that it was reported primarily in healthy, nonimmunosuppressed middle-aged adults and affected both genders equally (109). Clinically, a biphasic disease course may be seen; a nonspecific prodrome lasting several days is typical of the first phase. The prodrome itself is similar to many viral illnesses with fever, headache, nausea, and vomiting, but the duration of prodrome is longer in CNS Listeria illnesses compared with viruses. The second phase is characterized by progressive asymmetric cranial nerve palsies, cerebellar signs (e.g., ataxia or dysmetria), hemiparesis, and altered level of consciousness (109).
Treponema pallidum
Treponema pallidum, the cause of syphilis, is yet another bacterial infection that can potentially be confused with CNS viral etiologies. Syphilis, also known as the “great mimicker,” can be difficult to recognize; this is particularly true for neurologic presentations. Neurologic manifestations can occur during any stage of the infection and include meningeal syphilis, meningovascular syphilis, paretic neurosyphilis, and tabes dorsalis (see Chapter 38).
Borrelia Species
Borrelia burgdorferi, the causative agent of Lyme disease, is primarily endemic to the eastern United States, although reservoirs are also present in the Pacific Northwest and Midwestern states. Notably, the geographic range is expanding. Lyme disease can affect both the peripheral and central nervous system; CNS involvement is typically characterized by meningitis, although encephalitis can rarely occur (see Chapter 39).
Rickettsia
Rickettsial infections can also cause encephalitis. Of the rickettsial diseases, Rocky Mountain spotted fever (RMSF) and epidemic typhus are most commonly associated with neurologic manifestations (110). In patients with RMSF, an intense headache along with restlessness, irritability, confusion, and delirium often occur. General or focal neurologic impairment including vertigo, seizures, hemiparesis, and ataxia may also be present (111). In a study of 92 children hospitalized with RMSF in the southeastern and south central United States from 1990 through 2002, 33% had altered mental status, 18% photophobia, 17% seizures, and 10% coma (112). Ophthalmic features including photophobia, conjunctivitis, petechiae of the bulbar conjunctiva, exudates and retinal venous engorgement, papilledema, and ocular palsies are frequently described (113,114). Acute temporary hearing impairment may also occur (115). Similar neurologic complications have also been described for other rickettsial infections, although generally are not as severe (116) (see Chapter 27).
Parasites and Free-Living Ameba
A number of parasites can cause encephalitis via direct invasion of the brain. Helminthes including various ascaris, hookworms, Gnathostoma spinigerum, Angiostrongylus cantonensis, Spirometra spp., Alaria spp., and others can cause larva migrans, which refers to the prolonged migration and persistence of helminth larvae in the tissues of humans (117,118). Larva migrans can result in visceral (VLM), ocular (OLM), neural (NLM), and cutaneous larva migrans (CLM) based on the organ systems involved (118). VLM and NLM are usually diseases of childhood, affecting children ages 1 to 8 years old.
B. procyonis, a common ascarid roundworm in raccoons, causes an eosinophilic encephalitis in humans and other animals. It is most commonly identified in children and, although it is rare, often results in a severe and fatal illness. B. procyonis occurs in raccoons in North America, Europe, and parts of Asia (119). More than 90% of juvenile raccoons are infected in some areas of the United States (118). Humans become infected by ingesting raccoon roundworm eggs in raccoon feces, by soil or water contaminated with raccoon feces, or via contaminated hands. Small children are particularly vulnerable to infection because of their propensity to place dirt and other objects in their mouths.
Other important neurotropic helminthes associated with eosinophilic encephalitis or meningitis includes G. spinigerum and Angiostrongylus species. Gnathostomiasis, most commonly caused by the nematode G. spinigerum, is a cause of eosinophilic myeloencephalitis. G. spinigerum is endemic in Southeast Asia and is increasingly being recognized in Central and South America (120,121). Most cases are associated with the ingestion of raw or undercooked fish, frogs, snakes, chickens, or ducks. The median time from ingestion of infected food to onset of symptoms may be several weeks to several months (122). Common early symptoms may include sporadic episodes of cutaneous larva migrans (“creeping eruption”) with localized pain and pruritus. When CNS involvement occurs, it may result in the sudden onset of radicular pain or headache. Paralysis of the extremities and loss of bladder control may also occur (123). Cranial nerve abnormalities are also described. Intermittent symptoms can occur for 10 to 15 years after exposure because the larvae are long lived (124).
A. cantonensis, the rat lungworm, is the principal cause of human eosinophilic meningitis worldwide, and although many cases are self-limiting, severe forms of the disease occur (125,126). Angiostrongylus spp. have been reported in Louisiana and Hawaii as well as the South Pacific, Asia, Australia, and the Caribbean (127,128). Humans become infected by ingestion of the third stage larvae in the molluscan intermediate host (e.g., snails, crabs, freshwater prawns) or contaminated vegetables. Following ingestion, the larvae penetrate the intestinal wall and reach the CNS via the bloodstream. Clinical illness often consists of severe headache, photophobia, meningeal signs, hyperesthesia, and paresthesia. Coma, paralysis of extremities, and seizures are seen in the severe forms of the disease (125,126). Conjunctivitis, periorbital swelling, retinal hemorrhage, retinal detachment, or blindness may occur if the eye is infected (129,130) (see Chapter 46).
Free-living ameba are ubiquitous in nature, and a few have been associated with human disease. Those causing encephalitis are generally divided into two clinical entities: (a) primary amebic meningoencephalitis due to Naegleria fowleri (also known as “the brain-eating ameba”), and (b) granulomatous amebic encephalitis.
Primary amebic meningoencephalitis (PAM) is a fulminant disease occurring in children and young adults. The disease has been reported in Australia, Europe, Asia, Africa, and North America. In the United States, most of the cases have developed in the southern tier of the country, where warm water conditions are more likely to be encountered. Typically, humans become infected with N. fowleri while swimming or washing in warm, fresh water containing the ameba. Recent cases associated with the use of “neti” pots where N. fowleri–contaminated tap water were implicated as the source of infection have also been described (131). The onset of PAM is usually within 2 to 3 days after exposure, and symptoms include severe headache, fever, stiff neck, nausea, vomiting, diplopia, seizures, behavioral changes, and coma (132–135). Distortion of taste or smell may also be a clinical feature (136,137). The case fatality rate is very high; of 111 reported cases in the United States between 1962 and 2008, only one individual survived (131).
Two different closely related ameba cause granulomatous encephalitis: Acanthamoeba spp. and B. mandrillaris. Acanthamoeba granulomatous encephalitis is an opportunistic, chronic disease that may have a prodromal period of weeks to months. Predisposing factors to acanthamebiasis include steroid treatments, autoimmune conditions, organ transplants, chemotherapy, radiation therapy, alcoholism, and pregnancy (134,138). A small number of Acanthamoeba granulomatous encephalitis cases have been described in immunocompetent children. Clinical features of CNS Acanthamoeba infections are variable but typically have a subacute to chronic presentation with fever, headache, seizures, personality change, lethargy, or confusion. Cranial nerve palsies, meningeal signs, or hemiparesis may be seen on physical exam (138). Children infected with Acanthamoeba have exhibited headache, stiff neck, vomiting, abnormal behavior, fever, ataxia, and tonic-clonic seizures (139–141).
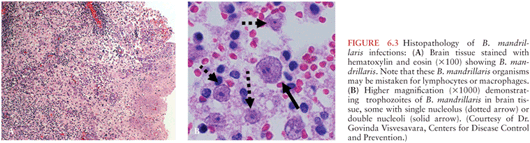
When first described, B. mandrillaris was considered to be very rare, but recent reports suggest it may be more common than previously recognized (142–145). Symptoms of Balamuthia granulomatous encephalitis include fever, headache, vomiting, ataxia, hemiparesis, tonic-clonic seizures, cranial nerve palsies (third and sixth cranial nerves), and diplopia (146). Otitis media has preceded the onset of Balamuthia granulomatous encephalitis in several pediatric cases (146,147). Hydrocephalus develops in many cases (147,148). Interestingly, two independent case reports involving Balamuthia encephalitis patients describe associated CNS aneurysms (149,150) (see Chapter 45).
Fungi
Although fungal CNS infections do not cause encephalitis per se and often manifest as meningitis and abscesses, some patients present with encephalitis-like symptoms. These illnesses are more common in immunocompromised individuals, but fungal neurologic infections can be seen in immunocompetent individuals. Important fungal causes of CNS infections in the United States include Cryptococcus neoformans, Coccidioides immitis, Histoplasma capsulatum, and Blastomyces dermatitidis. Cryptococcus gattii is an emerging fungal infection in the United States, particularly in the Pacific Northwest, and has a greater tendency to affect normal hosts than Cryptococcus neoformans (151) (see Chapter 40).
Other Agents
Hundreds of other infectious agents have been associated with encephalitis, but the frequency and significance of many of them are unknown. This is especially true when a nonneurotropic agent is found in a patient with encephalitis, particularly when the agent is identified outside the CNS. For example, Mycoplasma pneumoniae is one of the most commonly diagnosed infections among children with encephalitis (see Chapter 25). However, the significance of this association is unclear, particularly as most of the diagnoses are based on a positive immunoglobulin M (IgM) antibody to M. pneumoniae. M. pneumoniae is a ubiquitous pathogen, and there is a high background incidence of acute infection. Furthermore, there are many limitations of Mycoplasma serologic testing (152). Other similar examples of nonneurotropic agents implicated as causative agents include parvovirus B19, rotavirus, and human metapneumovirus (153–155).
Although the association of influenza viruses and encephalitis is better documented and more accepted than parvovirus B19, rotavirus, or human metapneumovirus, the mechanism by which influenza leads to neurologic illness is poorly understood. Influenza-associated encephalitis (IAE) and encephalopathy is typically characterized by a rapidly progressive neurologic illness. Cases have been described sporadically and follow the seasonal influenza pattern, with illnesses typically occurring during winter months in temperate climates. IAE is more common in the pediatric population than in adults. Evidence of neuroinvasion by influenza is rarely seen; these cases are better characterized as an encephalopathy rather than encephalitis. Many cases of IAE, especially acute necrotizing encephalopathy (ANE), have been reported from Japan, but cases of encephalitis and encephalopathy have been reported throughout the world, including the United States (156,157). Several case reports and case series describe neurologic illness associated with the pandemic H1N1 influenza virus, including encephalitis/encephalopathy (158–160).
Similarly, the neurologic illness associated with Bartonella is often an encephalopathy rather than encephalitis. Cat-scratch disease (CSD), typically caused by Bartonella henselae, is usually a self-limited infection associated with fever, regional lymphadenopathy, and malaise. As the name implies, many affected individuals have contact with a cat, often a kitten. Atypical presentations can occur especially in children and young adults and immunocompromised individuals. In a case series of 130 CSD cases in Japan, 19 (15%) had encephalopathy (161). Although lymphadenopathy is a hallmark of CSD, it is not always present in encephalopathy cases (161). Neuroretinitis can be an associated feature as well (162).
ACUTE DISSEMINATED ENCEPHALOMYELITIS
The incidence of acute disseminated encephalomyelitis (ADEM) is estimated to be 0.4 to 0.8 per 100,000 and it accounts for 10% to 15% of encephalitis cases in the United States (163–165). It is more common in children than adults, and many cases of ADEM have an identifiable trigger such as a recent illness or vaccination. Neurologic symptoms typically develop 2 to 4 weeks after the trigger with rapid progression of symptoms (165). Prior to the widespread use of vaccine-preventable diseases in the United States, measles and mumps were common triggers of ADEM. In regions of the world where vaccines are widely used to prevent measles and mumps, such as the United States and Canada, upper respiratory infections are now the most commonly identified triggers of ADEM.
ADEM affects multiple regions of the brain and spinal cord and is characterized by the rapid onset of encephalopathy along with multifocal neurologic deficits. Up to three quarters of patients with ADEM will have altered mental status, whereas seizures occur in 10% to 35% of patients (165). Motor deficits (e.g., acute hemiparesis), ataxia, decreased verbal output or mutism, cranial neuropathies, and urinary disorders are common (166). Recovery can be complete, although residual deficits can occur in up to 30% of patients.
NONINFECTIOUS ETIOLOGIES
There is growing recognition that immune-mediated conditions result in a substantial proportion of cases of encephalitis. Anti–N-methyl-D-aspartate receptor (NMDAR) encephalitis, a relatively newly recognized neuronal antibody-associated encephalitis, deserves special mention because of the seemingly high frequency of the syndrome. In the California Encephalitis Project, the frequency of anti-NMDAR encephalitis surpasses that of any single viral entity in the pediatric population and also contributes to cases in adulthood (167).
Clinically, anti-NMDAR encephalitis is characterized by abnormal behavior, seizures, and movement disorders followed by decreased level of consciousness and autonomic instability and may be associated with ovarian teratoma (168). Importantly, the development of prodromal symptoms of headache, low-grade fever, or a nonspecific viral-like illness prior to the onset of neurologic symptoms in many patients may initially suggest the diagnosis of an infectious, rather than autoimmune, encephalitis (168).
Another important cause of immune-mediated encephalitis is anti–voltage-gated potassium channel (VGKC) encephalitis. Patients are typically older than 50 years of age, present with symptoms of limbic encephalitis (memory dysfunction, behavioral changes, and seizures) and hyponatremia, and rarely have an underlying neoplasm. Although antibodies were initially thought to recognize the VGKC receptor, subsequent studies have demonstrated that the target of autoimmunity is usually a different antigen (i.e., LGI1 or CASPR2) that is tightly associated with the VGKC complex (168,169). Several other antibodies are also associated with limbic encephalitis, including those that recognize glutamic acid decarboxylase (GAD), AMPA receptor, and the γ-aminobutyric acid (GABAb) receptor. Intracellular onconeural antibodies associated with paraneoplastic conditions (e.g., anti-Hu, Yo, Ma2, CV2, amphiphysin, CRMP5, etc.) also need to be considered (170).
PATHOLOGY
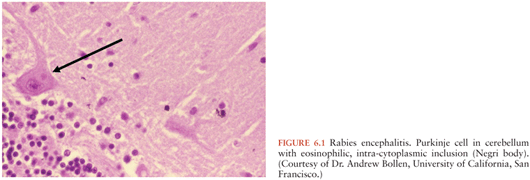
Given the many different etiologies of encephalitis, the pathology is highly variable and dependent not only on the underlying etiology but also the relative severity of the infection. The characteristic histology of patients with viral encephalitis includes perivascular mononuclear cell inflammation, phagocytosis of neurons, and microglial nodules. Distinctive characteristic histopathologic features are seen with some viral infections; intranuclear inclusion bodies are sometimes seen in herpes simplex and varicella zoster, whereas Negri bodies (eosinophilic cytoplasmic inclusions) are found within Purkinje cells and are pathognomonic for rabies (Fig. 6.1).
EVs, parechoviruses, herpesviruses, arboviruses, and rabies have well-established neurotropic potential where the virus directly invades the CNS and primarily affects the gray matter of the brain. Other viruses, such as measles and rubella viruses, primarily affect the white matter of the brain by triggering an autoimmune reaction and result in a postinfectious encephalitis (e.g., ADEM). Symptoms indistinguishable from viral encephalitis can be seen in patients with bacterial meningitis and rickettsial infections where associated vasculitis and elaboration of toxins can lead to CNS dysfunction. Intense inflammatory responses to fungi, free-living ameba, and parasites can also lead to CNS dysfunction.
Organisms enter the CNS by different routes. Most enter via the bloodstream, as is the case for EVs, HPeVs, and arboviruses as well as several bacteria, rickettsia, and fungal agents (171). Once the agent reaches the CNS, the blood–brain barrier is penetrated via the choroid plexus or through vascular endothelium (172).
The proposed mechanism for entry into brain of the amebae varies by organism. For example, Balamuthia is thought to enter the CNS via a hematogenous route, with ameba entering the bloodstream either from the lungs or from cutaneous lesions. Naegleria ameba, on the other hand, enter the nasal passages and directly extend into the CNS by penetrating the olfactory mucosa, entering the submucosal nervous plexus, migrating along the olfactory nerves, and traversing the cribriform plate. A distinct mechanism of entry via axon transport resulting in intraneuronal route is used by some viruses such as rabies and HSV-1.
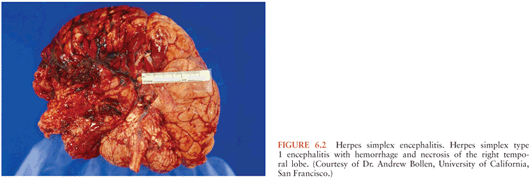
Within the CNS, the pathogen often targets specific cells and, depending on the brain region affected, variable clinical manifestations ensue (173). Agents with specific predilection to areas such as the brainstem (e.g., EV-71 and Listeria) can cause rapid decompensation with coma or respiratory failure. Herpes simplex encephalitis characteristically affect the temporal lobes and cause hemorrhage and necrotizing lesions (174) (Fig. 6.2).
WNND has a predilection for the gray matter of the brainstem and spinal cord, but cerebellum, temporal lobe, basal ganglia, and thalamus may also be affected (175). Indeed, many of the neuroinvasive flaviviruses such as JEV, TBEV, and WNV have a predilection for specific regions of the brain, including those regions important for motor control (thalamus, basal ganglia, brainstem, and anterior horn cells of spinal cord) (176).
In other instances, the infectious agents do not necessarily infect neurons. Nonneuronal cells, such as oligodendroglia, may be infected, with resultant demyelination (177). Alternatively, an infection may cause immune changes that result in damage. EBV-associated encephalitis, for example, may be a result of an immunologic phenomena rather than acute neuroinvasion. There is typically a 1- to 3-week delay in the onset of neurologic symptoms after acute EBV infection (40). Further, the virus itself is often not found in the CSF (40). In one cohort of five EBV encephalitis patients with CNS demyelination, four had prodromal symptoms for 2 weeks or more and did not have EBV detected by polymerase chain reaction (PCR) in CSF (40). Conversely, the development of neurologic symptoms either in the absence of, or within a few days of prodrome onset in the presence of, EBV in the CSF is suggestive of direct invasion. In the pediatric series cited previously, one such patient had EBV detected by PCR in brain tissue, suggesting that direct invasion of the brain may occur in some cases.
Viruses such as influenza are well known to be associated with CNS manifestations, but the mechanisms by which they cause neurologic signs and symptoms are not well understood. The lack of viral detection in most IAE cases in the CNS strongly points to a different pathogenesis; a number of potential mechanisms have been invoked, including excessive production of proinflammatory cytokines, vascular endothelial dysfunction, and mitochondrial dysregulation (178).
As discussed earlier, Listeria may be associated either with a pure meningitis or encephalitis. Pathologically, a suppurative reaction is seen in the meningitic form, whereas a granulomatous response is seen in the meningoencephalitis form (104).
Rickettsial agents invade and multiply in vascular endothelial cells, leading to vasculitis both within and outside the CNS (179,180). Vasculitis in the small vessels in the brain leads to meningeal irritation with perivascular mononuclear infiltrates. Characteristic pathologic lesions within the CNS include multifocal glial nodules and arteriolar microinfarctions (181).
In partially treated bacterial meningitis and in tuberculous and fungal meningitis, a chronic basilar meningeal inflammation can cause a subarachnoid exudate, leading to obstruction of CSF reabsorption with resultant communicating hydrocephalus and cranial nerve palsies. CNS vasculitis can also lead to infarcts and focal neurologic deficits.
N. fowleri, a free-living ameba, causes destruction of gray matter and devastation of the olfactory bulbs with purulent meningitis and pronounced brain edema (182). In Balamuthia and Acanthamoeba CNS infections, a granulomatous reaction occurs with affected areas including the cerebrum, cerebellum, and brainstem, where the amebae produce hemorrhagic necrotic lesions. Multinucleated giant cells, focal necrosis, and hemorrhage are seen in brain histopathology. In some instances, large sheets of ameba can be found in the perivascular areas of brain tissue (Fig. 6.3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree