Although the morbidity and mortality from infectious diseases declined dramatically during the 1900s related to the development and use of effective vaccines and antibiotics and a more hygienic environment, new infectious diseases have emerged, and old ones, such as tuberculosis, have reemerged. While factors influencing the balance between pathogens and host are always unique, some common factors can be described (
Table 13-1 and
Table 13-2).
Population Growth
Perhaps the most important factor in the emergence of infectious diseases has been the growth of the human population beginning in the latter half of the 1800s. The human population and subsequent human crowding had been stable for several centuries, but then increased gradually with the urbanization and concentration of the labor force with industrialization. At the end of the 1900s, an accelerated increase in the population and a dramatic growth in large urban populations occurred. The necessary sanitary infrastructure to support these changes— such as sewage disposal, water supply, and food distribution and storage—has not kept pace with them. Nearly all large cities in both the developed and developing world have substantial populations living in crowded slums. In particular, such crowding and marginal sanitary conditions have been associated with increases in infectious diseases.
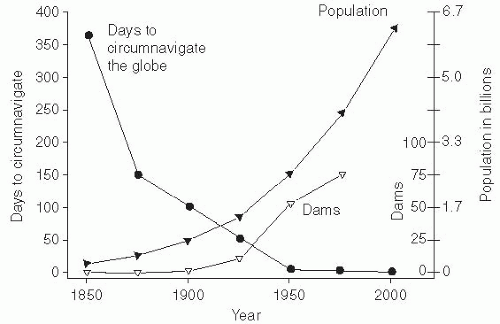
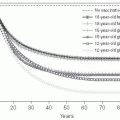
The world’s population was declared to have reached 7 billion on October 31, 2011. Most predictions are that this population increase will continue, as will the growth of megacities—those with more than 10 million inhabitants. It is estimated that the world population will increase to between 10 billion and 15 billion inhabitants and then stabilize. When it reaches 2.5 times the current population, crowding and overburdened health infrastructures would make it a challenge to control the emergence and spread of infectious diseases. Thus, limiting population growth is a key public health priority (
Figure 13-1).
Speed and Ease of Travel
Dramatic changes in the ability and ease of travel occurred in the 1900s. Airplane travel makes it possible to get from a tropical rainforest in Africa or South America to a suburban or rural area in the United States during the incubation period of Lassa fever, dengue, malaria, West Nile virus (WNV), encephalitis, and most other infectious diseases.
3 This factor has facilitated the introduction and spread of diseases from one area to another. In fact, autochthonous transmission of malaria has occurred occasionally in the temperate zone of the United States, secondary to the introduction of malaria by a visitor from an endemic area and subsequent focal transmission by local anopheline mosquitoes.
4The best example of the global spread of a new emerging infectious disease by airline travel was the severe acute respiratory syndrome (SARS) pandemic that affected 8450 persons and caused 850 deaths in 26 countries on 5 continents in 2003 before it was controlled. Also, air travel regularly effectively spreads new strains of influenza from one continent to another, typically from Asia to Australia, Europe, and North America, and then from North America to South America.
5,
6
Changes in the Food Industry
The global food supply can also be a source of infection. Fundamental changes in how food is produced have occurred over the last 100 years, such that today food is grown, processed, and delivered globally. Changes in global markets and the speed of transportation have made it economically profitable to transport food between the hemispheres—for example, enabling the U.S. consumer to have fresh fruits and vegetables on a year-round basis. The rise of agribusiness has also created extremely large farms and processing facilities. Because so much food passes through a relatively small number of facilities, the risk of cross-contamination is high. The United States also relies on a large population of migrant workers to perform manual labor on farms. These workers may come from other countries, and some may be carriers of pathogens from those countries. If proper hygiene is not maintained on the farms, these pathogens can enter the U.S. food supply.
Escherichia coli O157:H7
E. coli O157:H7 was first incriminated as the cause of infections in humans in 1982.
8 This organism has caused large-scale epidemics and sporadic cases of gastrointestinal illness in North America, Europe, and Japan.
9_16In the United States, 79,000 illnesses and 61 deaths each year are due to
E. coli O157:H7.
12 In contrast, this pathogen is not a significant problem in most developing countries.
This organism evolved from the ordinary intestinal flora of cattle by acquiring a large plasmid coding for two pathogenic traits—a hemolysin and Shiga toxin—and two traits that enhanced its survival— increased intestinal adhesion and acid stability (including survival in the human stomach).
9 Infections with
E. coli O157:H7 are markedly more severe than infections with other pathogenic
E. coli, being characterized by bloody diarrhea and, in some patients, hemolysis and acute renal failure, called
hemolyticuremia syndrome (HUS). Coupled with the fact that the infectious dose is very small (only 50 to 100 organisms), these traits gave this strain of
E. coli some of the qualities of a “super-bug.”
The
E. coli O157:H7 organism is carried without symptoms in the intestinal tract of 1% to 10% of healthy cattle and sheep in the United States and throughout the world.
11 It can be transmitted by food, raw milk, and water, as well as by direct person-toperson spread. Large outbreaks of
E. coli O157:H7 infection have occurred in the United States when hamburger contaminated during the slaughtering process was not thoroughly cooked. Contemporary food processing and distribution networks, collectively known as
corporate agribusiness, mix meat from multiple locations; as a consequence, large quantities of hamburger may be contaminated by a single cow. The cultural preference for rare steak has been carried into a preference for poorly cooked hamburger, which increases the risk of pathogens on the surface of the meat being transferred to the inner uncooked portion of the meat thereby increasing infection with this pathogen. After a large outbreak of
E. coli O157:H7 infection associated with a fastfood chain, the industry established standard cooking procedures and microbiologic monitoring of beef. No cases related to the fast-food industry have been reported since 1995.
13The
E. coli O157:H7 organism has been implicated in other food-borne outbreaks when cow manure was used as a fertilizer. The risk is highest in foods that are normally consumed raw, such as alfalfa sprouts or fruit juices. Apples used to produce cider may include those that have been contaminated by falling from the tree. Because
E. coli O157:H7 can
cause disease when the initial inoculum is very small and it is acid stable, there have been several apple juice-related outbreaks. This risk has led to recommendations that apple juice be pasteurized.
10
Escherichia coli O1O4:H4
A novel variant of
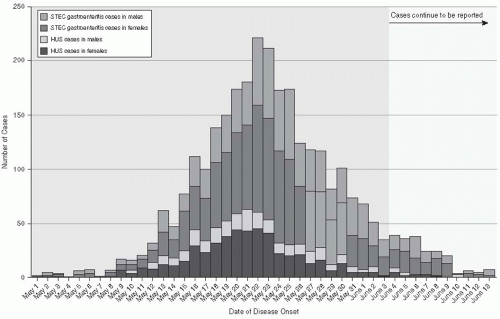
E. coli, E. coli O1O4:H4, caused a large epidemic of hemorrhagic gastroenteritis in Germany in 2011. On May 19, 2011, three hospitalized patients with acute renal failure and other symptoms of HUS were diagnosed in Hamburg, Germany. Active surveillance for acute hemorrhagic colitis and HUS identified 3222 cases and 39 deaths by June 18, 2011.
14 Of these cases, 810 (25%) involved HUS, adults (89%
5) and women (68%). The estimated median incubation period was 8 days, with a median of 5 days from the onset of diarrhea to the development of HUS. The outbreak strains were typed as an enteroaggregative strain of
E. coli that contained Shiga toxin and produced extended-spectrum beta-lactamase, conferring resistance to beta-lactam antibodies and third-generation cephalosporins (
Figure 13-2)
This outbreak differed from previous outbreaks of hemorrhagic colitis and HUS from
E. coli O157:H7 in several respects. Notably, it involved primarily adult women, whereas previous HUS cases reported in Germany and outbreak due to
E. coli O157:H7 strains elsewhere were concentrated among children. Also, at 4 to 6 days, the incubation period was somewhat longer in this outbreak than had been reported in previous
E. coli O157:H7 outbreaks. The proportion of patients with hemorrhagic colitis who developed HUS in this outbreak (25%) was also higher than in previous outbreaks (usually only 5% of cases). Importantly, enteroaggregative (EAEC) strains of hemorrhagic
E. coli were present only in humans, which indicates that the source of the outbreak was human fecal contamination, believed to be on bean and seed sprouts from an organic sprout farm in lower Saxsony.
13 In addition, human-to-human transmission likely contributed significantly to the cases in this outbreak.
14 A review of 90 confirmed outbreaks found 20% secondary transmission from an infected case,
15 highlighting the importance of secondary transmission during an outbreak.
Antibiotic treatment of patients with enterohemorrhagic
E. coli is contraindicated, because killing the organisms may cause increased release of the Shiga toxin. Instead, treatment of seriously ill patients includes hemodialysis, plasma exchange, and other strategies to remove circulating toxin. Recently, a few patients with HUS have been shown to improve after treatment with eculizumab, a monoclonal antibody to block complement complex formation.
16
Cyclosporiasis
Before 1996, most documented cases of
Cyclospora cayetanensis in North America occurred in travelers returning from overseas, and only three small U.S. outbreaks had been reported.
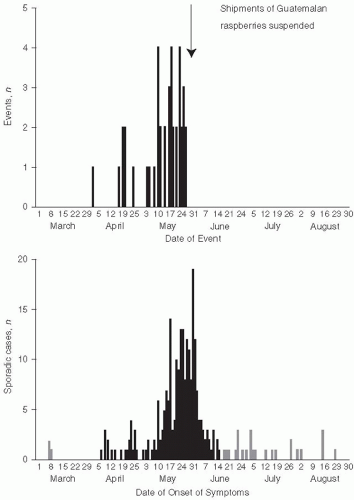
17 However, in 1998, several health departments reported cases of cyclosporiasis. Ultimately, 1465 cases, including 978 laboratory-confirmed cases, occurred in the spring and summer of 1996 and were reported to the CDC and the Canadian Health Department. Extensive investigation showed that raspberries imported from Guatemala were the source of these outbreaks.
18 Raspberries were first cultivated in Guatemala as a commercial crop in 1987, with exports markedly increasing in the mid-1990s. Another outbreak of cyclosporiasis related to Guatemalan raspberries occurred in 1997 (
Figure 13-3).
19 Although the exact mode of contamination of the raspberries is unclear, it is likely that rinsing with contaminated water prior to export occurred. These outbreaks emphasize the difficulty of controlling pathogens when a food is eaten uncooked and is not easily monitored or disinfected before eating. With the expansion of international commerce and importation of foods from many countries, this type of problem is certain to become even more frequent in the future.
Whether changes in U.S. consumer food habits— for instance, expansion of organic farming, which to date has been mostly on smaller farms, or the emphasis on “eating local”—will change the overall pattern of large outbreaks across multiple states remains to be seen. Nevertheless, some ingredients are unlikely to be affected by these factors and, therefore, large epidemics are unlikely to disappear. Furthermore, large agribusiness is better able to establish safety standards. The elimination of E. coli O157:H7 from the fast-food industry is an example of success in this sector.
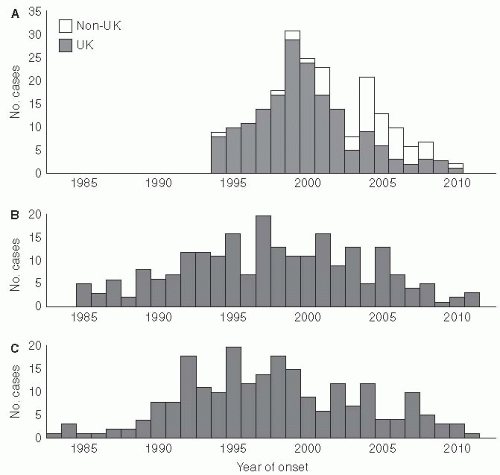
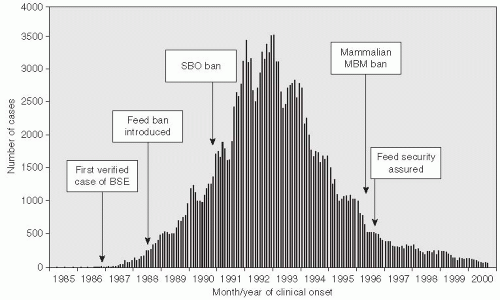
Variant Creutzfeldt-Jacob disease is an important example of a new type of foodborne illness that has affected humans (
Figure 13-4). In this situation, a change in animal feeding practices resulted in the emergence of a new disease in humans—variant Creutzfeldt-Jacob disease in the United Kingdom in 1996
21(
Figure 13-5). This human epidemic followed an epidemic of bovine spongiform encephalopathy in which nearly 1 million cattle died because of the transmission of an agent contained in their feed. The infectious agent was a member of a new class of organisms named “prions” by Stanley Pruisener, a neurologist, as they are proteinaceous infectious agents. Pruisener had earlier studied a unique epidemic among the Fore linguistic people in New Guinea involving a disease called Kuru. Kuru was spread by ritual mortuary cannibalism when the Fore consumed the remains, especially the brains, of their dead relatives to immortalize them. Pruisener found that the infectious material could be transmitted from infected humans by inoculating their infected brain material into primates. Although Kuru does not have many of the common characteristics of an infectious disease, no inflammatory response occurs in the affected brain and the infectious material does not contain nucleic acid, so it is now accepted that the transmissible spongiform encephalopathies are caused by prions.
22,
23
Antibiotic Use and Abuse
Antibiotic use in both humans and animals is a force in the emergence of resistant pathogenic microorganisms. Antibiotics are widely used therapeutically and prophylactically in humans and for growth promotion in domestic animals.
24 A recent study of
Campylobacter jejuni infections in Minnesota, between 1992 and 1998, linked human quinolone-resistant infections to the use of quinolones as a growth factor in chickens.
25 The widespread use of quinolone antibiotics in poultry feed, which began in the United States in 1995 and even earlier in Mexico, led to the emergence of these difficult-to-treat infections in humans in just a few years.
26That antibiotic resistance can develop quickly has been known since the development of penicillin. Penicillin inhibits the formation of the bacterial cell wall by interfering with peptidoglycan synthesis. Pencillinases render gram-negative bacteria resistant to penicillin. Methicillin, the next-generation antibiotic, was licensed in 1959 but methicillin-resistant
Staphylococcus aureus (MRSA) organisms had already been identified by the 1960s.
24 While methicillin is no longer in use, the name of this antibiotic is still used to describe those bacteria that are resistant to the peptidoglycan inhibitors.
In the last few decades, MRSA organisms have emerged as the gram-positive organisms that are most frequently responsible for invasive bacterial infections among hospitalized patients and those infected in the community in the United States.
27 Some of these MRSA infections are responsible for significant morbidity and mortality.
28 Originally MRSA strains were classified as either “hospital associated” or “community associated” based on epidemiological criteria—that is, where the infection was acquired— but convergence of the strains found in the two settings has made this designation no longer useful.
29 MRSA organisms can be divided into eight distinct clusters that were identified by pulsed-field gel electrophoresis, named USA-100 through USA-800.
29 Strains
USA-300 and USA-400 were classified as communityassociated MRSA (CA-MRSA), and the others were labeled as hospital-associated MRSA (HA-MRSA). All MRSA strains contain a gene coding for methicillin resistance, the mec-A gene complex, although the characteristics of the mec-A gene differ between strains. For example, strains USA-300 and -400 are similarly resistant to penicillinase-resistant antibiotics, but less frequently resistant to other antibiotics.
29 In addition, they more commonly carry a gene coding for the Panton-Valentine-leukocidin (PVL) toxin, which causes skin and soft-tissue necrosis.
In Europe, the rates of health care-associated MRSA infections are lower than in the United States.
30 However, one strain of MRSA, ST-398, has emerged in the Netherlands and Denmark as a zoonotic infection due to the incorporation of penicillinase-resistant antibiotics in pig feed for growth promotion.
31 The importance of the zoonotic reservoir of MRSA responsible for human infections in the United States has not been extensively studied.
32 This example illustrates the need for ecologic and public health-based decisions across health and agricultural industries to avoid new antibiotic-resistant human pathogens. Some studies suggest that restriction of the use of antibiotics in clinical settings might allow for the reemergence of an antibiotic-sensitive flora,
33 but such strategies have not proved entirely successful.
Dam Building
The construction of large dams to provide hydroelectric power to growing populations and irrigation for rural crops has had adverse consequences related to the emergence of infectious diseases. The number of large dams that were constructed in the United States, Asia, Africa, and elsewhere in the 1900s has increased in parallel with the population growth. The construction of such dams can displace rural and semirural populations and provide a propitious environment for the growth of mosquitoes and other vector species. The construction of the Aswan High Dam in upper Egypt, for example, was accompanied by a large expansion of the snail population and subsequently hundreds of thousands of Schistosoma haematobium infections in humans.
Expansion of Human Populations into Previously Uninhabited Forested and Suburban Areas
Not only has the number of humans increased, but their geographic dispersion has also expanded. Previously uninhabited areas may have disease cycles into which new inhabitants unwittingly intrude.
The emergence of Lyme disease, human granulocytic ehrlichiosis (HGE), human monocytic ehrlichiosis (HME), babesiosis, and Rocky Mountain spotted fever (RMSF) are linked to the growth of suburbia and recreation in tick-infested areas. In tropical Africa, the emergence of Ebola virus, Lassa fever, monkeypox, and HIV-1 and HIV-2 infections are related to the expansion of the human population into wilderness areas and increased exposure of humans to zoonotic pathogens.
Lyme Disease
Lyme disease was first recognized by Steere and colleagues in 1975, following the identification of a group of children living in Old Lyme, Connecticut, who were diagnosed with juvenile rheumatoid arthritis.
34 The geographic clustering of cases, the seasonal distribution with onset of illness in the summer, and a history of a prior distinctive skin lesion, erythema chronica migrans (ECM), eventually led to the hypothesis that Lyme disease was due to an infectious agent that was transmitted by ticks.
35 Willy Burgdorfer identified a spirochete organism, subsequently named
Borrelia burgdorferi, in the salivary glands of the tick
Ixodes scapularis on Shelter Island, New York. Lyme disease patients had immunofluorescent antibodies to this new organism, confirming its role as the causative agent of Lyme disease.
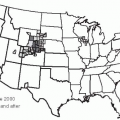
36In the 20 years since the recognition of Lyme disease, the number of reported cases has increased progressively, and the geographic areas of endemicity of the disease have expanded to include the coastal northeastern and mid-Atlantic states, several states in the Midwest (especially Minnesota, Wisconsin, and Michigan), and coastal California.
37,
38 Although education has increased awareness and diagnosis of this condition, there has also been a clear increase in the incidence of the disease.
38 Factors promoting the emergence of Lyme disease include the encroachment of human populations into areas infected with tick vectors and the growth of the deer populations, the preferred terminal host of
Ixodes scapularis ticks.
39
Other Tick-Borne Infections
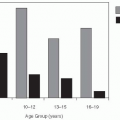
The same epidemiologic factors that led to an increase in Lyme disease have increased the incidence of other tick-borne diseases. For example, the incidence of Rocky Mountain spotted fever increased from about 200 to 400 cases per year from 1950 to 1960 to more than 1000 cases per year by 1980s.
46 Increased exposure to the disease vectors—primarily the Rocky Mountain wood tick (
Dermocenter andersonii) in the western United States and the American dog tick (
Dermocenter variabilis—in the eastern United States, occurred because of the expansion of human housing and recreation into the sylvan cycle.
Two forms of human ehrlichiosis—HME due to infection with
Ehrlichia chaffeeinsis and HGE due to infection with
Ehrlichia ewingii—have also been recognized with increased frequency in the past few years.
42 Like RMSF, these diseases are transmitted by ticks: HME by the dog tick and HGE by the deer tick. It is not unusual for a patient to be infected with Lyme disease and ehrlichiosis simultaneously.
Another tick-borne disease, babesiosis (an infection of red blood cells with
Babesia microti), can be transmitted to humans by tick bites in the endemic areas in the coastal northeastern United States.
43While Lyme disease and Rocky Mountain spotted fever are the only reportable tick-borne infections in the United States, other tick-borne illnesses have likely increased as well.
Relocation of Animals
Movement of animal species, either through intentional transportation by humans or as a reaction to changes in their habitat, can increase human contact with their associated pathogens. For example, an epidemic of raccoon rabies was recognized in the northeastern United States; it was attributable to the intentional transportation of raccoons from the South, especially Georgia, Alabama, Florida, and the Carolinas, to Virginia and West Virginia for hunting by sportsmen.
44 Unfortunately, the raccoons were from rabies-endemic areas and raccoon rabies subsequently spread throughout the northeastern United States. Potential rabies exposure from raccoons is the most common cause of human postexposure rabies vaccine prophylaxis. For reasons that are unclear, despite more than 1000 documented exposures of humans to rabid raccoons in the northeastern United States, there has been only one documented transmission of rabies.
45 This outcome may represent either a success of the vaccine program or an unrecognized species barrier between this strain of rabies and humans.
More exotic viruses have also been introduced with animal importation. Ebola virus from Philippine rhesus macaques (
Macaca fascicularis) caused an outbreak in a United States Army primate facility in Reston, Virginia.
46 In Germany, Marburg virus infected laboratory workers after exposure to infected monkeys imported from Africa.
47
Contact with Exotic Animals
Exotic pets may also introduce new pathogens to communities. Monkeypox in humans was first identified in 1970 in the Democratic Republic of the Congo.
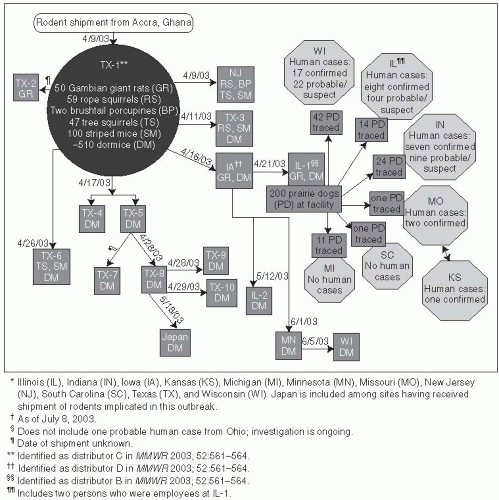
48 The causative agent is an Orthopox virus that is related to the smallpox (variola) virus and a number of other human and animal viruses. During May and June of 2003, the first cluster of human cases of monkeypox in the United States was reported.
49 The outbreak occurred across six Midwestern states—Wisconsin (39 cases), Indiana (16 cases), Illinois (12 cases), Missouri (2 cases), Kansas (1 case), and Ohio (1 case)
50—and ended in early July 2003. Monkeypox was imported to the United States with African rodents that were shipped with prairie dogs to pet stores. While rodents were the source of infection, all 71 cases of human disease resulted from prairie dog contact (
Figure 13-6).
All of the monkeypox cases were associated with prairie dogs purchased from an animal distributor in Illinois. These prairie dogs appear to have been infected with monkeypox virus through contact with Gambian rats and deer mice from Ghana. The CDC identified several animals with monkeypox from this shipment. A total of 178 (28%) African rodents could not be traced from the original shipments because records were not available. However, no cases of monkeypox were detected as a result of contact with these African rodents.
The Food and Drug Administration, CDC, and state regulatory authorities implemented several public health strategies to control this outbreak. These strategies included a joint order banning importation and movement of the implicated animal species as well as state-enacted measures to prohibit intrastate shipment and trade of the animals, establish premises quarantine, and begin animal euthanasia. The rapid diagnosis of monkeypox and coordinated public health response by CDC and FDA was effective in preventing the establishment of monkeypox as an epizootic disease in the United States. Many animals are susceptible to monkeypox, including squirrels, which are believed to be an important natural reservoir in Africa.
51 Additionally, smallpox vaccine was given to 30 persons who had been exposed to infected animals to prevent infection. One of these 30 persons developed a rash confirmed to be monkeypox within 2 weeks of immunization.
50The monkeypox epidemic emphasized, in a highly dramatic way, the potential for animal-to-human transmission of an unusual pathogen made possible by the unimpeded ability to transport animals from remote areas into the United States. The risk of introducing and spreading exotic diseases into a new environment by importation of infected animals has likely increased in the last few decades. The introduction of West Nile virus into the United States in 1999, for example, may have followed a similar pathway, although its origin has not been conclusively determined. How are future importations of new diseases to be prevented? It may not be easy to control these importations because of global trade and commerce.
Global Climate Change
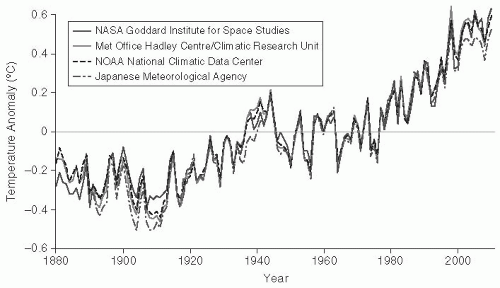
Human activity has modified the climate of the planet. Climatologic researchers believe that greenhouse gases, such as carbon dioxide, methane, and nitrous oxide, have played a major role in the climate change experienced over the past several decades (
Figure 13-7).
Often referred to as
global warming, these climactic changes are highly complex and are likely to include increases and decreases in temperatures and other changes in weather patterns. Currently, the planet is within 1°C of the previous maximum temperature, the Holocene maximum from 5000 to 9000 years ago; this suggests that further increases will result in dramatic changes in sea levels and extermination of species.
52 Global climate change might also lead to disruption in the earth’s hydrologic cycle, leading to droughts in some areas and major increased rainfall with flooding in other areas. Storm intensity is also predicted to increase. These extreme weather conditions would affect human populations in a range of ways that could increase susceptibility to new and emerging diseases. Disruptions in farming, for example, could lead to food shortages and ultimately malnutrition. Emergency situations during and after storms could promote crowding in shelters and contaminated water sources. Vector populations may be enhanced as well. Whether global warming will continue at an accelerated rate, as some have predicted, and whether this trend will lead to an expansion of infectious diseases are not certain. However, the evolving scenario has sufficient biologic plausibility that it should be taken seriously.
Several epidemics have occurred in which climate change is believed to have been an important factor. In 1991, cholera occurred in Latin America after an absence of more than 100 years.
53 Within 2 years, cholera had spread from Peru to Mexico. Theories on the emergence of cholera pointed to a ship from Asia introducing the pathogen by release of contaminated bilge water. However, the cholera outbreak in Peru occurred simultaneously all along the western coast of the country, making this theory an unlikely explanation. Subsequently, Colwell et al. reported that the recent El Niño weather phenomenon was a critical factor in the rapid spread of cholera.
53 The 1993 El Niño warmed the surface of the South Pacific Ocean, leading to an algal bloom in the surface waters along the Pacific coast of South America. Zooplankton in the algal bloom can harbor
Vibrio cholera in their exoskeleton
53 and gut, with as many as 10
4 organisms per copepod being noted. These ubiquitous copepods disseminated cholera along coastal areas of Latin America soon after its reintroduction to this region.
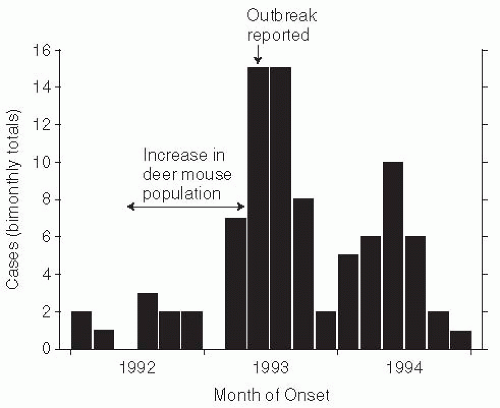
53At the intersection of the states of New Mexico, Colorado, Arizona, and Utah, known as the Four Corners area, El Niño increases in rainfall led to increases in food supply for the mouse reservoir of Sin Nombre hantaviruses.
54 The resulting outbreak of hantavirus pulmonary syndrome was believed to be the first ever, as antibody serosurveys of persons living in New Mexico were unable to document any previous infections (
Figure 13-8).
To a point, mosquitoes will benefit from increases in temperature and rainfall. Large rainfalls in Tanzania in 1997 increased mosquito vector populations and were linked to an epidemic of Rift Valley fever (RVF). At least 478 deaths occurred, and an estimated 89,000 persons were infected.
55 The relationship among RVF epidemics, rainfall, and vector expansion has been shown to be present in previous east African epidemics as well.
56 Other mosquito species, such as various
Anopheles species,
Aedes aegypti, and
Aedes albopictus, may expand their numbers or range under such circumstances, which could lead to epidemics of malaria, dengue, yellow fever, and viral encephalitis (such as Eastern encephalitis [EE]). Warmer temperatures also increase the epidemic’s potential to spread through impact on the pathogens themselves. Dengue virus and the malaria virus have shortened extrinsic incubation periods at higher temperature, which means mosquitos are infectious sooner and for a longer percentage of their lives.
57,
58
War and Societal Disruption
Nearly every major war has been accompanied by significant epidemics of infectious diseases involving both the military combatants and the civilian populations in the war-torn regions. Often, casualties from infectious disease exceed the number of deaths in battle and overshadow military factors in the outcome of the conflict.
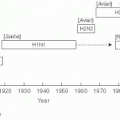
59Many examples of this interaction can be cited, but the “swine influenza” pandemic of 1918-1919 that occurred during World War I in Europe may be the most graphic one. The origin of this virus was likely a bird source in Kansas that was carried to the European theater by infected U.S. troops.
60 The resulting epidemic targeted healthy young adults, and was often rapidly fatal. The “swine influenza” was estimated to have infected 20% of the world’s population and killed more than 20 million persons worldwide and at least 43,000 U.S. military personnel. More than 80% of the American war casualties were due to influenza.
59 Germany’s General Erich Von Rudendroff blamed influenza for the defeat of the German army in the war, as more than 2000 men in each of his army divisions were ill in June 1918.
Other infectious diseases have affected troops as they are preparing to deploy or in the crowded and not always hygienic conditions of war. World War II soldiers were exposed to hepatitis B when they received yellow fever vaccine stabilized with human serum from an HBV carrier. During World War II, soldiers and civilians in the Pacific Rim experienced large epidemics of dengue. During the Soviet invasion of Afghanistan in 1979-1989, waterborne hepatitis was extremely common.
61 The causative agent was not identified until 1983, when Balyon described hepatitis E as a cause of enteric hepatitis in Asia.
62 In the first and particularly the second Gulf Wars, epidemics of visceral leishmaniasis due to
Leishmania tropica occurred in U.S. troops.
Hantaviruses
A new viral infection first came to the attention of Western medicine during the Korean War in 1951, when U.S. troops stationed in Korea developed a new disease that was subsequently named
Korean hemorrhagic fever. This disease was manifested by a febrile course with influenza-like symptoms. In approximately one-third of the cases, hemorrhagic symptoms and hypertension occurred, followed by severe renal failure. More than 3000 troops were infected, and the mortality rate was about 5%.
63 The etiologic agent was not identified until 1976, when Lee et al. working in Seoul, Korea, showed the etiologic agent to be a virus when it was isolated in Vero cell cultures.
84 The virus was named Hantan virus after the Hantan River, which transects the epidemic area at the demilitarized zone separating North and South Korea. It was isolated from the lungs of the striped field mouse,
Apodermus agrarius. This common animal is now recognized as the major rodent host of the Hantan virus in rural Korea. The rodents commonly acquire the infection early in life and continuously excrete the virus in their urine for the rest of their lives. Persons who may be exposed to infected rat urine, such as soldiers in a foxhole, are at risk of infection. There was no evidence of person-to-person spread of infection or of waterborne or foodborne transmission of Hantan virus in Korea.
Subsequently, another rodent-associated virus with some similarities to Hantan virus was isolated from urban rats (
Rattus norwegicus and
Rattus rattus).
84 Isolated cases of hemorrhagic fever were identified in urban residents and in laboratory workers who were exposed to these rats; the virus was named
Seoul virus. It has now spread worldwide owing to rodents stowed away on commercial ships. In the United States, this virus has been identified in Baltimore and has been shown in a preliminary casecontrol study to have an association with hypertension and chronic renal disease
65; however, these data are awaiting further confirmation.
Another related virus, Puumala virus, was isolated from patients in Sweden and voles in Finland. This virus caused a syndrome, termed nephropathica epidemica, consisting of an acute febrile disease with renal involvement.
66 Subsequently, WHO grouped the syndromes caused by these three related viruses together under the rubric
hemorrhagic fever with renal syndrome (HFRS).
In almost all conflicts, civilians are at increased risk of disease. Displaced war refugees are at risk for malnutrition and diseases that flourish in crowded unsanitary conditions, such as cholera, shigellosis, and malaria. The United Nations High Commission on Refugees estimates that there are currently more than 16 million refugees and 5.4 million internally displaced persons throughout the world.
67 Many of these refugees are highly vulnerable to epidemics of infectious diseases. A massive outbreak of El Tor cholera occurred among Rwandan refugees in Goma, Zaire, during the war in Rwanda, resulting in 12,000 deaths—a 14.9% case-fatality rate in July 1994.
68 This outbreak once again demonstrated the potential for cholera to cause severe mortality in a situation of extreme civil disruption. In contrast, the cholera epidemic in Latin America at the same time had mortality of less than 1% because peaceful conditions allowed for good diagnostic and medical services, including oral dehydration therapy.
69