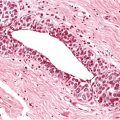
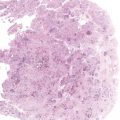
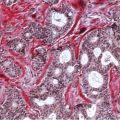
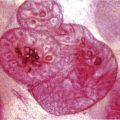
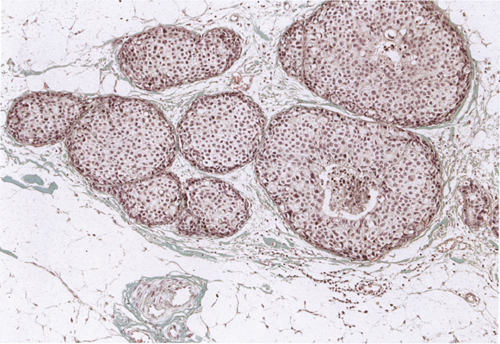
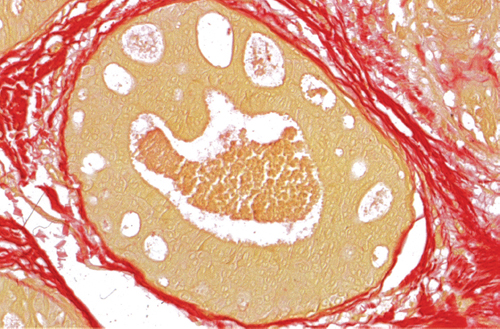
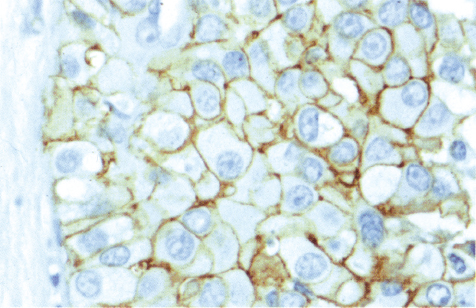
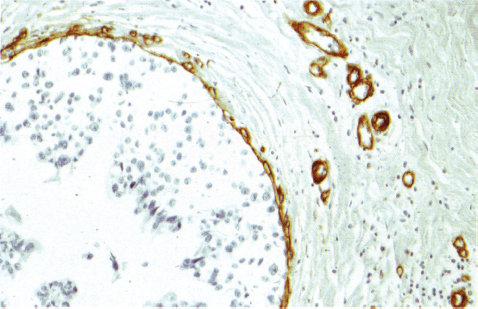
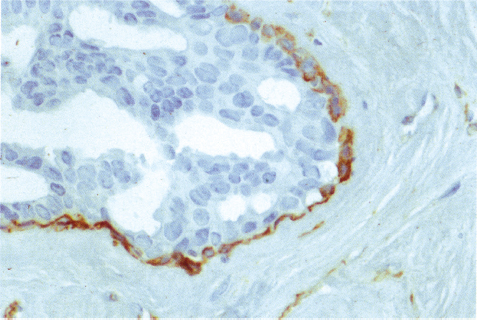
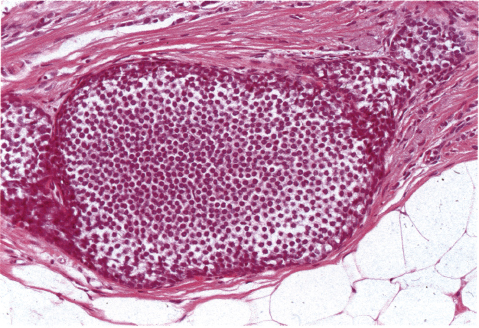
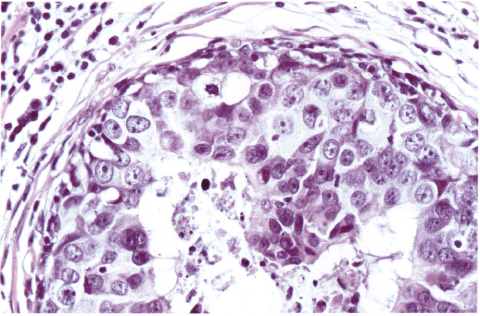
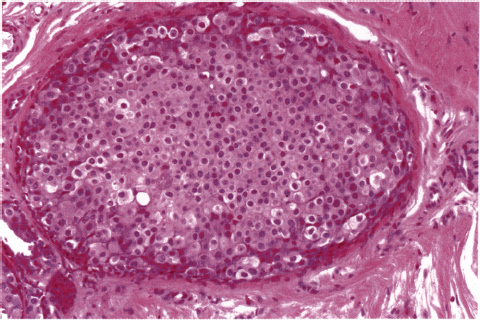
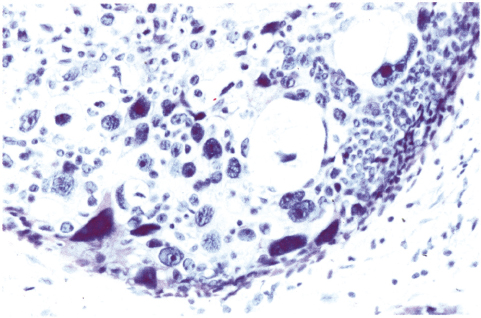
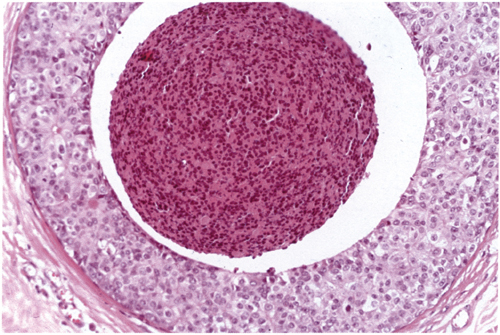
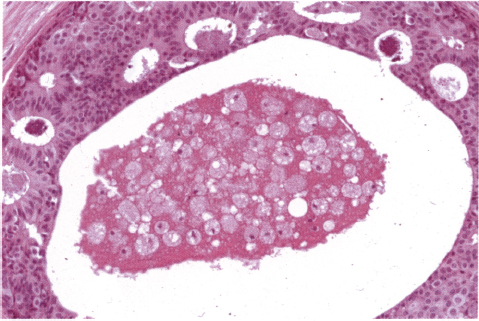
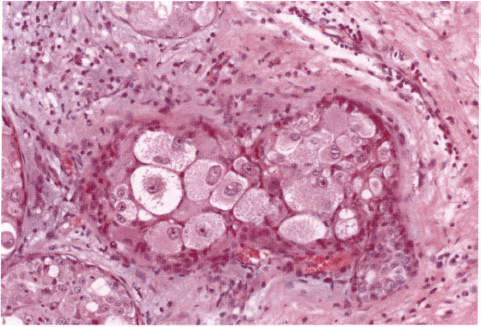
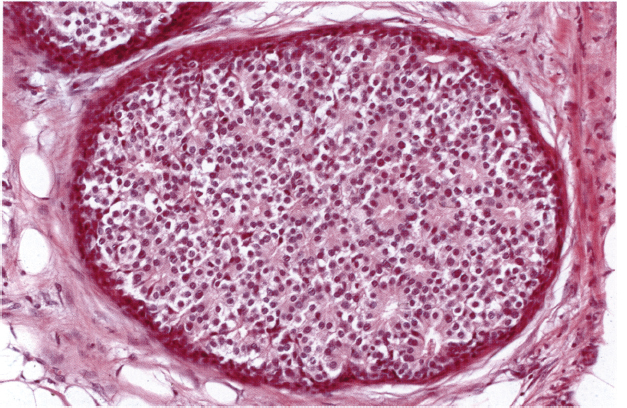
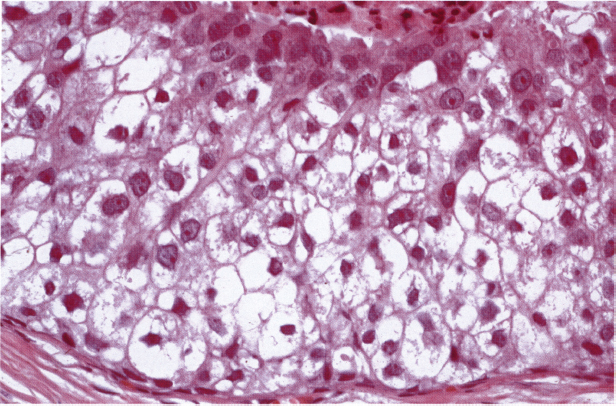
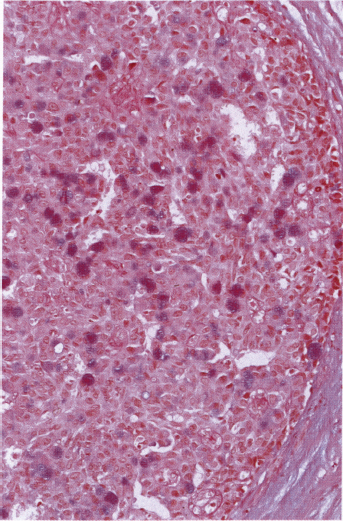
Chapter 4 Ductal Carcinoma In Situ (DCIS) Carcinoma in situ is a malignant tumor growing in spaces surrounded by an intact basement membrane. These spaces are either preformed, pre-existing ducts or lobules, or newly formed acini, lobules, or ducts (Fig. 4.1). The diagnostic criteria for carcinoma in situ are: Malignancy of the tumor cells can be illustrated immunohistochemically-for example, by staining on oncogene cerbB-2(Fig. 4.3). Fig. 4.1 The criterion of an intact basement membrane (Fig. 4.4, collagen IV stain) is more accurate for determining the invasiveness of breast carcinoma because the myoepithelial cell layer may be discontinuous or focally absent (Fig. 4.5, smooth muscle actin stain). Fig. 4.2 Fig. 4.3 Fig. 4.4 As with almost all pathologic processes in the breast, ductal carcinoma in situ (DCIS) and lobular carcinoma in situ (LCIS) initially develop in a lobule. The word “ductal” indicates a tissue differentiation other than “lobular.” “Ductal differentiation” means that the tumor cells are larger and more cohesive with a tendency to form gland-like or papillary structures. LCIS, on the other hand, contains smaller cells, often with a cytoplasmic vacuole; these cells are less cohesive and never form gland-like or papillary structures (see also Chapter 3). These characteristics allow the pathologist to delineate the two basic types of carcinoma in situ, DCIS and LCIS, in the vast majority of cases. However, DCIS itself is a heterogeneous disease that needs to be further stratified by grading and subtyping. Fig. 4.5 When grading DCIS, the most important histopathologic prognostic factor is the nuclear grade, as follows: Low nuclear grade: monomorphous, small nuclei, no or very few regular mitoses, no or few apoptotic bodies (Fig. 4.6). The nuclei are usually diploid and estrogen-receptor positive. High nuclear grade: polymorphous, large nuclei with high mitotic rate and many apoptotic bodies (Fig. 4.7). Irregular mitoses can be present. The nuclei are usually aneuploid and estrogen-receptor negative. Intermediate nuclear grade: somewhat enlarged and somewhat polymorphous nuclei with few mitoses and few apoptotic bodies (Fig. 4.8). The nuclear grade may vary considerably in the same DCIS (Fig. 4.9). In these cases, the use of the highest nuclear grade is recommended. Fig. 4.6 Fig. 4.7 Fig. 4.8 Fig. 4.9 Fig. 4.10 The second most important histopathologic prognostic factor in grading DCIS is the presence or absence of central necrosis in the lumen of the ducts and acini. This necrosis is the result of cell death, as indicated by the presence of apoptotic nuclear fragments in the necrotic debris (Fig. 4.10). Secretion in the lumen of the ducts with DCIS, which often contain degenerated cells (Fig. 4.11), must be recognized as not representing necrosis. Fig. 4.11 A simple practical grading system of DCIS is recommended, as follows: DCIS grade I: low nuclear grade without central necrosis DCIS grade II: low nuclear grade with central necrosis, or intermediate nuclear grade (with or without central necrosis) DCIS grade III: high nuclear grade (with or without central necrosis) The tumor cells of DCIS may express phenotypic variations enabling the recognition of DCIS subtypes, such as: – apocrine DCIS (Fig. 4.12) – endocrine DCIS (Fig. 4.13) – clear-cell DCIS (Fig. 4.14) – signet-ring cell DCIS (Fig. 4.15) Fig. 4.12 Fig. 4.13 Fig. 4.14 This subtyping, though morphologically simple and straightforward, has only minimal clinical value. Fig. 4.15 The growth pattern of DCIS is important to the histologist for the differential diagnosis of DCIS, benign hyperplasia, atypical ductal hyperplasia, and LCIS. The patterns are:
< div class='tao-gold-member'>
Ductal Carcinoma In Situ (DCIS)
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree