Francesco R. Simonetti, Robin Dewar, Frank Maldarelli
Diagnosis of Human Immunodeficiency Virus Infection
Human immunodeficiency virus (HIV) infection results in a progressive immune deficiency that has caused more than 30 million deaths worldwide1,2 during the past 30 years. By the end of 2011, more than 34 million individuals were living with HIV infection throughout the world.3 HIV detection methods are a cornerstone of the medical and public health response to the HIV epidemic. Accurate, sensitive, and precise assays have been designed for three general purposes: patient diagnosis and clinical management, epidemiologic surveillance, and donor screening for blood and tissue products. This chapter surveys the methods, strategies, and circumstances for detection of HIV infection.
Diagnosis of HIV infection is not simply the result of interpretation of a laboratory test but proceeds as for the evaluation of any other illness, from a careful history and physical examination to indicated laboratory studies; laboratory detection of HIV infection is a two-step process that requires sequential use of a highly sensitive screening test followed by a highly specific confirmatory assay. Evaluation of HIV infection should be conducted in a confidential fashion with voluntary participation, appropriate counseling, and informed consent of the individual. HIV testing is not a static methodology, and methods continue to undergo technologic developments in sensitivity to detect early HIV infection and in new strategies and formats for screening/confirmation. During the period 2006-2013, advances in diagnostic methods, regulatory approvals, and testing implementation guidelines have fundamentally changed the approach to HIV diagnosis in the United States. In developing countries, expansion in testing availability and acceptance of voluntary counseling and testing for HIV has resulted in increases in numbers of individuals screened. At the same time, fundamental changes have occurred in the approach to newly diagnosed HIV infection and in the use of antiretroviral therapy to prevent HIV infection as preexposure prophylaxis (PrEP) and as postexposure prophylaxis (PEP). A thorough understanding of the capability and limitations of HIV testing modalities in these and other settings is essential in establishing a diagnosis of HIV. As testing is expanded and new therapeutic approaches are implemented, health care professionals will have more choices and settings for HIV testing, and specialists will evaluate and counsel patients under a variety of circumstances. In addition, because HIV is a worldwide epidemic, physicians may evaluate patients with test results from outside their geographic locale and should be aware that recommendations on the use and interpretation of HIV testing may vary according to governing jurisdiction (Centers for Disease Control and Prevention [CDC], World Health Organization [WHO], other national agencies). Numerous screening and confirmatory tests have been developed, but only a subset has been U.S. Food and Drug Administration (FDA) approved or recommended for use by the WHO. Regardless of approval, no assay or test strategy is perfect and familiarity with assay limitations is essential to ensure accurate identification of HIV infection; misdiagnosis of HIV infection has profound consequences for patients and their contacts; and liability issues have been substantial.4,5
Background/Perspective
The current state of the field of HIV diagnosis is the product of concerted advances in laboratory techniques coordinated with progressive expansion in implementation.
Advances in Laboratory Science
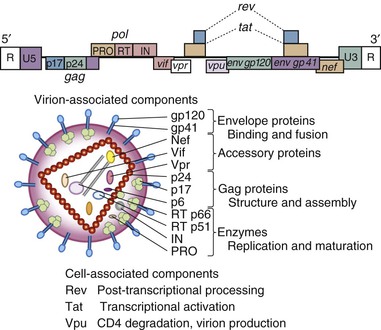
In early studies of patients with acquired immune deficiency syndrome, retrovirus infection was demonstrated by transmission of infection to cultures of susceptible cells with reproducible cytopathology associated with virus-like particles containing reverse transcriptase enzyme activity characteristic of the retrovirus family.6,7,8,9,10,11 As such, virus isolation was the first diagnostic “gold standard” of infection in patients with AIDS. Serum from patients expressing infectious retroviruses contained antibodies that recognized viral gene products,6,7,12,13 and development of infectious molecular clones of HIV14–16 permitted detailed virus characterization of the AIDS virus (Fig. 122-1). During the development of molecular techniques for HIV detection, the use of CD4/CD8 cell ratios as a marker of immunodeficiency was utilized in screening.17
Several standard laboratory procedures used to detect HIV gene products, including radioimmunoprecipitation, Western blot (WB), enzyme-linked immunosorbent assays (ELISA), and immunofluorescence, were adapted to form the earliest HIV detection systems,18 which were FDA approved in 1985; testing in blood donations revealed 0.25% of all donations were repeatedly reactive.19 Two principal technical issues of the first generation of HIV ELISA assays were (1) a high rate of false-positive results arising from a variety of clinical conditions and laboratory artifacts20 and (2) a small but important number of false-negative results resulting from the inability to detect the presence of HIV antibodies early in infection before full seroconversion (so-called window period).21,22
To address false-positive results, highly specific tests including WB, immunofluorescence, and radioimmunoprecipitation were incorporated as confirmatory assays on all repeat ELISA-reactive samples.23–25 Although all confirmatory assays are relatively labor intensive, WB procedures proved most useful, efficient, and specific; WB technique was soon established as a widely utilized “gold standard” test for confirmation and was FDA approved for use in the United States in 1987.
Technical advancements in ELISAs also improved sensitivity for early HIV infection during the window period. A second generation of ELISAs decreased false-positive rates by employing recombinant antigens and synthetic peptides instead of infected cell lysates, resulting in greater sensitivity.26–30 Introduction of an assay to detect the presence of viral antigen (p24) in 1989 was especially useful during early virus infection and improved detection of recent HIV infection31–35; p24 antigen detection kits were FDA approved in 1989.
In 1986, a second human immunodeficiency virus (HIV-2) was identified in West African natives with AIDS-like immunodeficiency who had nonreactive or unusual HIV-1 serologic results.36,37 Shortly thereafter, cases of HIV-2 were detected in the United States, Canada, and Europe38–43; changes in ELISAs to include HIV-2 antigens ensued, and combination ELISA kits were introduced in 1991. The ELISA approach was also adapted in a number of distinct formats to produce flexible, rapid tests that would be useful in resource limited settings44 and for use with other body fluids including urine and saliva.45–49
HIV testing was developed to detect HIV subtype B, the most common subtype in the United States and Europe. However, the majority of infections worldwide are non-B subtype and early-generation assays had variable sensitivity to detect these non-B subtypes; inclusion of additional antigens and peptides has improved sensitivity, such that the current versions of ELISA, WB, and nucleic acid testing (NAT) assays will detect B and non-B subtypes with the same sensitivity and specificity.
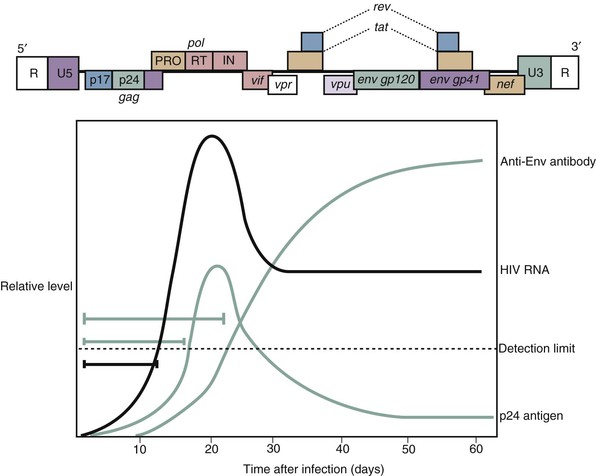
Inability to detect HIV during early infection (window period) remains a critical limitation in HIV testing.50 Methods to detect HIV p24 antigen decreased the window period and were required for blood donor screening in 1996, but they remained relatively insensitive. Improvements in ELISA approaches using “sandwich” antibody techniques resulted in greater specificity in screening and greater sensitivity in detecting antibody during the window period and ushered in a new third generation of HIV ELISA assays.51,52 Detection of HIV nucleic acid, denoted NAT, was developed for diagnosis and found to be more sensitive than third-generation ELISAs in detecting HIV during the window period.53 NAT (for HIV-1 only) was approved for use in screening plasma donors in 2001 and for use in individual blood donors in 2002. Qualitative HIV-1 RNA detection for diagnosis was approved in 2006, which reduced the window period, on average, by 6 days compared with p24 assays in seroconversion panels.54
In independent efforts to increase detection of infections during the window period, fourth-generation assays have been developed that employ a combination of third-generation ELISA and sensitive p24 antigen detection.52,55–58 The first Ab/Ag diagnostic assay was FDA approved in 2010 for detection of both HIV-1 and HIV-2.59 Although not as sensitive as NAT, these fourth-generation tests are marked improvements in detecting early infection.60,61 Fourth-generation assays perform well in detecting HIV-262 infection and in group O infection.63 Fourth-generation testing has excellent performance characteristics,64 but several studies of direct field testing reported decreased sensitivity in detecting early HIV infection. Modeling estimates that the use of fourth-generation tests is cost effective,65 although estimates suggest NAT may offer greater cost savings.66
HIV diagnosis has involved two-stage screening and confirmation strategy for more than 20 years. In the United States and many developed countries, confirmation has involved laboratory assays, typically WB or immunofluorescence assays. Dual ELISA formats have been standard practice in many developing countries with WHO oversight and have been implemented with useful advantages in rapid field diagnosis of HIV infection. Two-step algorithms to diagnose HIV infection have undergone recent progress in developed countries, with FDA approval of specific ELISA assays for confirmation. The impact of this advance remains uncertain but has great potential to change the tempo of detection as rapid testing becomes available for screening and confirmation.67
Advances in Guidelines, Recommendations, and Regulation
Implementation of HIV testing has evolved over the past 25 years, although certain goals have remained: Under the majority of circumstances, testing is voluntary and confidential. The first ELISA HIV test kits received FDA approval for use in blood donor screening in the United States in March 1985.19 All blood donations reactive in a single test were discarded, and units with repeat reactivity were considered positive for viral antibodies. Within approximately 3 months, more than 1 million units of donated blood were screened in the United States with 0.25% reported to be repeatedly reactive.19
An early challenge presented by HIV testing was expanding diagnostics beyond blood donation facilities and implementing a public health measure that was confidential, voluntary, and effective. In the United States and elsewhere, so-called alternative sites were established, independent of blood centers, and were permitted to perform voluntary and confidential HIV testing with pre- and post-test counseling procedures.68 The logistics of screening at alternative sites69 were often controversial, but mandatory testing of patient populations was rejected.
As testing procedures for blood donation expanded, variations in laboratories carrying out these evaluations were expected and test kit evaluation procedures were established with the CDC Model Performance Evaluation Program to evaluate and limit variation70,71 and worldwide under WHO auspices.72 High-performance and quality control measures were delineated,73,74 and guidelines for screening donor organs before transplantation were established by the FDA with the first interim rule in 1993 that required donor screening and record keeping. Worldwide, HIV testing of donated blood products is required in 42 of 121 participating countries, representing 66% of the world’s population.75
Rapid testing methods began in the United States with agglutination assays,44 and home collection procedures were established in 1996. The first home collection kits contained written pretest counseling and obtained dried blood spots for shipping and testing. Results and post-test counseling were provided by telephone,76 and “home testing” for HIV became FDA approved in 2012.59 Home test kits represent ELISA screening assays, which require confirmation if reactive.
As described later, recommendations for voluntary HIV testing in the United States have shifted to opt-out testing with inferred assent. Issues surrounding mandatory testing and partner notification remain areas of controversy. In general, mandatory (without consent) testing has been rejected on the basis of ethical concerns for patient rights77 outweighing the public health issues. In South Korea, where mandatory testing has been discontinued, a decrease in numbers of individuals identified was accompanied by an increase in late presentation of HIV infection (CD4 <200 cells/µL).78 Mandatory testing has been supported under governmental authority to protect the community interest against epidemic disease.79 Mandatory testing of inmates in federal corrections facilities began in 1998, and approximately 24 states have mandatory testing in state prisons.80–82 In certain circumstances, especially sexual offenses, HIV testing has been court ordered. Test results are made available to victims and in some cases to prison administrators. HIV testing of infants is mandatory in several states including New York, New Jersey, and Connecticut. Testing as a part of military recruitment is required in the United States and at least 26 other countries.77 Mandatory testing is required by a number of governments for immigrants entering as a worker, student, or temporary or permanent resident, and policies for migrants and ethnic minorities vary by country.83,84 A list of the requirements for HIV testing for travel to individual countries is maintained.85 Premarital testing is required for marriage in certain denominations.86 Regulatory bodies such as the WHO have categorically rejected mandatory HIV testing,77 and the issue remains debated.81,82,87,88
Initially, reporting of HIV infection cases for epidemiologic purposes was deemed unnecessary because the HIV-1 epidemic was tracked using AIDS case definition and mortality statistics. With the advent of effective antiretroviral therapy resulting in the dramatic decline in death and in AIDS diagnoses, the epidemic can no longer be tracked using these modalities, and HIV diagnoses represent a key marker of prevalence. In the United States, reporting takes place on state and federal levels.89 Initial recommendations were to report names in a partner notification strategy; however, concerns for privacy and stigmatization were proposed as reasons to maintain complete confidentiality with unique identifiers throughout the diagnostic process. This was referred to as “HIV exceptionalism” and contrasted with certain public health considerations.90–93 Name reporting has become standard practice in the United States; names of individuals newly diagnosed with HIV infection are reported to state health departments, where data are maintained in confidential databases; personal identifying information is removed from the dataset when the data are reported to the CDC.
In combination with strong public health commitments, HIV testing has been an essential aspect of successful measures to reduce HIV incidence throughout the world, especially in countries with high transmission rates, such as Uganda, Zambia, Cote d’Ivoire, Senegal, and Thailand.94 Reduction in transmission has not been clearly observed in all countries and is more difficult in areas with relatively low-level prevalence at baseline, such as the United States. Multiple factors contribute to the failure to reduce transmission rates; in attempts to address potential barriers to testing, the CDC launched a new initiative, “Advancing HIV Prevention: New Strategies for a Changing Epidemic.”95,96 One cornerstone of this effort is the availability of rapid/simple testing, which may be performed outside traditional health facilities. Initially, test results and counseling were delivered when serostatus had been confirmed and not based on screening assays.97 Experience with screening, poor follow-up for return visits, and the advent of rapid testing procedures prompted review of this recommendation. The Association of State and Territorial Public Health Laboratory Directors reviewed this policy and in 1998 recommended that results of screening tests be provided to patients before confirmation in circumstances where providers believe patients may benefit from the information.98 The development of convenient sampling and ELISA methods has made HIV sample collection and HIV testing outside health care facilities, so-called point-of-care testing, possible. In the United States, the introduction of rapid HIV-1 testing procedures was combined with new guidelines in 2001 to recommend screening of all pregnant women and simplification of the consent process. This was followed by additional recommendations in 2003 to make testing routine in health care settings and to use rapid testing during labor and delivery. This strategy expanded HIV testing beyond traditional health care facilities and permitted support of point-of-care, real-time HIV diagnosis. Benchmark results of these advances have been analyzed using a number of statistical approaches. The National Center for Health Statistics surveyed more than 188,000 noninstitutionalized individuals aged 18 to 64 years and asked questions about HIV testing. After an initial increase in individuals tested over the years 1987-1995, the total proportion of the population who had ever undergone testing and the rate at which individuals were tested within a year have been routinely measured over time.99 The percent of individuals ever tested for HIV increased in 2000-2011 from 36% to 45%, but the yearly testing rate has remained relatively stable at 9.6% to 10.4%,100,101 despite the promotion of rapid testing modalities and expansion of testing resources. Although the proportion of people with HIV aware of their status is about 80% in the United States,102 the majority of HIV-infected persons in Africa are unaware of their status. The United Nations estimates that only 30% of infected individuals worldwide are aware of their status.103 The risks of a large population of infected undiagnosed individuals include ongoing transmission and clinical progression.100,104 Long-term failure to identify infected patients is directly reflected in the proportion of individuals who are diagnosed with AIDS at the time of, or within a year of, HIV diagnosis and of individuals who are likely to have been infected for prolonged periods. Despite the expansion of services and new CDC recommendations in 2001 and 2003, the average proportion of all AIDS diagnoses made within 12 months of a diagnosis of HIV in 2002-2007 did not decline. More than a third of all patients presented with AIDS (38.1%, range 36% to 39%); similar data are reported in other developed and developing nations with endemic HIV where late presenters approximate 30% to 50% of new infections.105–115 Analysis of longitudinal data is useful in identifying trends in effectiveness of HIV detection. Analysis of 1997-2007 data from the North American-AIDS Cohort Collaboration revealed overall increases in CD4 count at diagnosis, suggesting some improvement in screening.116 Meta-analysis of CD4 counts at presentation117 found only a slight increase in CD4 counts at diagnosis during 2000-2011, suggesting challenges persist in testing implementation.
In an effort to reach the substantial proportion of undiagnosed individuals, the CDC revised recommendations in 2006100 to make HIV testing a part of routine screening for all individuals presenting for care, incorporating an opt-out strategy, where patients implicitly agree to testing on presenting for care; testing is done unless the patient specifically declines. Testing outside of any medical facility has been possible since the approval of home collection services in 1996 and has been expanded using home testing, approved in the United States in 2012. Opt-out testing is cost effective,118 but the effectiveness in improving diagnoses remains incompletely studied. In pilot studies, Haukoos and co-workers119 did identify an increase in diagnoses, mostly in late presenters. Additional efforts were initiated to overcome barriers to testing. Home collection for HIV testing was introduced in the United States in 1996. It required individuals to prepare dried blood spots using a lancet, ship samples for testing, and receive results by telephone; its use was not widespread.120 Introduction of HIV self-testing completed in the home was introduced in 2012 and has enabled HIV testing to become a routine over-the-counter screening event. Sampling is via oral fluid, has simple technical manipulations, and has obvious advantages of confidentiality. Early studies suggest utility of home testing in informing patient decisions,121 but cost (≈$40 to $50) has been reported as affecting willingness to test.122 Diverting test kits for partner testing121,123 may also affect sexual activity in patients at elevated risk for HIV but also represents an unintended use and highlights concerns for counseling and confidentiality. Subsequent linkage to care represents an additional challenge to home testing and highlights new roles for health care professionals in post-test counseling.
The potential for success of the 2006 “opt-out” CDC guidelines remains uncertain. Since the incidence of HIV infection in the United States has remained stable at 50,000 to 55,000 infections/year, additional increases in testing frequencies will be essential to achieve meaningful decreases in incidence. Initially, opt-out testing was at odds with guidelines and laws in several states, regarding requirements for written consent.124 Pilot programs of active screening are relatively labor intensive but have demonstrated success in identifying HIV infection.125 However, the combinations of legislative and regulatory decisions in 2011 and 2013 have completely recast HIV testing in the United States. The Affordable Care Act mandates that all new insurance plans cover, without deductible or co-pay, preventive services with a strong (“A” or “B”) recommendation from the U.S. Preventive Services Task Force (USPSTF). In April 2013, the USPSTF recommended HIV testing for all individuals from 15 to 65 years with an “A” rating. As these rulings stand, opt-out (not mandatory) testing will be performed for all individuals as part of a routine health maintenance program throughout the United States. Similar counseling and testing programs have had early success in programs in developing countries.126 Even if fully implemented, universal testing may still not provide sufficient surveillance and active surveillance programs may be required.
HIV diagnostic testing has taken on several new roles with the advent of postexposure and preexposure prophylaxis. HIV Prevention Trials Network Study 052, which evaluated the effect of immediate versus delayed initiation of antiretroviral therapy (ART) on heterosexual transmission from HIV-infected persons to their HIV-uninfected partners, found that immediate initiation of ART resulted in a 96% reduction in sexual transmission of HIV in discordant couples.127,128 The combination of tenofovir and emtricitabine is now FDA approved for use as preexposure prophylaxis. HIV diagnostic testing is an essential part of any preexposure prophylaxis strategy to determine whether the PrEP recipient is actually HIV uninfected.129 Similarly, postexposure prophylaxis strategies must incorporate appropriately timed testing strategies to ensure HIV transmission does not go undiagnosed.130,131
HIV Diagnostic Terminology and Performance Characteristics
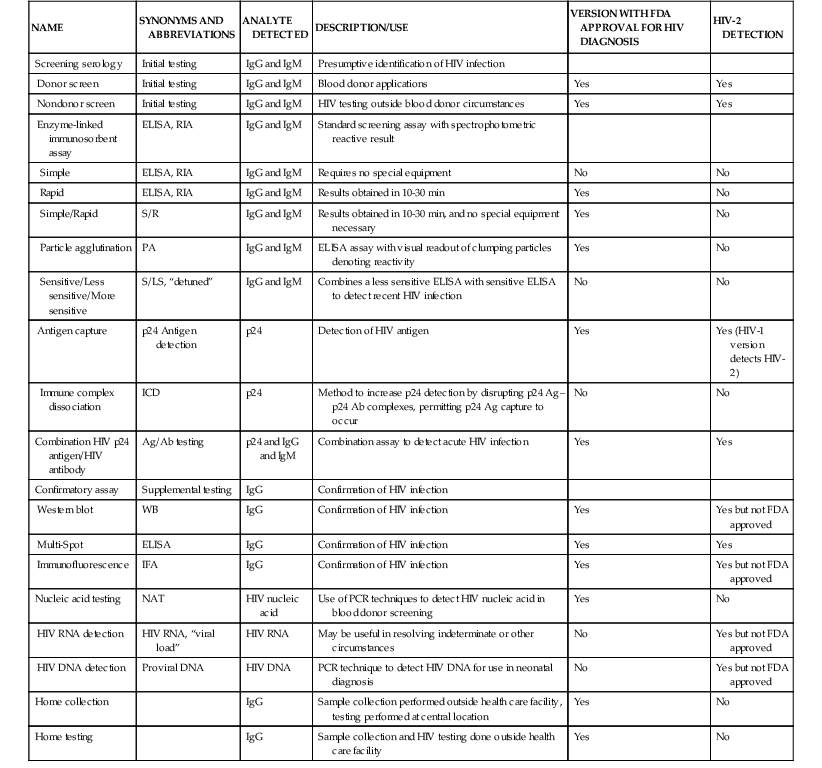
Laboratory approaches for detecting the presence of HIV infection have been developed (Table 122-1) typically for a particular application, such as blood safety, patient diagnosis, or epidemiologic surveillance.132 A number of specific terms have been applied in describing HIV testing, procedures, and results (Table 122-2). In the United States, the FDA has regulatory oversight for testing and not all modalities have FDA-approved versions. Serologic tests are broadly divided into screening assays and confirmatory tests (see Table 122-1); the qualitative HIV RNA detection assay introduced in 2006 is approved for both screening and confirmation (but not both in an individual patient). Serologic screening assays are available in a number of ELISA or p24 antigen assay formats for initial testing. Results of screening tests are considered reactive or nonreactive. In the United States, confirmatory tests consist of WB, qualitative HIV RNA determination (diagnostic test), or the Multi-Spot (Meso Scale Diagnostics, Gaithersburg, MD) ELISA assay or immunofluorescence assays; results of confirmatory tests are termed positive, negative, or indeterminate. Home sample collection of dried blood spots for HIV testing is available, as is home HIV testing. NAT has been introduced (see Table 122-1), with DNA- and RNA-based formats.
TABLE 122-2
Test Performance Definitions and Derivations
| TEST PERFORMANCE CHARACTERISTICS | DEFINITION | FORMULA |
| True HIV positive | Number of individuals who are actually HIV infected | A |
| True HIV negative | Number of individuals who are actually HIV-uninfected | B |
| False HIV positive | Number of positive HIV test results/total samples without HIV infection | C |
| False HIV negative | Number of negative test results/total samples with HIV infection | D |
| Total number of individuals with HIV infection | HIV-infected population | A + D |
| Total number of individuals without HIV infection | HIV-free population | B + C |
| Total number of positive tests | — | A + C |
| Total number of negative tests | — | B + D |
| Sensitivity | Number of positive test results/total samples with HIV infection | A/A + D |
| Specificity | Number of negative test results/total samples without HIV infection | B/B + C |
| Positive predictive value | Proportion of those with HIV-positive assay who are actually HIV infected | A/A + C |
| Negative predictive value | Proportion of those with HIV negative assay who are actually HIV uninfected | B/B + D |
| Test efficacy | Test performance under ideal conditions | |
| Test effectiveness | Test performance under practical conditions | |
| Number of persons with HIV infection who test positive | — | A |
| Number of persons without HIV infection who test negative | — | B |
| Number of HIV-positive test results and not infected | — | C |
| Number of HIV-negative test results with the disease | — | D |
| Total number of persons | — | A + B |
HIV, human immunodeficiency virus.
No test is perfect, and assay imperfections are quantitated using a number of characteristics: sensitivity, specificity, false-positive rate, and false-negative rate. For assays of HIV infection, both false-negative rates and false-positive rates have profound implications. Sensitivity (see Table 122-2) relates how many infections are missed by testing; inadequate sensitivity has great impact both in blood surveillance and in individual diagnosis; in both circumstances inability to detect infection potentially exposes others to infection and misses critical opportunities for counseling and therapy. Specificity (see Table 122-2) describes the proportion of uninfected individuals who test negative for HIV. Decreased specificity results in false-positive findings, prompting profound patient distress and extensive additional evaluation. Decreased specificity compromises HIV testing from a patient diagnosis (fear of positive testing) and public health standpoint (inadequate description of the epidemic, unnecessary use of resources). In general, the optimization of any test ultimately pits sensitivity at odds with specificity; the greater the sensitivity of a test, the more likely the possibility that false positives may occur, whereas increasing specificity results in increased numbers of false negatives (Fig. 122-2). In order to maximize both specificity and sensitivity for detection of HIV infection, a single test is inadequate. Instead, a sequential strategy has been designed. Testing is initiated with a highly sensitive ELISA screening (>99.5% sensitive); in this phase, false positives may be significant (1% to 10%). The screening phase is followed, however, by a highly specific confirmatory test (>99.5% specific), and the initial false positives are excluded. As a result, HIV testing has the highest level of sensitivity and specificity of any medical diagnostic procedure.