Abstract
Pleural metastases and malignant pleural effusion may occur with metastatic breast cancer. Presentation can vary widely from an incidental finding on imaging to a large effusion with severe dyspnea. Any pleural effusion in a breast cancer patient can be suspected to be a malignant effusion until proven otherwise. The focus of the clinician should be to provide the most efficient, accurate diagnosis with the least risk of complication and pain for the patient. This chapter reviews diagnosis and management of malignant pleural and pericardial effusions.
Keywords
pleural effusion, pericardial effusion, metastasis, palliation
Pleural metastases and malignant pleural effusion may occur with metastatic breast cancer. Presentation can vary widely from an incidental finding on imaging to a large effusion with severe dyspnea. Any pleural effusion in a breast cancer patient can be suspected to be a malignant effusion until proven otherwise. The focus of the clinician should be to provide the most efficient, accurate diagnosis with the least risk of complication and pain for the patient. Overall, malignant pleural effusions account for approximately 20% to 25% of all effusions. Infectious or postinfectious etiologies are the most common cause of exudative effusion and pleural malignancies, both primary and metastatic, are the second leading cause of exudative pleural effusions, estimated to number approximately 150,000 annually in the United States. Breast cancer is second only to lung cancer as the leading cause of all pleural metastases and thus accounts for approximately one-fourth of all malignant effusions. In women, it is the most common cause of a malignant effusion, accounting for up to 40%. In this chapter, we review the magnitude and presentations of pleural metastases and malignant pleural effusion (MPE) in breast cancer, their biochemical profiles, and methods of diagnosis and management. A similar related clinical entity, malignant pericardial effusion (MPCE), is also covered.
The estimated incidence of malignant pleural involvement in breast cancer ranges from 2% to 12%. Although cancer cell–positive effusions have been noted as the initial presentation of a malignancy, this is not commonly reported as the initial diagnosis of breast cancer. Conversely, malignant breast cancer pleural metastases are a common initial presentation of disease progression or recurrence, occurring in 42% to 43% of patients. Overall, in advanced breast cancer, pleural metastases are a common occurrence and are found in 36% to 65% of advanced disseminated diseases. The time of initial breast cancer diagnosis to the development of malignant pleural involvement varies but averages 35 to 42 months. The contribution of malignant pleural involvement by breast cancer toward the overall mortality and morbidity of the disease depends on the number of metastatic disease sites, total tumor burden within the pleural space, and underlying pulmonary reserve of the patient.
Pathogenesis
Although MPE can be a significant problem for those affected, the details of its pathogenesis are not clear. It appears to be a combination of factors leading to an overall increase in pleural fluid production that overwhelms its removal, thereby causing an accumulation manifest as MPE. Interestingly, pleural effusion does not occur in every patient with pleural metastases. Current research has shed light on the fact there may be certain genetic characteristics or “secretomes” carried by these tumors that do cause effusions. Tumor cells may produce vascular endothelial growth factor (VEGF) along with a host of concomitant factors. These factors interact with inflammatory cells in the mesothelium and endothelium leading to capillary leak into the pleural space that overwhelms the lymphatic system’s ability to reabsorb. There is also some thought that direct tumor invasion of the lymphatics may disrupt this drainage system as well.
Clinical Presentation
Symptoms associated with breast cancer pleural metastases may be related to local and systemic effects. Unlike primary pleural tumors, metastatic cancers to the pleural space, including breast cancer, rarely present as bulky metastases without pleural effusion. Dyspnea is, in general, the most common presenting symptom and is often related to the size of the pleural effusion. However, up to one-fourth of patients may be asymptomatic at presentation.
Pleural metastases from breast cancer result in dyspnea by causing a restrictive pulmonary physiology and gas-exchange abnormalities. The incompressible pleural fluid collection and the limited outward chest wall excursion result in compression and atelectasis of the underlying lung parenchyma. The reduction in vital capacity reduces the effective gas exchange. Although the pulmonary circulation has an adaptive hypoxic vasoconstrictive response, this is incompletely effective when there is a large effusion and when an atelectatic lobe or lung leads to significant shunting and ventilation/perfusion mismatching. Other causes of subjective dyspnea without significant hypoxemia include mediastinal shifting and reflex stimulation of the chest wall and lungs as a result of altered compliance ( Box 72.1 ).
Atelectasis of lung parenchyma resulting from malignant pleural effusion
Inflammatory pleurisy and chest wall pain leading to splinting
Lung parenchymal metastases
Pneumonia obscured by effusion
Pulmonary thromboembolism
Pneumonitis secondary to radiation
Left ventricular dysfunction leading to pulmonary edema
Cardiotoxicity due to antineoplastic agents
Malignant pericardial effusion and tamponade
Anemia secondary to advanced cancer or antineoplastic therapies
Aside from the aforementioned causes of dyspnea, other concomitant factors may contribute to breathlessness. Anemia reduces the oxygen-carrying capacity and systemic oxygen delivery and may induce a hyperdynamic cardiac response and strain. Patients are generally older and may have underlying degrees of congestive heart failure, which is often manifested as dyspnea. Risks of pulmonary thromboembolism are increased secondary to hypercoagulable states of cancers, effects of hormonal therapy, and the decreased mobility of many cancer patients. Pulmonary embolism (PE) is listed as the fourth most common cause of pleural effusions by some authors and must be considered in the differential diagnosis of a “paramalignant effusion” ( Table 72.1 ). One-fourth of PE-associated effusions may be transudative, but three fourths of them are exudative and may confound the diagnosis of a breast cancer–associated malignant effusion.
| Cause | Transudates | Exudates |
|---|---|---|
| Congestive heart failure from all causes, especially bilateral effusions | ++++ | Rarely |
| Parapneumonic effusions | — | ++++ |
| Pulmonary thromboembolism | +++ | + |
| Postobstructive atelectasis | ++++ | — |
| Hypoalbuminemia resulting from cachexia | ++++ | — |
| Associated with ascites, malignant or cirrhosis | +++ | + |
| Chylothorax resulting from thoracic duct obstruction | — | ++++ |
| Mediastinal adenopathy, including compression of pulmonary arteries | + | +++ |
| Superior vena cava syndrome | ++++ | — |
| Status post chest wall or mediastinal radiation | — | ++++ |
| Drug-induced pleural reactions (bleomycin, cyclophosphamide, methotrexate, mitomycin, procarbazine) | — | ++++ |
Malignant pericardial involvement should be suspected in a patient with persistent dyspnea after drainage of MPE. The patient with an MPCE may or may not have hemodynamic instability. The true incidence of MPCE associated with all breast cancer patients is unclear, but it has been reported as high as 19% in an autopsy series. Of the subgroup of patients with known metastatic pleural breast cancer with pericardial spread of disease, 63% to 100% also have lung and pleural metastases at the time of MPCE diagnosis. These patients with both breast MPE and MPCE appear to have a higher frequency of bilateral malignant effusions. Dyspnea may also be a result of antineoplastic therapies. Pulmonary parenchyma can be quite radiosensitive, and radiotherapy directed against breast cancer may scatter and lead to subacute radiation pneumonitis and delayed fibrosis with restriction. Some cytotoxic chemotherapy agents have associated pulmonary toxicities, or cardiotoxicity. In addition to intrinsic lung dysfunction, cytotoxic and radiation-induced immunosuppression with concomitant structural lung damage may predispose patients to pulmonary infections. In a clinicopathologic review of the pattern of metastatic diseases and cause of death in breast cancer patients, the pulmonary system is the number one or two site of metastases, and infections account for about one-fourth of the deaths.
Other nonspecific chest symptoms attributable to pleural metastases often include cough and, much less commonly, pleurisy and chest wall pain. Tachypnea, tachycardia, and cyanosis may be related to impaired gas exchange and hypoxemia. A large pleural effusion may transmit increased pressure to the pericardium and rarely cause a tamponade-like effect; in general, however, hypotension is not expected. Likewise, fever and hemoptysis should prompt a search for concomitant processes such as empyema, pneumonia, sepsis, pulmonary thromboembolism, or endobronchial metastases.
Diagnosis
Radiographic Findings
Plain chest radiography is the most common radiographic means of identifying malignant pleural involvement. There is opacification of various extent of the hemithorax, ranging from blunting of the costophrenic angle on the frontal view and posterior gutter on the lateral view to complete opacification of the hemithorax, with or without a midline shift of the mediastinal structures. Decubitus films to confirm a free-flowing liquid layer may be used to follow up suspicious blunting without the classic meniscus fluid sign. With the ready availability of computed tomography (CT) scans that provide superior discrimination of tissue versus fluid, advanced three-dimensional imaging can help direct diagnosis and treatment. Contrast enhancement of the parietal pleural is useful in separating exudative from transudative effusions. More specific features of malignant pleural involvement include pleural nodularity and irregularity and pleural thickness greater than 1 cm. Pleural surfaces thus assessed with CT scanning have a sensitivity of 87% and specificity of 100% for malignant involvement, although the sensitivity is lower for metastatic cancers versus primary pleural cancers. A large pleural effusion normally shifts the mediastinum away toward the contralateral chest, sometimes causing critical compression of vascular and conducting airway structures. Therefore a midline undeviated mediastinum or even ipsilaterally deviated mediastinum in the presence of a large effusion suggests central airway obstruction leading to complete atelectasis of the lung. This should prompt an airway examination to look for endobronchial obstruction resulting from tumor or volume loss resulting from inspissated mucus. Ultrasound can complement chest films and/or CT scans to guide bedside sampling and drainage of fluid pockets, especially when these are small or may have become loculated, or when the patient physiology and physiognomy such as chronic obstructive pulmonary disease and obesity will increase the risk of complications from thoracentesis.
Most metastatic pleural effusions from breast cancer are unilateral and arise in the hemithorax ipsilateral to the initial site of disease 50% to 83% of the time. Although bilateral pleural effusions have usually been attributed to left ventricular dysfunction and congestive heart failure, up to 10% of patients may have bilateral malignant effusions. It should be qualified that these studies are from the era before CT scans were routinely used to assess disease progression or to identify pleural pulmonary involvement. The routine use of CT scanning may detect many smaller pleural effusions. Distribution of metastatic implants on the pleural surfaces have been studied in vivo during diagnostic and therapeutic thoracoscopy, and this has demonstrated that a majority of the visible lesions stud the visceral pleura and the parietal pleura. Given the differential blood supply and lymphatic drainage of the visceral and parietal pleural surfaces, this suggests that most pleural metastases occur in combination with and perhaps subsequent to hematogenous and lymphangitic spread of disease to the lungs. Canto-Armengod observed a preponderance of ipsilateral pleural metastases studding the costal pleura, whereas metastases to the contralateral pleura more commonly affect the mediastinal pleura.
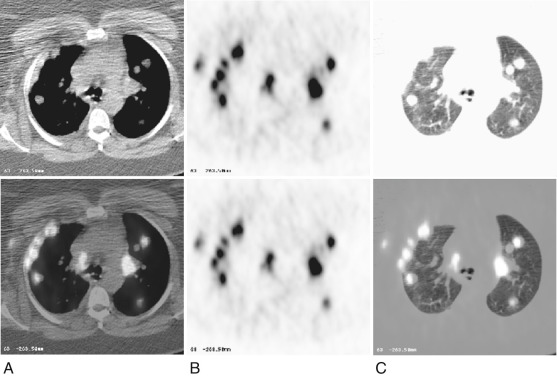
18-Fluorodeoxy glucose positron emission tomography (FDG-PET) is accepted as an effective metabolic imaging adjunct to help characterize abnormal-appearing tissue as likely being neoplastic, inflammatory, or benign. FDG-PET has high sensitivity and specificity rates in the imaging of primary pleural cancers, as in mesothelioma. FDG-PET is superior to CT alone scanning in the detection of pleural metastases in primary bronchogenic carcinoma, with a sensitivity approaching 90% and a specificity and accuracy of 94.1% and 91.4%, respectively. In terms of breast cancer, FDG-PET has been used for imaging and staging of primary breast cancer, although the results for axillary and mammary nodal staging vary and depend on nodal size and the tumor proliferation index. In its evaluation of disseminated metastatic disease in breast cancer, FDG-PET is at least as effective as Tc 99m -MDP bone scanning, but with regard to the pleural space, although there are case reports, its efficacy in detecting metastatic disease has not been formally evaluated.
An example of FDG-PET positive pleural, lung parenchymal, and extrathoracic soft tissue metastases in a breast cancer patient is presented in Fig. 72.1 . The use of PET-CT scanners with fused PET-CT imaging facilitates the localization of pleural metastases and help distinguish these from metastases to the lung periphery, chest wall osseus, and soft tissue structures.
Tissue Confirmation
Thoracentesis and Studies on the Pleural Fluid
The radiologic advances outlined earlier have greatly improved our ability to detect earlier and characterize the extent of possible pleural metastases from breast cancer. However, the broad differential of possible paramalignant effusions generally makes it necessary to obtain a tissue diagnosis to confirm regional spread of disease before proceeding with appropriate regional and/or systemic therapy.
Because the presentation of malignant pleural metastases from breast cancer is most often a pleural effusion ( Fig. 72.2 ), symptomatic or otherwise, the initial diagnostic step is removal and analysis of fluid for diagnosis and as needed for the relief of symptoms. Thoracentesis can be performed “blind,” without real-time radiologic guidance, after review of a chest film or CT scan; however, beside ultrasonography by physicians can be a valuable tool, especially in patients with smaller or loculated effusions. The diagnostic yield of pleural fluid cytology varies and ranges from 40% to 90%, depending on the tumor type, tumor burden, and number of thoracentesis performed. Breast cancer appears to have a higher pleural cytologic yield than metastatic lung cancer. There is debate as to whether a large volume of fluid improves diagnostic yield; practice currently varies from sending only 10 to 20 mL to more than 1 L. Furthermore, local laboratory practice differs as to whether only a small aliquot is processed as a smear or a cytospin slide or whether a larger volume is processed into a cell block. There is evidence that the preparation of a cell block increases the diagnostic yield from 11% to 38%. An additional advantage of a cell block is having additional material available for immunostaining. Routine tests performed on the pleural fluid include chemistries to distinguish an exudate from a transudate. Glucose, pH, lactate dehydrogenase (LDH), and cultures may be ordered to rule out an infected or complicated postobstructive parapneumonic effusion, one of the several causes of a paramalignant effusion (see Table 72.1 ). A malignant effusion with low glucose, low pH, and a high LDH generally portends a worse prognosis, but it should not discourage the clinician from attempting to drain and sclerose the affected space for palliation.

Immunohistochemistry
Given the rich source of tumor markers associated with adenocarcinomas, and markers with greater specificity for breast cancer in particular, there have been ongoing attempts to improve on the diagnostic sensitivity and prognostic value of identifying a malignant breast effusion with such molecular markers. The 2007 American Society of Clinical Oncology (ASCO) recommendations for the use of tumor markers include the measurement of steroid hormone receptors (estrogen and progesterone receptor status) and HER2/neu status of the primary tumor. Although metastatic effusions from breast cancer also have a high frequency of positive estrogen receptor (ER) and progesterone receptor (PR) staining (72% and 52%, respectively), so do ovarian metastatic effusions, thus limiting the specificity of ER and PR staining.
Biomarkers of tissue proliferation have also been looked for in suspicious pleural fluid, both as an aid to diagnosis and, perhaps in the future, as a target for specific therapy. Ki67 is a human nuclear antigen present in cycling but not resting cells, and positive immunohistochemical labeling of suspicious but cytologically negative effusions may obviate more invasive surgical procedures. VEGF has been found and measured in various malignant serous effusions, including pleural effusions, and reaches levels 10 times higher than that in matched sera. The use of anti-VEGF neutralizing antibodies in in vitro systems point the way toward possible future targeted therapy.
Tissue Biopsies
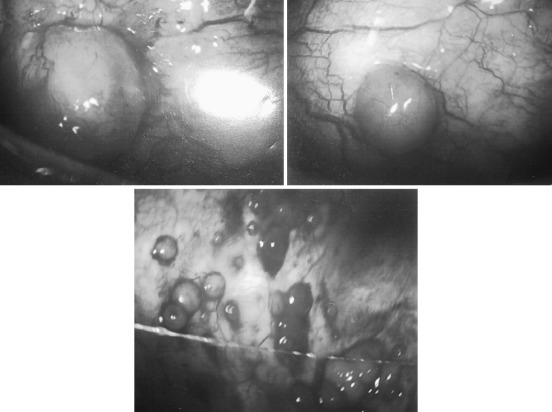
Pleural needle biopsy with a variety of needles had been performed “blind” after confirming entry into a pocket of pleural fluid with a finder 21-gauge or smaller needle or directly with ultrasound. The yield of pleural needle biopsy is generally lower than that provided by fluid cytology. Unlike diffuse granulomatous inflammation, sampling the patchy pleural metastases, and especially the finding of predominantly visceral pleural implantation ( Fig. 72.3 ), including the inaccessible mediastinal pleural surfaces, may explain the low additional yield. Given the potential risk of parenchymal lung puncture, occasional life-threatening bleeding, and the availability of more accurate minimally invasive image-guided procedures, blind pleural needle biopsies are generally of historical interest and unwarranted.
Surgical approaches for tissue diagnosis may be required when repeated thoracentesis with or without image-guided pleural biopsy fails to provide a tissue confirmation or when the initial presentation suggests a multiloculated complex malignant effusion. Thoracoscopic approaches, either with simple single-entry thoracoscopy with the patient under local anesthesia and conscious sedation or video-assisted thoracic surgery (VATS) with the patient under general anesthesia, have 86% to 100% diagnostic accuracy in diagnosing a malignant pleural effusion. Confirmation of malignant pleural involvement based on histologic examination of fresh-frozen tissue or the appearance under visual examination may expedite long-term management of the involved pleural space by pleurodesis at the end of the case, either by mechanical or chemical means.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree