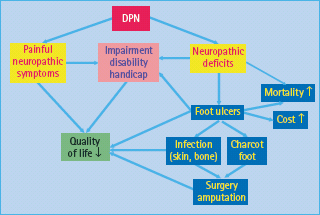
- Approximately one in three people with diabetes is affected by distal symmetric polyneuropathy (DPN), which represents a major health problem as it may present with excruciating neuropathic pain and is responsible for substantial morbidity, increased mortality and impaired quality of life.
- Neuropathic pain exerts a substantial impact on quality of life, particularly by causing considerable interference with sleep, daily activities and enjoyment of life.
- Treatment is based on four cornerstones: (1) intensive diabetes therapy and multifactorial risk intervention; (2) treatment based on pathogenetic mechanisms; (3) symptomatic treatment; and (4) avoidance of risk factors and complications.
- Recent experimental studies suggest a multifactorial pathogenesis of diabetic neuropathy. From the clinical point of view, it is important to note that, based on these pathogenetic mechanisms, therapeutic approaches could be derived, some of which are currently being evaluated in clinical trials.
- Management of chronic painful DPN remains a challenge for the physician and should consider the following practical rules: the appropriate and effective drug has to be tried and identified in each patient by carefully titrating the dosage based on efficacy and side effects; lack of efficacy should be judged only after 2–4 weeks of treatment using an adequate dosage. Analgesic combination therapy may be useful, and potential drug interactions have to be considered given the frequent polypharmacy in people with diabetes.
- Epidemiologic data indicate that not only increased alcohol consumption but also the traditional cardiovascular risk factors such as visceral obesity, hypertension, hyperlipidemia and smoking have a role in the development and progression of diabetic neuropathy and hence need to be prevented or treated.
Classification and epidemiology
Diabetic neuropathy has been defined as a demonstrable disorder, either clinically evident or subclinical, that occurs in the setting of diabetes without other causes for peripheral neuropathy. It includes manifestations in the somatic and/or autonomic parts of the peripheral nervous system [1] which are being classified along clinical criteria; however, because of the variety of the clinical syndromes with possible overlaps there is no universally accepted classification. The most widely used classification of diabetic neuropathy proposed by Thomas [2] has subsequently been modified [3]. This proposal differentiates between rapidly reversible, persistent symmetric polyneuropathies and focal or multifocal neuropathies (Table 38.1). Diabetic distal symmetrical sensory or sensorimotor polyneuropathy (DPN) represents the most relevant clinical manifestation affecting approximately 30% of community-based people with diabetes [4]. There is emerging evidence to suggest that intermediate hyperglycemia is associated with an increased risk of DPN. In the general population living in the region of Augsburg, Southern Germany, the prevalence of DPN was 28.0% in subjects with diabetes, 13.0% in those with impaired glucose tolerance (IGT), 11.3% in those with impaired fasting glucose (IFG) and 7.4% in those with normal glucose tolerance (NGT) [5]. The incidence of DPN is approximately 2% per year.
Table 38.1 Classification of diabetic neuropathies. Adapted from Sima et al. [3].
1 Rapidly reversible Hyperglycemic neuropathy 2 Persistent symmetric polyneuropathies Distal somatic sensory/motor polyneuropathies involving predominantly large fibers Autonomic neuropathies Small-fiber neuropathies 3 Focal/multifocal neuropathies Cranial neuropathies Thoracoabdominal radiculopathies Focal limb neuropathies Proximal neuropathies Compression and entrapment neuropathies |
The most important etiologic factors that have been associated with DPN are poor glycemic control, visceral obesity, diabetes duration, height, hypertension, age, smoking, hypoinsulinemia and dyslipidemia [4]. The clinical impact of DPN is illustrated in Figure 38.1. DPN is related to both lower extremity impairments such as diminished position sense and functional limitations such as walking ability [6]. There is accumulating evidence suggesting that not only surrogate markers of microangiopathy such as albuminuria, but also those used for DPN such as nerve conduction velocity and vibration perception threshold (VPT) may predict mortality in people with diabetes [7,8]. Elevated VPT also predicts the development of neuropathic foot ulceration, one of the most common causes for hospital admission and lower limb amputations among patients with diabetes [9].
Pain is a subjective symptom of major clinical importance as it is often this complaint that motivates patients to seek health care. Pain associated with diabetic neuropathy exerts a substantial impact on quality of life, particularly by causing considerable interference in sleep and enjoyment of life [10]; however, in one UK survey, only 65% of patients with diabetes received treatment for their neuropathic pain, although 96% had reported the pain to their physician [11]. Pain treatment consisted of antidepres-sants in 43.5% of cases, anticonvulsants in 17.4%, opiates in 39% and alternative treatments in 30%. While 77% of the patients reported persistent pain over 5 years, 23% were pain-free over at least 1 year [11]. Thus, neuropathic pain persists in the majority of patients with diabetes over periods of several years.
Chronic painful DPN is present in up to 26% of patients with diabetes [11–14]. As well as the high prevalence of painful neuropathy among people with diabetes and intermediate hyperglycemia described previously, subjects with macrovascular disease appear to be particularly prone to neuropathic pain [14]. Among survivors of myocardial infarction (MI) from the Augsburg MI Registry, the prevalence of neuropathic pain was 21.0% in the people with diabetes, 14.8% in those with IGT, 5.7% in those with IFG and 3.7% in those with NGT [15]. The most important risk factors of DPN and neuropathic pain in these surveys were age, obesity and low physical activity, while the predominant co-morbidity was peripheral arterial disease, highlighting the paramount role of cardiovascular risk factors and diseases in DPN.
Clinical manifestations
Distal symmetric polyneuropathy
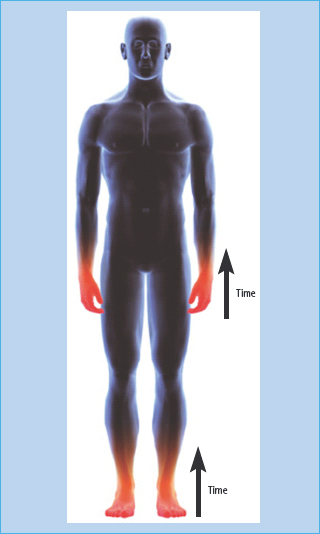
The term “hyperglycemic neuropathy” has been used to describe sensory symptoms in people with poorly controlled diabetes that are rapidly reversible following institution of near-normoglycemia [2]; however, the most frequent form is DPN, commonly associated with autonomic involvement. Its onset is insidious and, in the absence of intervention, the course is chronic and progressive. It seems that the longer axons to the lower limbs are more vulnerable to the nerve lesions induced by diabetes (length-related distribution). This notion is supported by the correlation found between the presence of DPN and height. DPN typically develops as a “dying-back” neuropathy, affecting the most distal extremities (toes) first. The neuropathic process then extends proximally up the limbs and later it may also affect the anterior abdominal wall and then spread laterally around the trunk. Occasionally, the upper limbs are involved with the fingertips being affected first (“glove and stocking” distribution; Figure 38.2).
Figure 38.2 The typical “glove and stocking” distribution of diabetic distal symmetric sensory or sensorimotor polyneuropathy (DPN).
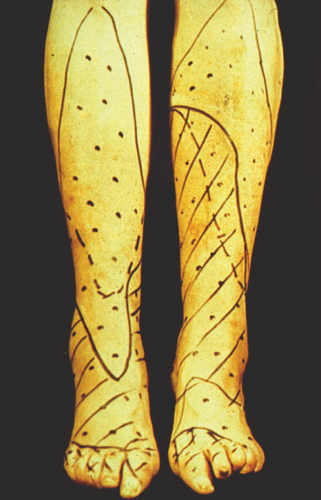
Variants including painful small-fiber or pseudosyringomyelic syndromes and an atactic syndrome (diabetic pseudotabes) have been described. Small-fiber unmyelinated (C) and thinly myelinated (Aδ) fibers as well as large-fiber myelinated (Aα, Aβ) neurons are typically involved. It is as yet uncertain whether the various fiber type damage develops following a regular sequence, with small fibers being affected first, followed by larger fibers, or whether the small-fiber or large-fiber involvement reflects either side of a continuous spectrum of fiber damage. Nevertheless, there is evidence suggesting that small-fiber neuropathy may occur early, often presenting with pain and hyperalgesia before sensory deficits or nerve conduction slowing can be detected [2]. The reduction or loss of small fiber-mediated sensation results in loss of pain sensation (heat pain, pinprick) and temperature perception to cold (Aδ) and warm (C) stimuli. Large-fiber involvement leads to nerve conduction slowing and reduction or loss of touch, pressure, two-point discrimination and vibration sensation which may lead to sensory ataxia (atactic gait) in severe cases. A typical example for the distribution of sensory deficits is shown in Figure 38.3. Sensory fiber involvement causes “positive” sensory symptoms such as paresthesia, dysesthesia and pain, as well as “negative” symptoms such as reduced sensation.
Figure 38.3 A typical example for the distribution of sensory deficits (dots: reduced thermal sensation; lines: reduced pain sensation; crossed lines: reduced touch sensation) in a patient with distal symmetric sensory or sensorimotor polyneuropathy (DPN).
Persistent or episodic pain that typically worsens at night and improves during walking is localized predominantly in the feet. The pain is often described as a deep-seated aching but there may be superimposed lancinating stabs or it may have a burning thermal quality. In a clinical survey including 105 patients with painful DPN, the following locations of pain were most frequent: 96% feet, 69% balls of feet, 67% toes, 54% dorsum of foot, 39% hands, 37% plantum of foot, 37% calves and 32% heels. The pain was most often described by the patients as “burning/hot,” “electric,” “sharp,” “achy” and “tingling” and was worse at night time and when tired or stressed [10]. The average pain intensity was moderate, approximately 5.75/10 on a 0–10 scale, with the “least” and “most” pain 3.6 and 6.9/10, respectively. Allodynia (pain from a stimulus that does not normally cause pain; e.g. stroking) may occur. The symptoms may be accompanied by sensory loss, but patients with severe pain may have few clinical signs. Pain may persist over several years [16] causing considerable disability and impaired quality of life in some patients [10], whereas it remits partially or completely in others [17,18]. despite further deterioration in small-fiber function [18]. Pain remission tends to be associated with sudden metabolic change, short duration of pain or diabetes, preceding weight loss and less severe sensory loss [17,18].
Compared with the sensory deficits, motor involvement is usually less prominent and restricted to the distal lower limbs resulting in muscle atrophy and weakness at the toes and foot. Ankle reflexes are frequently reduced or absent. At the foot level, the loss of the protective sensation (painless feet), motor dysfunction and reduced sweat production, resulting in dry and chapped skin and which is caused by autonomic involvement, increase the risk of callus and foot ulcers. Thus, the neuropathic patient has a high-risk of developing severe and potentially life-threatening foot complications such as ulceration, osteoarthropathy (Charcot foot) and osteomyelitis as well as medial arterial calcification and neuropathic edema. Because DPN is the major contributory factor for diabetic foot ulcers, and the lower limb amputation rates in subjects with diabetes are 15 times higher than in the non-diabetic population, early detection of DPN by screening is of paramount importance [9]. This is even more imperative because many patients with DPN are asymptomatic or have only mild symptoms. In view of these causation pathways, the majority of amputations should be potentially preventable if appropriate screening and preventative measures were adopted.
Acute painful neuropathy
Acute painful neuropathy has been described as a separate clinical entity [19]. It is encountered infrequently in patients with type 1 (T1DM) and type 2 diabetes mellitus (T2DM) and presents with continuous burning pain particularly in the soles (“like walking on burning sand”) with nocturnal exacerbation. A characteristic feature is a cutaneous contact discomfort to clothes and sheets which can be objectified as hypersensitivity to tactile (allodynia) and painful stimuli (hyperalgesia). Motor function is preserved, and sensory loss may be only slight, being greater for thermal than for vibration sensation. The onset is associated with and preceded by precipitous and severe weight loss. Depression and impotence are constant features. The weight loss has been shown to respond to adequate glycemic control and the severe manifestations subsided within 10 months in all cases. No recurrences were observed after follow-up periods of up to 6 years [19]. The syndrome of acute painful neuropathy seems to be equivalent to diabetic cachexia as described by Ellenberg [20]. It has also been described in girls with anorexia nervosa and diabetes in association with weight loss [21].
The term “insulin neuritis” was used by Caravati [22] to describe a case with precipitation of acute painful neuropathy several weeks following the institution of insulin treatment. In a recent series, painful symptoms gradually improved in all patients, allowing discontinuation of analgesic therapy within 3–8 months. Thus, careful correction of glucose levels should be considered in patients with long-standing uncontrolled diabetes [23]. Sural nerve biopsy shows signs of chronic neuropathy with prominent regenerative activity [24] as well as epineurial arteriovenous shunting and a fine network of vessels, resembling the new vessels of the retina which may lead to a steal effect rendering the endoneurium ischemic [25]. This may happen in analogy to the transient deterioration of a pre-existing retinopathy following rapid improvement in glycemic control.
Focal and multifocal neuropathies
Most of the focal and multifocal neuropathies tend to occur in long-term patients with diabetes of middle age or older. The outlook for most of them is for recovery, either partial or complete, and for eventual resolution of the pain that frequently accompanies them [26]. With this in mind, physicians should always maintain an optimistic outlook in dealing with patients with these afflictions.
Cranial neuropathy
Palsies of the third cranial nerve (diabetic ophthalmoplegia) are painful in about 50% of cases. The onset is usually abrupt. The pain is felt behind and above the eye and at times precedes the ptosis and diplopia (with pupillary dysfunction in 14–18% of the cases) by several days. Oculomotor findings reach their nadir within a day or at most a few days, persist for several weeks and then begin gradually to improve. Full resolution is the rule and generally takes place within 3–5 months. The fourth, sixth and seventh cranial nerves are next in frequency [26].
Mononeuropathy of the limbs
Focal lesions affecting the limb nerves, most commonly the ulnar, median, radial and peroneal may be painful, particularly if of acute onset, as may entrapment neuropathies, such as the carpal tunnel syndrome which is associated with painful paresthesia [26].
Diabetic truncal neuropathy
Mononeuropathy of the trunk (thoracoabdominal neuropathy or radiculopathy) presents with an abrupt onset with pain or dysesthesia being the heralding feature, sometimes accompanied by cutaneous sensory impairment or hyperesthesia. Pain has been described as deep, aching or boring, but also the descriptors of jabbing, burning, sensitive skin or tearing have been used. The neuropathy is almost always unilateral or predominantly so. As a result, the pain felt in the chest or the abdomen may be confused with pain of pulmonary, cardiac or gastrointestinal origin. Sometimes, it may have a radicular or girdling quality, half encircling the trunk in a root-like distribution. Pain may be felt in one or several dermatomal distributions and, almost universally, it is worst at night. Rarely, abdominal muscle herniation may occur, predominantly in middle-aged men, involving 3–5 adjacent nerve roots between T6 and T12 (Figure 38.4). The time from first symptom to the peak of the pain syndrome is often just a few days, although occasionally spread of the pain to adjacent der-matomes may continue for weeks or even months. Weight loss of 15–40 pounds (7–18kg) occurs in >50% of the cases. The course of truncal neuropathy is favorable, and pain subsides within months to a maximum of 1.5–2 years [26].
Figure 38.4 Diabetic truncal neuropathy (thoracoabdominal neuropathy or radiculopathy) leading to herniation of the oblique abdominal muscle.
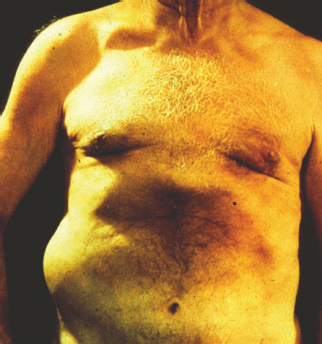
Diabetic amyotrophy
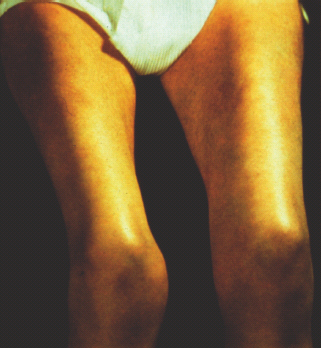
Asymmetric or symmetric proximal muscle weakness and muscle wasting (iliopsoas, obturator and adductor muscles) are easily recognized clinically in the syndrome of lower limb proximal motor neuropathy (synonyms: Bruns–Garland syndrome, diabetic amyotrophy, proximal diabetic neuropathy, diabetic lumbosacral plexopathy, ischemic mononeuropathy multiplex, femoral–sciatic neuropathy, femoral neuropathy). Pronounced bilateral atrophy and paresis of the quadriceps muscle is shown in Figure 38.5. Pain is nearly universal in this syndrome. Characteristically, it is deep, aching, constant and severe, invariably worse at night and may have a burning raw quality. It is usually not frankly dysesthetic or cutaneous. Frequently, pain is first experienced in the lower back or buttock on the affected side or may be felt as extending from hip to knee. Although severe and tenacious, the pain of proximal motor neuropathy has a good prognosis. Concurrent distal sensory polyneuropathy is frequently present. Weight loss is also a frequently associated feature and may be as much as 35–40 pounds (16–18 kg). The weight is generally regained during the recovery phase [27,28].
Figure 38.5 Diabetic amyotrophy (proximal neuropathy): pronounced bilateral atrophy and paresis of the quadriceps muscle.
Central nervous system dysfunction
Relatively little attention has been directed toward impairment of the central nervous system (CNS) in patients with DPN. Previous autopsy studies in people with diabetes have demonstrated diffuse degenerative lesions in the CNS, including demyelination and loss of axon cylinders in the posterior columns [29,30], degeneration of cortical neurons [31] and abnormalities in the midbrain and cerebellum [31,32] which have been described as diabetic myelopathy [30] and diabetic encephalopathy [32].
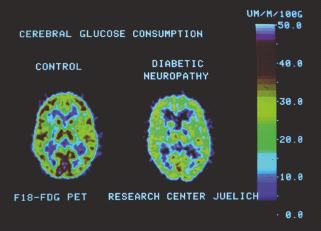
Studies that evaluated CNS function in people with diabetes using evoked potentials in response to stimulation of peripheral nerves, event-related potentials and neuropsychologic tests have yielded variable results as to the existence of spinal or supraspinal (central) conduction deficits or cognitive dysfunction; however, the author has shown that the degree of dysfunction along the somatosensory afferent pathways in patients with T1DM depends on the stage of peripheral neuropathy, is not related to the duration of diabetes or glycemic control and can be characterized by an alteration of the cortical sensory complex and peripheral rather than spinal or supraspinal conduction deficits [33]. Magnetic resonance imaging (MRI) showed an increased frequency of subcortical and brainstem lesions in patients with T1DM with DPN [34]. Moreover, patients with DPN showed a smaller cross-sectional chord area at C4/5 and T3/4 [35]. Using positron emission tomography (PET) and [18F]-2-deoxy-2-fluoro-D-glucose (FDG), we have demonstrated reduced cerebral glucose metabolism in patients with T1DM with DPN as compared with newly diagnosed people with diabetes and healthy subjects (Figure 38.6) [36]. Spectroscopic measurement of brain metabolites such as N-acetyl aspartate (NAA) in the thalamus revealed a lower NAA: creatine ratio, suggesting thalamic neuronal dysfunction in DPN [37]. Thus, there is accumulating evidence suggesting that neuropathic involvement at central and spinal levels is a feature of DPN, but it is not clear whether these are primary or secondary events.
Figure 38.6 Positron emission tomography (PET) and [18F]-2-deoxy-2-fluoro-D-glucose (FDG) showing reduced cerebral glucose metabolism in a patient with T1DM with polyneuropathy compared to a healthy subject.
Pathogenetic mechanisms
Recent experimental studies suggest a multifactorial pathogenesis of diabetic neuropathy [38–40]. Most data have been generated in the diabetic rat model on the basis of which two approaches have been chosen to contribute to the clarification of the patho-genesis of diabetic neuropathy. First, it has been attempted to characterize the pathophysiologic, pathobiochemical and structural abnormalities that result in experimental diabetic neuropathy. Secondly, specific therapeutic interventions have been employed to prevent the development of these alterations, to halt their progression or to induce their regression despite concomitant hyperglycemia. At present, the following six pathogenetic mechanisms are being discussed which, in contrast to previous years, are no longer regarded as separate hypotheses, but as a complex interplay with multiple interactions between metabolic and vascular factors (see Chapter 35):
- Increased flux through the polyol pathway that leads to accumulation of sorbitol and fructose, myo-inositol depletion and reduction in Na+-K+-ATPase activity.
- Disturbances in n-6 essential fatty acid and prostaglandin metabolism which result in alterations of nerve membrane structure and microvascular and hemorrheologic abnormalities.
- Endoneural microvascular deficits with subsequent ischemia and hypoxia, generation of reactive oxygen species (oxidative stress), activation of the redox-sensitive transcription factor nuclear factor kB (NFkB), and increased activity of protein kinase C (PKC).
- Deficits in neurotrophism leading to reduced expression and depletion of neurotrophic factors such as nerve growth factor (NGF), neurotrophin-3, and insulin-like growth factor I (IGF-I) and alterations in axonal transport.
- Accumulation of non-enzymatic advanced glycation end-products on nerve and/or vessel proteins.
- Immunologic processes with autoantibodies to vagal nerve, sympathetic ganglia and adrenal medulla as well as inflammatory changes.
From the clinical point of view it is important to note that, based on these pathogenetic mechanisms, therapeutic approaches could be derived, some of which have been evaluated in randomized clinical trials.
Diagnostic assessment
As a result of the increasing recognition of diabetic neuropathy as a major contributor to morbidity and the recent burst of clinical trials in this field, several consensus conferences have been convened to overcome the current problems that arise from the lack of agreement about the definition and diagnostic assessment of neuropathy. The Consensus Development Conference on Standardized Measures in Diabetic Neuropathy [41] recommended the following five measures to be employed in the diagnosis of diabetic neuropathy:
Clinical measures
Clinical measures include:
- Sensory (pain, light touch, vibration, position);
- Motor (graded as normal = 0, weak = 1–4 [25–100%]);
- Reflexes (present or absent); and
- Autonomic (bedside tests including heart rate variation during deep breathing and postural blood pressure response) examination.
The basic neurologic assessment comprises the general medical and neurologic history, inspection of the feet and neurologic examination of sensation using simple semi-quantitative bedside instruments such as the 10 g Semmes–Weinstein monofilament (Figure 38.7a), e.g. the Neuropen (touch) [42], NeuroQuick (Figure 38.7b) [43] or Tiptherm (temperature) [44]. calibrated Rydel–Seiffer tuning fork (vibration) (Figure 38.7a), pinprick (pain) and tendon reflexes (knee and ankle). In addition, assessment of joint-position and motor power may be indicated. The normal range for the tuning fork on the dorsal distal joint of the great toe is ≥5/8 scale units in persons ≤20–40 years of age, >4.5/8 in those aged 41–60 years, ≥4/8 in individuals aged 61–71 years and ≥3/8 scale units in those aged 72–82 years [45]. An indicator test for the detection of sudomotor dysfunction is the Neuropad which assesses plantar sweat production by means of a color change from blue to pink. The patch contains the complex salt anhydrous cobalt-II-chloride. In the presence of water, this salt absorbs water molecules, normally changing its color from blue to pink. If the patch remains completely or partially blue within 10 minutes, the result is considered abnormal (Figure 38.7b) [46].
Figure 38.7 (a) Bedside tests for assessment of large fiber function. (b) Bedside tests for assessment of small fiber function.

Clinical assessment should be standardized using validated scores for both the severity of symptoms and the degree of neuropathic deficits such as the Michigan Neuropathy Screening Instrument [47], Neuropathy Symptom Score for neuropathic symptoms and Neuropathy Disability Score for neuropathic deficits (impairments) [48] which appear to be sufficiently reproducible. The neurologic history and examination should be performed once a year. Minimum criteria for the clinical diagnosis of neuropathy according to the Neuropathy Symptom Score and Neuropathy Disability Score are:
- Moderate signs with or without symptoms; or
- Mild signs with moderate symptoms.
This means that the exclusive presence of neuropathic symptoms without deficits is not sufficient to diagnose DPN. Therefore, early stages of DPN or a painful small-fiber neuropathy without or with minimal deficits can only be verified using more sophisticated tests such as thermal thresholds or skin biopsy.
The intensity (severity) of neuropathic pain and its course should be assessed using an 11-point numerical rating scale (Likert scale) or a visual analog scale. Various screening tools (with or without limited bedside testing) have been developed to identify neuropathic pain such as the PainDetect, LANSS, NPQ, DN-4, ID-Pain). These questionnaires use verbal descriptors and pain qualities as a basis for distinguishing neuropathic pain from other types of chronic pain such as nociceptive pain [49].
The following findings should alert the physician to consider causes for DPN other than diabetes and referral for a detailed neurologic work- up:
- Pronounced asymmetry of the neurologic deficits;
- Predominant motor deficits, mononeuropathy, cranial nerve involvement;
- Rapid development or progression of the neuropathic impairments;
- Progression of the neuropathy despite optimal glycemic control;
- Symptoms predominantly in the upper limbs;
- Family history of non-diabetic neuropathy; or
- Diagnosis of DPN cannot be ascertained by clinical examination.
The most important differential diagnoses from the general medicine perspective include neuropathies caused by alcohol abuse, uremia, hypothyroidism, vitamin B12 deficiency, peripheral arterial disease, paraneoplastic syndromes, inflammatory and infectious diseases and neurotoxic drugs.
Clinical measures are used to:
- Establish the presence or absence of neurologic dysfunction in diabetes;
- Exclude non-neuropathic causes of neurologic dysfunction;
- Eliminate non-diabetic causes of neuropathy;
- Distinguish and classify the different forms of diabetic neuropathy; and
- Monitor progression and provide a clinical correlate of outcome in trials.
The limitations to clinical measures include:
- Lack of sensitivity to change once they become abnormal; and
- Limited reliability and reproducibility.
Positive symptoms may reflect different pathophysiology than deficits (i.e. pain or paresthesia may be related to the degree of compensatory regeneration rather than to the degree of nerve fiber damage). Hence, it has been suggested that symptom or pain scores should not be used to evaluate overall presence or progression of diabetic neuropathy but only to assess pain severity [41].
Electrodiagnostic measures
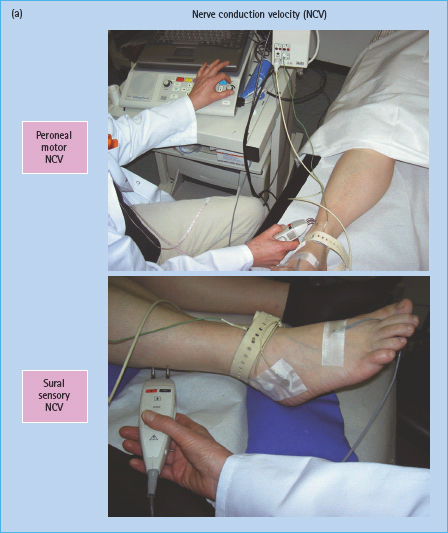
Electrophysiologic techniques have the advantage of being the most objective, sensitive, specific and reproducible methods that are available in many neurophysiologic laboratories worldwide (Figure 38.8).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree