- The greatest long-term risk in diabetes is cardiovascular disease (CVD), with macrovascular disease being the cause of 80% of mortality in people with diabetes.
- Epidemiologic studies have established that glycemic control, nephropathy and lipids are risk factors for CVD in type 1 diabetes.
- Low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, smoking and hypertension are the principal risk factors in type 2 diabetes mellitus (T2DM).
- In T2DM, optimized glycemic control has modest effects in reducing CVD endpoints.
- Reduction of LDL cholesterol with statins has consistently shown cardiovascular event reductions >30%.
- The dyslipidemia of T2DM is associated with elevated triglycerides, reduced HDL cholesterol and small dense particles.
- Fibrates, when used as monotherapy, have shown a modest reduction in events in T2DM in the FIELD study.
- Post hoc analysis of the Coronary Drug Project with niacin suggests possible benefits on cardiovascular endpoints.
- Trials now underway will allow the efficacy of combination lipid-lowering therapies in diabetes to be determined.
- Optimal control of all risk factors can reduce cardiovascular events and mortality in diabetes by 50%.
Introduction
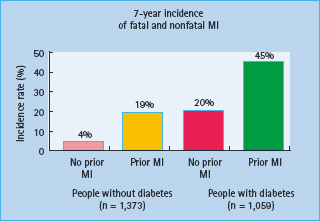
An association between diabetes and heart disease was described more than a century ago. Two decades later, in 1906, it was hypothesized that this association was caused by atherosclerosis. The importance of diabetes as a cardiovascular disease (CVD) risk factor became established following the Framingham Study and this was subsequently confirmed by other landmark studies [1,2]. The magnitude of diabetes as a CVD risk factor is substantial, with the increase in cardiovascular risk being two- to fourfold. Many guidelines regard diabetes as a coronary heart disease (CHD) risk equivalent [3–5]. This concept is based originally on a Finnish cohort [6], which showed comparable risk of CHD outcomes such as myocardial infarction (MI) and CHD death between subjects with type 2 diabetes mellitus (T2DM) for > 10 years and subjects with established CHD (Figure 40.10). This was still apparent after adjusting for known risk factors such as age, sex, hypertension, total cholesterol and smoking. The Organization to Assess Strategies for Ischemic Syndromes (OASIS) study showed that patients with diabetes and no previous CVD have the same long-term morbidity and mortality as patients with established CVD but no diabetes after hospitalization for unstable coronary artery disease (CAD) [7]; however, there is a wide variation in the rate of CHD in diabetes which depends on the population studied, duration of diabetes, as well as existing risk factors [8,9]. This equivalence has not been confirmed by a subsequent study and it also seems less valid in older subjects where those with existing CHD have a greater risk than non-CHD patients with diabetes [9,10]. Most of the literature that reports on CVD risk and diabetes only considers T2DM. Even though people with type 1 diabetes mellitus (T1DM) are clearly at increased risk for CVD [11,12], no study has specifically examined whether subjects with T1DM have a CVD risk that is comparable or higher than those with T2DM. In T1DM there may even be a higher risk of premature CVD. In a cohort of 292 patients with T1DM followed for 20–40 years, the cumulative mortality rate from CAD was 35% by age 55 [11]. As T1DM mostly presents at an earlier age, it remains more difficult to assess and compare this but rates of CVD are increased at all ages [13].
Figure 40.10 Equivalence of cardiovascular risk in patients with previous coronary heart disease (CHD) and those with diabetes. MI, myocardial infarction. Reproduced from Haffner et al. [6], with permission from Massachusetts Medical Society.
Concomitant CVD risk factors also differ according to the type of diabetes. For example, those with T1DM have a two- to threefold increase in risk of developing CHD and stroke in later life. This risk is notably increased in those developing diabetic nephropathy. Other markers of CVD risk in people with diabetes include diabetic retinopathy, autonomic neuropathy, erectile dysfunction, microalbuminuria and proteinuria [14].
In general, people with diabetes have a two- to fourfold CVD risk compared with the non-diabetic population [15]. Even though guidelines do not recommend formal CVD risk estimation in those with diabetes because of the significant risk these patients already have and the tendency of the Framingham algorithm to underestimate risk in this group, clinicians may still opt to estimate the risk by employing various risk calculators of which the UK Prospective Diabetes Study (UKPDS) is the most used [16]. It should be noted however, that these risk calculators predict risk with variable accuracy [17]. As all methods of CVD risk estimation suffer from distinct limitations, clinical judgment remains necessary to assess risk accurately and select and titrate appropriate treatment [18,19].
Cardiovascular disease risk factors in diabetes
Glucose
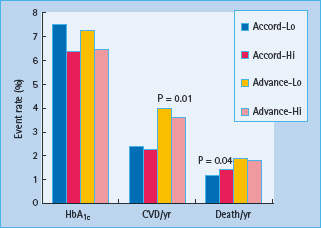
A risk continuum exists across a broad glucose concentration range which incorporates individuals without diabetes, with the risk of CVD being the lowest when the fasting blood glucose is 4–4.9 mmol/L [20–22]. Despite the well-established association between blood glucose and atherosclerosis, surprisingly few studies have been able to show an improvement in cardiovascular outcome by reduction in blood glucose. In T1DM, the Epidemiology of Diabetes Interventions and Complications (EDIC) study [23] which followed subjects after the completion the Diabetes Control and Complications Trial found that glucose lowering was associated with a long-term benefit with regard to cardiovascular complications that became apparent only years after recruitment. In T2DM, 10-year follow-up data from the UKPDS intensive glucose therapy showed long-term beneficial effects on macrovascular outcomes [24]; however, unlike the microvascular benefits, risk reductions for MI and death from any cause were observed only with extended post-trial follow-up (Figure 40.11). These results suggested that improved glucose control may result in a larger cardiovascular risk reduction in patients with T1DM than among those with T2DM, which is consistent with the results of one meta-analysis. Furthermore, neither the recent Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) [25] nor the Action to Control Cardiovascular Risk in Diabetes (ACCORD) [26] trials, each including in excess of 10 000 participants, could not show a significant beneficial effect on CVD outcome when targeting near-normal glucose levels in T2DM as determined by a HbA1c <6.5% (48mmol/mol) (Figure 40.12). More worrying was the finding in the ACCORD trial that near-normal glucose control was actually associated with a significantly increased risk of death from any cause and death from cardiovascular causes, the very outcomes the trial was designed to prevent [27]. Several other outcomes trials are underway, which should improve our understanding of the problem of glycemic control and CVD [28].
Figure 40.11 Results of the UK Prospective Diabetes Study (UKPDS) at 10 years follow-up. Reproduced from Holman et al. [24], with permission from Massachusetts Medical Society.
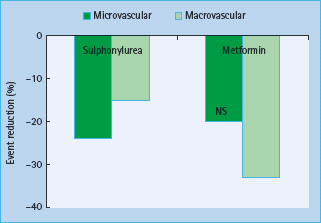
Figure 40.12 Effects of improved diabetes control to an HbA1c <6.5% (48 mmol/mol) in the ACCORD and ADVANCE studies. CVD, cardiovascular disease; Lo, intensive glycemic control; Hi, conventional glycemic control. Data from ACCORD group N Engl J Med 2008; 358:2545 and ADVANCE group N Engl J Med 2008; 358:2560.
Dyslipidemia
Compared with hyperglycemia, targeting dyslipidemia has proven much more effective in preventing the macrovascular complications of diabetes; however, for many years the benefits of intervention on lipoproteins as cardiovascular risk factors in diabetes were uncertain. The principal reason was that people with diabetes were excluded from trials of lipid-lowering therapies. Thus, virtually no data exist from early studies with bile acid sequestrants, fibrates or nicotinic acid.
The reasons for the excess CVD risk in diabetes are numerous and varied and in part relate to the lipid abnormalities seen in diabetes. Enhanced glycation of lipoproteins has direct effects on lipoprotein metabolism as glycated lipoproteins are handled differently by lipoprotein receptors, particularly of the scavenger group, thus promoting atherogenesis [29]. Enhanced glycation also amplifies the effects of oxidative stress on lipoproteins and therefore affects both T1DM and T2DM [30]. The term diabetic dyslipidemia refers to the lipid abnormalities typically seen in persons with T2DM and is synonymous with atherogenic dyslipidemia [31,32]. It is characterized by elevated triglyceriderich remnant lipoproteins (routinely measured as hypertriglyceridemia), small dense LDL particles and low HDL cholesterol concentrations. Several factors are likely to be responsible for diabetic dyslipidemia: insulin effects on liver apolipoprotein production, downregulation of lipoprotein lipase (LPL) as opposed to hepatic lipase, increased cholesteryl ester transfer protein (CETP) activity, and peripheral actions of insulin on adipose and muscle.
Low density lipoprotein cholesterol
LDL cholesterol is identified as the primary target of lipid-lowering therapy. Analysis of the UKPDS showed that LDL cholesterol was the strongest risk factor for CHD in this population and HDL cholesterol was the second strongest [33]. Even relatively modern studies discouraged recruitment or restricted entry to patients with hypercholesterolemia and reasonable glycemic control (HbA1c <8% [64 mmol/mol]) as in the Scandinavian Simvastatin Survival Study (4S) [34]. Only recently have studies been performed recruiting large groups of people with T2DM. The 4S trial included only 202 people with diabetes out of 4444 participants. In this small group of subjects, simvastatin therapy was associated with a 55% reduction in major CHD (fatal and non-fatal CHD) (P = 0.002) compared with a 32% reduction in major CHD in subjects without diabetes [35]. It was concluded that the absolute benefit of cholesterol lowering in patients with diabetes may be greater than that in patients without diabetes because the patients with diabetes have a higher absolute risk of atherosclerotic events and CHD. This notion was later confirmed in several other studies that also recruited people with diabetes as a subgroup [36].
A number of studies have been specifically performed with statins in diabetes. Most notable of these were the Collaborative Atorvastatin Diabetes Study (CARDS) [37] and the Heart Protection Study (HPS) [38] where subjects were randomized in a double-blind placebo-controlled fashion to 10mg/day atorvastatin in CARDS and to 40 mg/day simvastatin in the HPS. This produced, respectively, a 40% and 33% reduction in LDL cholesterol associated with a 37% and 31% reduction in combined cardiovascular endpoints. The HPS is the largest statin study to date and included a subgroup of 5963 patients with diabetes (29% of the total study group) [39]. The CARDS trial only included people with diabetes and more than one additional risk factor (e.g. uncontrolled hypertension and/or microalbuminuria) but without prior overt CVD. The study was terminated 2 years prematurely having shown unexpected early benefit. By contrast, the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus (ASPEN) study of similar design to CARDS and also using atorvastatin showed a non-significant 15% reduction in events [40]. In end-stage diabetes with renal failure, aggressive LDL cholesterol reduction reduced events by a non-significant 8% despite a 41% reduction in LDL cholesterol [41]. Thus, the benefits of statin therapy seem to occur early in disease in diabetes.
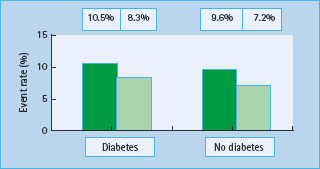
Overall, the accumulated evidence therefore supports the efficacy of statin therapy in reducing cardiovascular risk in patients with diabetes. A meta-analysis, which evaluated the efficacy of cholesterol-lowering therapy in 18 686 people with diabetes in 14 randomized trials of statins, reported a 9% proportional reduction in all-cause mortality and a 21% proportional reduction in major vascular events per 1 mmol/L reduction in LDL cholesterol (Figure 40.13) [42].
Figure 40.13 Comparison of the effects of reducing low density lipoprotein (LDL) cholesterol on cardiovascular events in patients with and without diabetes. Reproduced from Cholesterol Treatment Triallists Collaborators. Lancet 2005; 366:1267–1278, with permission from Elsevier.
There are few data on diabetes and other drugs that reduce LDL cholesterol as people with diabetes were excluded from trials of bile acid sequestrants. The cholesterol absorption inhibitor, ezetimibe, works by reducing the upper intestinal cholesterol absorption to produce in monotherapy a 20–25% reduction in LDL cholesterol and, in contrast to bile acid sequestrants, it has remarkably little effect on other lipid fractions. People with diabetes were excluded from the Simvasatatin Ezetimibe Aortic Stenosis study [43]. More recently, it has received several potential setbacks with respect to surrogate marker measurements as well as pointers of potential detrimental effects [44]. Two ongoing trials, the Improved Reduction of Outcomes: Vytorian Efficacy International Trial (IMPROVE-IT), in which simvastatin plus ezetimibe is compared with simvastatin plus placebo, and the Study of Heart and Renal Protection (SHARP) trial, in which simvastatin plus ezetimibe is compared with placebo, will hopefully provide answers to these questions.
Low density lipoprotein subtractions
The LDL class comprises a heterogeneous population of particles [45]. LDL is heterogeneous with respect to lipid composition, charge, density, and particle size and shape. The sizes of LDL particles fall between the large triglyceride-enriched very low density lipoprotein (VLDL) particles and the dense and small protein-rich HDL. In addition, these small dense LDL particles may be more atherogenic than would be suspected by their concentration alone, because in vitro and cell culture studies suggest they may be more readily oxidized and glycated. Oxidized LDL delivers cholesterol to the atherosclerotic plaque in an unregulated way through uptake by the macrophage and is increased in diabetes [46].
Another aspect is the fatty acid composition of the LDL particle. Linoleic acid is the main polyunsaturated fatty acid in the LDL particle, and this is increased in diabetes. The reason for this may be because of the decreased activity of the insulin sensitive enzyme, 5α-desaturase. There is a strong correlation between linoleic acid in the LDL particle and propensity of the LDL particle to be oxidized. Uncontrolled diabetes results in increased free radical formation which leads to increased oxidation. The fact that the LDL particles are smaller and denser means that they carry relatively less cholesterol per particle. Estimation of the LDL cholesterol may therefore be misleading as there will be more LDL particles for any cholesterol concentration.
Large numbers of studies, including the Quebec Cardiovascular study [47]. have confirmed the association of small dense LDL with CVD which reported that men with small dense LDL particles had an increased risk of CAD compared with men with normal-sized LDL particles, independent of LDL cholesterol, triglyceride and the total cholesterol: HDL cholesterol ratio; however, no prospective studies have specifically examined whether altering particle size profiles results in benefits on cardiovascular events although analysis of the Veterans Affairs High Density Lipoprotein Cholesterol Intervention Trial (VA-HIT) study does suggest some role for this mechanism [48].
Even with effective LDL cholesterol treatment, the residual risk of further cardiovascular events remains high, emphasizing the importance of improving other abnormalities and other CVD risk factors commonly observed in these patients.
Triglycerides
The reason for the elevated triglycerides in diabetes is complex, however, because of this derangement, it has been suggested that diabetes should not be called mellitus but rather lipidus [49]. Defects in insulin action and hyperglycemia can lead to changes in plasma lipoproteins in patients with diabetes. Alternatively, especially in the case of T2DM, the obesity and insulin-resistant metabolic disarray that are at the root of this form of diabetes could, themselves, lead to lipid abnormalities exclusive of hyper-glycemia [32]. This molecular interplay between lipid and carbohydrate metabolism has led to what might be termed a “lipocentric” view of the pathogenesis of insulin resistance and T2DM [50]. As fatty acids have such a central role in insulin sensitivity, obesity and T2DM, it follows that the major disturbance in lipoprotein metabolism in diabetes is found in the triglyceride-rich lipoproteins, stemming from abnormalities in chylomicron synthesis and clearance [51].
Triglycerides (also referred to as triacylglycerol) are formed from a single molecule of glycerol combines with three fatty acids and represent a heterogeneous group of molecules which most frequently are measured collectively, as a “family” of analytes [52]. Elevated serum triglyceride levels are associated with increased risk for atherosclerotic events [53,54]. As high serum triglyceride levels are associated with abnormal lipoprotein metabolism, as well as with other cardiovascular risk factors including obesity, insulin resistance, diabetes and low levels of HDL cholesterol, it becomes more difficult to distinguish between cause and effect and to establish hypertriglyceridemia as an independent cardiovascular risk factor. Some causes of hypertriglyceridemia have no apparent effect on atherosclerotic vascular disease, making it difficult to prove that elevated triglycerides are a risk factor [55]. Nevertheless, several meta-analyses have found that triglycerides are an independent risk factor for CHD [54,56,57].
The two main sources of plasma triglycerides are exogenous (i.e. from dietary fat) carried in chylomicrons and endogenous (from the liver) and carried in VLDL particles. In capillaries within fat and muscle tissue, these lipoproteins and chylomicrons are hydrolyzed by LPL into free fatty acids. LPL is activated by apolipoprotein C-II, cleaving the triglyceride core and releasing free fatty acids, which can be oxidized by muscle for energy or kept in adipose tissue for future use, and inhibited by the action of apolipoprotein C-III [58]. In routine clinical practice, hyper-triglyceridemia is the most frequent lipoprotein abnormality found in uncontrolled diabetes. The mechanisms for these include increased production or absorption, or reduced catabolism (mainly because of decreased activity of LPL). Liver apoli-poprotein B production (the major protein component of VLDL and LDL) is increased in T2DM. This is indirectly brought about by increased lipolysis which occurs in adipose tissue, a consequence of insulin resistance and/or insulin deficiency. The increased lipolysis results in increased fatty acid release from fat cells with increase in fatty acid transport to the liver. Studies in tissue cultures, animal experiments [59] and humans [60] suggest that fatty acids modulate liver apolipoprotein B secretion.
Microsomal triglyceride transfer protein (MTP) assembles the chylomicron in the intestine and the VLDL particle in the liver. MTP has been shown to be increased in the intestine of subjects with diabetes [61]. Cholesterol absorption also seems to be adversely influenced in subjects with diabetes. The Niemann-Pick C1-like 1 protein, which has a critical role in the absorption of cholesterol, is increased in people with diabetes. The ATP-binding cassette transporters ABC – G5 and ABC – G8 dimerize to form a functional complex necessary for efflux of dietary cholesterol and non-cholesterol sterols from the intestine and liver. These proteins have been shown to be reduced in diabetes in both liver and intestine. LPL is an insulin-dependent enzyme being responsible for the conversion of lipoprotein triglyceride into free fatty acids. It has several other activities relating to lipid and carbohydrate metabolism [62]. Both patients with T1DM and T2DM have reduced LPL activity which is further suppressed by adipose-derived cytokines such as tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6) [32].
Statins form the mainstay of lipid management based on their efficacy in lowering LDL cholesterol, but their effects on components of the atherogenic dyslipidemia associated with T2DM are more modest, reducing triglycerides at most by 15–30% and raising HDL cholesterol typically by less than 10%. There is no clear consensus on the benefits of directly targeting hypertriglyceridemia [63]. As the interventions usually affect both triglycerides and HDL cholesterol, it also becomes more difficult to distinguish between the individual benefits.
Fibrates
The “fibrate” class of lipid-towering drugs is useful for lowering elevated triglyceride or non-HDL cholesterol levels as these agents, which act on peroxisomal proliferator-activated receptor α (PPARα), increase lipoprotein lipase activity, reduce apolipo-protein C-III and may increase HDL cholesterol or decrease fibrinogen [64]. Despite this, clinical trials of these drugs have reported mixed results in general, and most early trials recruited only a few patients with diabetes [65,66].
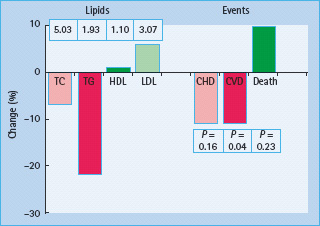
The VA-HIT study evaluated the potential benefits of gemfibrozil in 2531 men with an acute MI. Patients with relatively low LDL cholesterol (<3.6 mmol/L) and low HDL cholesterol (<1.0 mmol/L) were recruited. A significant reduction in the primary endpoint (fatal and non-fatal MI) of 22% was achieved [67]. One-third of participants had T2DM. These outcomes were achieved despite relatively small changes in HDL cholesterol (8%) and no change in LDL cholesterol (0%). An exploration of the effect of gemfibrozil showed that the principal effect of the fibrate treatment was a 31% reduction in triglycerides which reflects changes in particle sizes, but was not related to event reduction [68]. A subgroup analysis of the subjects with diabetes showed a relative risk reduction of 32% compared to 18% in the non-diabetic group [69], however, the enthusiasm for fibrate use has been considerably dampened by results of the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study [70]. This trial randomized 9795 subjects with T2DM to fenofibrate (200 mg/day) or placebo who were not on statin treatment at the beginning of the study, and participants in the trial were treated for 5 years. The study reported a non-significant 11% reduction in the primary endpoint of CHD although a significant reduction in total cardiovascular events was achieved with fenofibrate therapy (P = 0.035) (Figure 40.14). The study was confounded by asymmetrical statin drop-in with many more patients on placebo arm being initiated on statin therapy during the trial (17%) than those on the fenofibrate arm (8%) [65]. It is therefore difficult to compare fibrate trials as they seem to give heterogenous results depending on the compound used, and no fibrate trials have shown to reduce all cause mortality. Nevertheless, meta-analayses suggest they may reduce non-fatal MI [71]. It is hoped that the ACCORD trial, which randomized fenofibrate in addition to baseline 20–40 mg simvastatin, can provide more definitive answers.
Figure 40.14 Reductions in mortality, coronary heart disease (CHD) and cardiovascular events with fenofibrate therapy in the FIELD study. HDL, high density lipoprotein; LDL, low density lipoprotein; TC, total cholesterol; TG, triglycerides. Reproduced from Keech et al. [70], with permission from Elsevier.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







