OUTLINE
Introduction, 268
Clinical Manifestations, 268
History, 269
Physical Examination, 269
Pulmonary, 269
Ear, Nose, and Throat, 269
Neurological, 269
Gastrointestinal, 269
Diagnostic Workup and Investigation, 269
Management, 271
Symptomatic Support, 271
Home Oxygen, 272
Medications, 272
Respiratory Support, 273
Coronavirus Disease–Associated Acute Respiratory Distress Syndrome and Intubation, 273
Invasive Procedures and Hemodynamic Support, 274
Disposition, 274
Special Considerations, 276
Emergency Medical Services, 276
Personal Protective Equipment, 277
Space Constraints and Modifications, 277
Telehealth, 279
COVID-19 Vaccination in the Emergency Department, 279
Conclusion, 279
Introduction
The challenges posed by the COVID-19 pandemic have affected all of humanity, as well as the full range of medical specialties, yet one of the single most affected specialties has been emergency medicine. Emergency medicine specialists have been at the forefront for decades confronting all human crises related to physical and mental health and are trained in a variety of life-, limb- and vision-saving interventions. However, the COVID-19 pandemic added additional burden to already strained emergency medicine departments. The pandemic adversely affected the care of non–COVID-19 patients, just as it affected the patients seeking service from other fields of medical specialties, including definitive and often elective treatments and procedures in oncology, cardiology, and orthopedics, to name a few.
COVID-19 can affect multiple organ systems and often manifests with confusing clinical scenarios and diverse ramifications.
The following sections of this chapter attempt to capture the various manifestations of COVID-19 infection as emergency situations for the patients; the challenges in arriving at a proper diagnosis; appropriate workup, including laboratory and imaging studies; and the rather uncharted territory of interventions and management protocols, including supportive care, medications, and invasive procedures. As more experience is gained in dealing with this pandemic, many aspects of clinical management will evolve and change, for the better, just as in the past 2 years.
Clinical Manifestations
The clinical manifestation of COVID-19 is highly variable and includes a wide range of reported symptoms, making early clinical differentiation of this disease process challenging within the emergency department (ED). In one review that included over 40,000 patients, at least 26 different clinical manifestations were reported. Symptomatic manifestations can also range in severity from mild to critical. Additionally, time to recovery is also highly variable, with some individuals experiencing only a few days of symptoms and others having persistent symptoms and long-term sequalae. Of those individuals who develop symptoms, approximately 97.6% will do so within 11.5 days of the infection.
The most common symptom of all patients diagnosed with COVID-19 is fever. After fever, in the general population, the following symptoms were most common: cough, dyspnea, fatigue, malaise, myalgias, nausea and/or vomiting or diarrhea, headache, and anosmia or ageusia. Less frequent symptoms reported include sore throat, chest pain, and abdominal pain. ,
History
Patient-provided history is a crucial piece for any potential or confirmed COVID-19 patient. Initial history questions regarding travel, sick contacts, or known COVID-19–positive exposure can be collected during the triage process and can help guide appropriate clinical care, diagnostics, and subsequent isolation needs. Next, signs and symptoms provided by the patient, such as fevers, cough, shortness of breath, body aches, nausea and/or vomiting, and loss of taste or smell, will help further guide diagnostic workup and testing. A detailed investigation into immunocompromised status and additional comorbidities is also needed to help stratify risk as well as guide potential treatment modalities. Finally, general demographics questions such as occupation, age, race, surrounding population statistics, travel history, and socioeconomic status can all be helpful at this stage in an ED evaluation. In the advent of widespread vaccine availability, ascertaining a patient’s previous positive or negative testing status along with their current vaccine status can additionally provide key details for the clinician.
Physical Examination
Physical examination findings are very diverse and can be nonspecific, and similar to those of other viral illnesses and common infections such as influenza, pneumonia, bronchitis, or gastroenteritis. Special attention to vital signs is an important first step of any ED evaluation as abnormalities in any of the vital signs can be seen with COVID-19. The most common, however, is fever (>38°C).
Pulmonary
COVID-19 predominately affects the respiratory system, making dyspnea (53%–80%) and cough (60%–86%) the most common symptoms and contributors to clinical presentation. , Special consideration to respiratory rate and pulse oximetry is critical in the ED because these play a critical role in disposition and potential inpatient and outpatient treatment strategies. Despite predominately affecting the respiratory system, auscultatory findings can be either normal or abnormal. Coarse breath sounds are the most common finding identified during auscultation. Fine and coarse crackles were the next most common. Hypoxia with or without associated symptoms was frequently reported. When hypoxia was observed without additional clinical manifestations, it was often referred to as “silent hypoxemia,” which came to be an initial hallmark of SARS-CoV-2–infected individuals.
Ear, Nose, and Throat
Ear, nose, and throat (ENT) symptoms and clinical findings are less common. A unique and distinctive feature of COVID-19 was sensory alterations to smell and/or taste. Although a loss of taste and smell has a wide variation in prevalence, ranging from 4.23% to 98.33%, it is a clear pointer to COVID-19 when elicited during a history or examination. Other ENT symptoms that have been documented are more nonspecific, making them less useful in isolation. These include sneezing, rhinitis, sore throat, rhinorrhea, and nasal congestion. Objective ENT physical examination findings are more uncommon with tonsillar swelling, throat congestion, and lymphadenopathy seen in less than 2% of patients.
Neurological
Several neurological manifestations have been associated with COVID-19 infection. Reported symptoms include myalgias, headaches, and dizziness. Encephalopathy also can occur and is more frequent in the elderly. Cerebrovascular occlusions have been documented and attributed as a complication to COVID-19 manifesting with associated neurological deficits. , ,
Gastrointestinal
The most common gastrointestinal (GI) symptoms include anorexia, diarrhea, and nausea and/or vomiting. Abdominal pain and vomiting are recorded in approximately 5% of patients. In one study, patients with GI symptoms had a longer time from onset to admission, which was thought to be related to delayed diagnosis given nonspecific symptoms. These nonspecific symptoms made diagnosis more challenging because 7% of the COVID-19 patients with GI symptoms had no other respiratory or ENT complaints. ,
Diagnostic Workup and Investigation
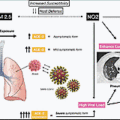
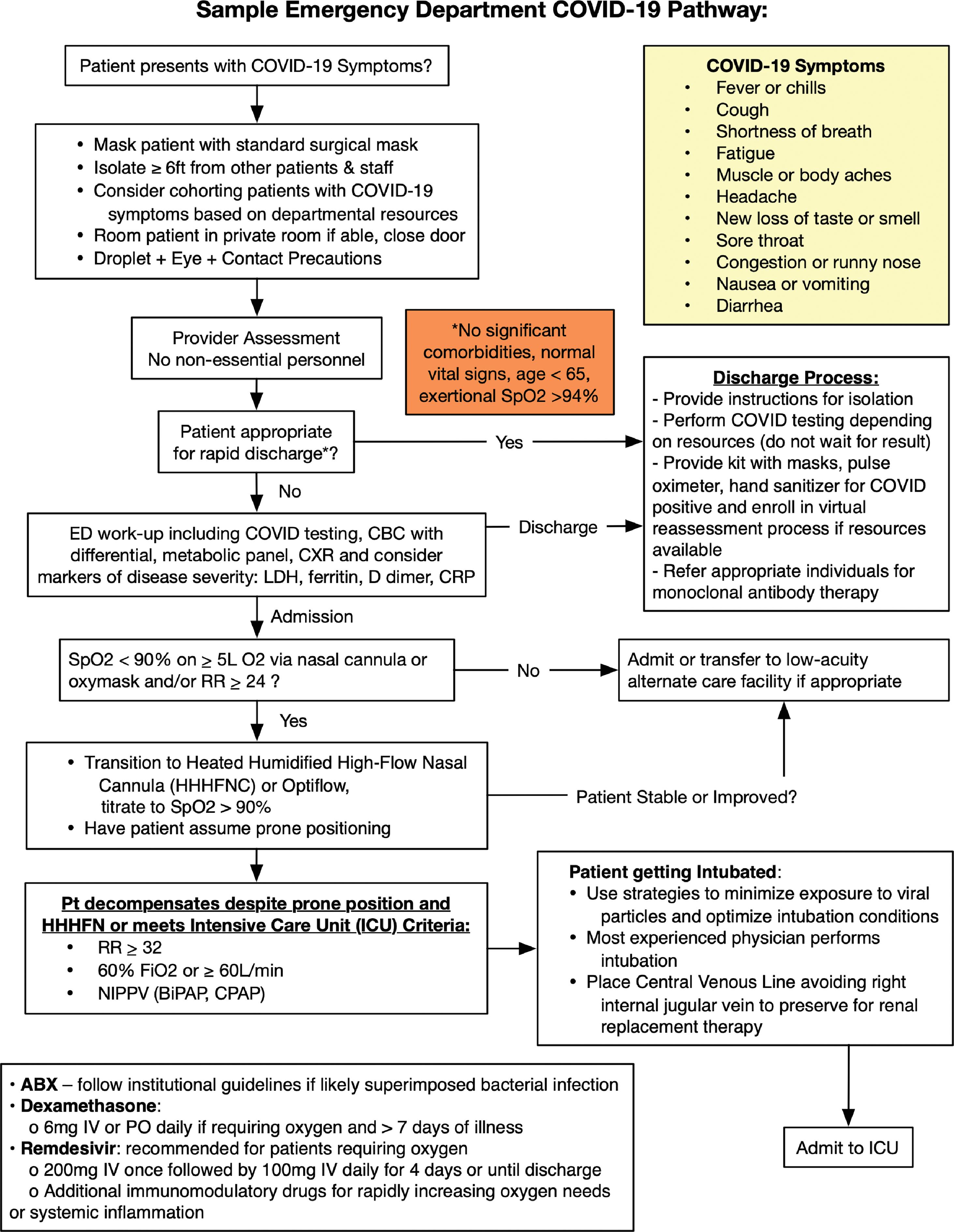
The diagnostic evaluation of suspected patients with COVID-19 in the ED is discussed in the following section. These diagnostic tools serve as adjuncts to the clinical evaluation and treatment of life-threatening situations by the ED provider. A sample diagnostic and treatment pathway is shown in Fig. 15.1 .
Laboratory Evaluation
COVID-19 Testing
COVID-19 testing should be performed in the emergency department (ED) to assist in the diagnosis of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). While Although testing continues to evolve, there are two basic types of testing: viral testing (nucleic acid amplification tests ([NAATs)] and antigen tests) and antibody tests (serological tests). Antibody testing is mostly limited to identifying past infections and is less beneficial for ED testing of suspected patients; current literature suggests that they should not be used as a definitive test in a point-of-care hospital setting. Included in NAATs is the reverse transcriptase polymerase chain reaction (RT-PCR) test. RT-PCR and other NAATs are generally considered the preferred testing method for the ED as long as the hospital’s laboratory has the appropriate equipment; laboratory-based NAATs are generally considered the most sensitive form of testing. Sensitivity and specificity are affected by several factors that include the analytical testing instrument used and the quality of acquisition of the sample. Nasopharyngeal samples are considered superior to throat samples. , Turnaround times (TATs) affect the utility of the test for the ED provider as because they can range from minutes to days. Ideally, an ED must perform rapid NAAT testing to confirm a diagnosis of COVID-19. Depending on capacity, an ED may choose to prioritize cohorts of patients into more rapid testing depending on disposition, severity, or other factors.
Supplemental Testing
A complete blood count is a commonly ordered study on many patients. A study with both outpatient- and inpatient-confirmed COVID-19 cases found a median white blood cell (WBC) count of 4700 mm 3 , and another retrospective study of hospitalized patients found the median to be 7000 mm 3 . A lymphopenia has been seen associated with COVID-19 in up to 83% of cases. The basic metabolic panel, another commonly ordered test, does not seem to show any pathognomonic findings. Other laboratory tests that are often ordered, some to determine severity of disease, include d -dimer, C-reactive protein (CRP), lactate dehydrogenase (LDH), and procalcitonin. d -Dimer has been found to be elevated (≥0.5 mg/L) in 46.4% of all patients and 59.6% of those with severe disease. CRP of 10 mg/L or greater has been seen in 60.7% of patients, with a higher percentage of those with elevation with severe disease to 81.5%. LDH is found to be elevated in 41% of patients with a threshold of 250 U/L. Procalcitonin is often not found to be elevated; only 5.5% of all patients and 13.7% with severe disease had procalcitonin levels 0.5 ng/mL or greater. Testing for other respiratory infections, such as influenza, also may be performed concurrently based on prevalence of other infections at the time.
Imaging
Chest x-ray is a common test ordered on patients with respiratory complaints and abnormalities, which are commonly noted in patients with COVID-19. A majority (59.1%) of patients who had a chest x-ray examination demonstrated an abnormality; among patients with increased disease severity, a higher proportion (76.7%) showed evidence of radiographical abnormalities. The most frequently found abnormalities in descending order of prevalence are bilateral patchy shadowing, local patchy shadowing, ground-glass opacity, and interstitial abnormalities. As expected, these findings increase with disease severity. Chest computed tomography (CT) is another imaging modality used to better evaluate lung pathological conditions in COVID-19 patients. Given the increased sensitivity of this imaging modality, a higher percentage of patients (86.2%) had a noted abnormality. In severely ill patients, this increased to almost 95%. Similar abnormalities to the chest radiographs were noted on chest CT. It should be noted, however, that one study found that 56% of patients who presented within 2 days of diagnosis had normal a CT image. Pulmonary embolus is a known sequela of COVID-19 infection, and CT pulmonary angiograms should be considered in patients for whom there is a high clinical suspicion or who have not shown improvement along the expected recovery pattern.
Consultation
Consultation practices should follow the locally determined pathways for engagement with critical care services, infectious disease, and other specialists. Extracorporeal membrane oxygenation (ECMO) may be considered in the severely ill patient, and the appropriate teams should be considered based on the specific hospital’s guidelines. Telemedicine consultation with specialists or other providers may be used in the appropriate circumstances to limit in-person contact and provide additional recommendations.
Management
Treatment of SARS-CoV-2 (COVID-19) in the ED ranges from symptomatic and supportive care to identifying and treating associated end-organ dysfunction and invasive life-sustaining care for severe and critically ill patients. A sample diagnostic and treatment algorithm is shown in Fig. 15.1 . Several medications are currently under investigation and may be appropriate for select patients (see medications section). The National Institutes of Health (NIH) has published information on caring for patients with COVID-19, and detailed information can be found at online at the NIH Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. ,
Symptomatic Support
Patients meeting criteria for mild to moderate disease without oxygen requirement or progressive dyspnea can be managed at home. Treatment consists of supportive care, education on preventing the spread of COVID-19 to others, and guidance on when to seek input from a health care provider or in-person evaluation. Patients with dyspnea may find prone positioning helpful while sleeping or resting. Supportive care includes antipyretics, analgesics, antitussives, and antiemetics. Rest and hydration should be emphasized, as well as signs and symptoms of dehydration that would prompt a need for return visit to the ED. Regarding antipyretics, there is current controversy over the use of nonsteroidal antiinflammatory drugs (NSAIDs), including ibuprofen; therefore it is reasonable to suggest acetaminophen as first-line therapy and using NSAIDs sparingly or for breakthrough fever and pain. Patients who are currently taking NSAIDs for comorbid conditions may continue using as directed. Opioids for pain are usually not indicated and may cause respiratory depression.
Home Oxygen
Depending on hospital capacity and the availability of alternative care facilities, some patients with mild to moderate dyspnea and limited supplemental oxygen requirement may be discharged from the ED with supplemental oxygen with planned virtual reassessment within 24 to 48 hours.
Medications
Since the emergence of the COVID-19 pandemic, recommendations for or against various treatment options have changed dynamically along with our understanding of this novel virus. As such, the recommendations found in this chapter are up to date as of its publication.
Antivirals
Remdesivir is currently the only medication approved by the FDA for the treatment of COVID-19. It is approved for mild to moderate COVID-19 in high-risk nonhospitalized patients and in hospitalized patients (aged > 12 years and weighing >40 kg). The FDA does not recommend using remdesivir in patients with an eGFR of < 30 mL/min due to lack of data. It may be initiated in the ED as a 200-mg oral loading dose. The outpatient regimen is a 3-day course initiated within 7 days of symptom onset (200 mg IV on Day 1, followed by 100mg IV once daily on Days 2 and 3). Patients should be observed for >1 hour after infusion.
Ritonavir-boosted nirmatrelvir (Paxlovid) and molnupiravir have both received Emergency Use Authorizations from the FDA for the treatment of mild to moderate COVID-19 in patients aged >12 years and weighing > 40 kg who are within 5 days of symptom onset and at high risk of progressing to severe disease. The NIH COVID-19 Treatment Guidelines Panel recommends using nirmatrelvir 300mg with ritonavir 100mg (Paxlovid) orally twice daily for 5 days in nonhospitalized patients with mild to moderate COVID-19. Emergency physicians should carefully review the patient’s medications to evaluate potential drug-drug interactions. Resources include the Liverpool COVID-19 Drug Interactions website, the EUA fact sheet for the ritonavir-boosted nirmatrelvir or pharmacist consultation. Molnupiravir should only be used if ritonavir-boosted nirmatrelvir or remdesivir cannot be used. The NIH COVID-19 Treatment Guidelines Panel recommends using molnupiravir 800mg orally (PO) twice daily for 5 days only when alternative therapies cannot be used. Treatment should be started as soon as possible and within 5 days of symptom onset.
Anti–SARS-CoV-2 Monoclonal Antibodies
Anti-Sars-CoV-2 monoclonal antibodies (mAbs) can be considered for the treatment of COVID-19 under Emergency Use Authorization by the FDA for nonhospitalizaed patients with mild to moderate COVID-19 who are at high risk for severe disease. Patients at risk for clinical decompensation who may benefit from monoclonal antibody treatment include those with a body mass index greater than 35, chronic kidney disease, diabetes mellitus, immunosuppressive disease or treatment, sickle cell anemia, age older than 65 years or older than 55 years with coronary artery disease, hypertension, or chronic lung diseases, including chronic obstructive pulmonary disease (COPD). Of note, SARS-CoV-2 variants may have reduced susceptibility to monoclonal antibodies because of mutations in the spike protein, and this may have an impact on effectiveness of treatment. Vaccination should be deferred for at least 90 days after treatment with anti–SARS-CoV-2 monoclonal antibodies. High-risk patients may receive monoclonal antibody therapy in the ED or be referred to an infusion clinic to initiate treatment. The NIH COVID-19 Treatment Guidelines Panel recommends bebtelovimab 175 mg IV in patients aged > 12 years as an alternative only when ritonavir-boosted nirmatrelvir (Paxlovid) and remdesivir are not available. Therapy should be initiated as soon as possible within 7 days of symptom onset and in a setting where the patient can be monitored and treated for severe hypersensitivity (including anaphylaxis) for one hour after injection. Treatment with bamlanivimab plus etesevimab or casirivimab plus imdevimab have been paused because the Omicron variant of concern and its subvariants are not susceptible to these agents
Antibiotics
There is insufficient evidence to recommend for or against empiric broad-spectrum antimicrobial therapy in the treatment of COVID pneumonia. However, if clinical suspicion suggests superimposed bacterial pneumonia (such as the presence of a lobar infiltrate on chest X-ray, leukocytosis, elevated serum lactate, microbiologic laboratory results or shock), clinicians should then follow routine Infectious Diseases Society of America (IDSA) guidelines for appropriate treatment of bacterial pneumonia.
Anticoagulation
Inpatients should be prophylactically anticoagulated with unfractionated or low-molecular-weight heparin if no contraindication exists. Patients with COVID-19 and a diagnosis of thromboembolism should receive the treatment dose of anticoagulation per usual guidelines. Patients being discharged from the ED or hospital with COVID-19 (and no thromboembolism) do not require anticoagulation unless they are at high-risk for thromboembolic disease, in which case, the decision to initiate anticoagulation therapy should be made on a case-by-case basis.
Steroids
There is no indication for steroid treatment in patients with COVID-19 being discharged from the ED who do not require supplemental oxygen. Additionally, the NIH recommends against the use of dexamethasone or other corticosteroids in hospitalized patients who do not require supplemental oxygen. Patients requiring supplemental oxygen, high-flow nasal cannula (HFNC), noninvasive ventilation, invasive mechanical ventilation, or ECMO should be treated with dexamethasone 6 mg orally (PO) or intravenously (IV) daily. If dexamethasone is unavailable, an alternative corticosteroid such as prednisone 40 mg, methylprednisolone 32 mg, or hydrocortisone 160 mg may be substituted. For patients being treated for septic shock and not already receiving a corticosteroid for treatment of COVID-19, the NIH recommends adding a steroid. Patients with COVID-19 being treated for asthma or COPD exacerbations who do not meet criteria for dexamethasone treatment for COVID-19 alone may be treated with an appropriate corticosteroid per the usual protocol.
Respiratory Support
Patient presentations to the ED with COVID-19 range from mild disease to overt respiratory failure. Mortality for intubated patients with COVID-19 is high, around 50%. Many patients have been shown to tolerate hypoxia , ; therefore it is reasonable to titrate from nasal cannula to nonrebreather, HFNC, and then noninvasive positive pressure ventilation (NIPPV) before progressing to intubation. In patients with acute hypoxemic respiratory failure, HFNC is recommended over NIPPV. Symptomatic improvement and decreased work of breathing may be helpful indicators of respiratory status in the presence of permissive hypoxia.
Proning
Nebulizers and Metered-Dose Inhalers
Mild to moderate asthma and COPD exacerbations during the COVID pandemic can be managed with metered-dose inhalers (MDIs) and usual therapy, including steroids and magnesium. If a patient presents with acute respiratory failure or severe asthma or COPD exacerbation, nebulized bronchodilators can be given using inline administration by NIPPV or invasive mechanical ventilation with a viral filter. This should take place in a negative-pressure room, and staff must wear personal protective equipment (PPE) appropriate for aerosol-generating procedures. Finally, subcutaneous terbutaline or epinephrine may be viable alternatives to nebulized bronchodilators if negative-pressure rooms are unavailable. ,
Coronavirus Disease–Associated Acute Respiratory Distress Syndrome and Intubation
There is some evidence that patients with COVID-19 who manifest acute respiratory syndrome represent a different pathophysiology than previously described patients with ARDS not related to COVID-19. Some have recommended referring to this unique entity as coronavirus disease–associated acute respiratory distress syndrome (CARDS). A subset of these patients may retain normal lung compliance and thus can tolerate larger tidal volumes. Research is evolving in this area, and it is recommended that readers refer to the most up-to-date guidance from the Centers for Disease Control and Prevention (CDC) and the NIH.
Emergency physicians should proceed with intubation after a goals-of-care discussion with the patient and family giving careful consideration of age and comorbidities because of the high mortality associated with CARDS. If intubation becomes necessary, a provider with extensive airway management experience should perform the intubation. Strategies that can be implemented to optimize success and limit viral exposure are shown in Table 15.1 .