- There is a strong link between congestive heart failure (CHF), and diabetes and both conditions are becoming increasingly more common.
- The prevalence of the combination of CHF and diabetes was 0.5% in men and 0.4% in women increasing by age in a recent population-based study. Diabetes was found in 12% of those with CHF compared with only 3% of the controls without CHF.
- Diabetes is a serious prognostic factor for cardiovascular mortality in patients with left ventricular dysfunction caused by ischemic heart disease.
- A 1% (11 mmol/mol) reduction in glycated hemoglobin (HbA1c) has been found to reduce CHF by 16%. In this study, the prognosis improved with a decrease in HbA1c without any threshold or observed upper limit.
- Available data favor a proportionately similar efficacy of evidence-based CHF therapy in patients with and without diabetes. The impact expressed as number of patients needed to treat to avoid one event is lower among people with diabetes because of their higher absolute risk.
- Further studies have to be conducted before an aggressive glucose normalization can be recommended as a possibility to improve the prognosis in patients with diabetes and CHF.
- Thiazolidinediones can provoke or worsen CHF. These drugs should be used with great caution in patients with diabetes and heart failure.
Introduction
The concept that there may be a direct relation between diabetes and congestive heart failure (CHF) is not new. In 1954, Lundbaek [1,2] published an article on clinically important complications in patients with diabetes underlining that heart disease was common in patients with diabetes; indeed, it was present in two-thirds of elderly subjects. He was the first to suggest the presence of a diabetes-specific cardiomyopathy. Twenty years later, Rubler et al. [3] published supporting data, concluding that myocardial disease seemed to be a complication of the diabetic state and not merely caused by coronary artery disease (CAD). Shortly thereafter, the Framingham study presented epidemiologic evidence for a strong relation between CHF and diabetes. The latter study clearly indicated that the relation between diabetes and CHF was not only caused by traditional risk factors for coronary heart disease (CHD), but also involved other mechanisms [4].
The prevalence of CHF is increasing in Western societies because of an aging population and also because of increased survival following ischemic heart disease (IHD), and in particular myocardial infarction (MI) [5]. There are many known causes of CHF, including hypertension, CAD, valvular dysfunction, arrhythmias, anemia, renal failure and thyroid dysfunction [5–9]. Risk factors for the development of CHF include increasing age, valvular heart disease and IHD, particularly previous MI, electrocardiographic signs of left ventricular hypertrophy, cardiomegaly detected by chest X-ray, increased heart rate, hypertension and decreased pulmonary vital capacity; diabetes should be added to this list as a strong risk factor. The Framingham study used several of these risk factors to construct a multivariate risk formula to identify high-risk candidates for CHF [10].
Currently, IHD is the leading cause of CHF in industrialized societies with diabetes as a rapidly emerging risk factor for both CHF and IHD [8,11]. Considering the rapidly growing prevalence of diabetes [12], this means that the combination of diabetes and CHF will become increasingly more common in the future. Poor glucose control contributes to the development of CHF as reflected by the relation between an increase in glycated hemoglobin (HbA1c) and the risk of developing CHF [13]. In an epidemiologic study of an elderly Italian cohort, 9.5% of the participants had CHF and 14.7% diabetes. Interestingly, the prevalence of diabetes among subjects with CHF was high, almost 30%. The association was strengthened during follow-up, indicating that CHF predicted the appearance of diabetes [14].
In summary, there is a strong link between the existence of CHF and diabetes, and both conditions are becoming increasingly more common. The links between these disorders are complex and not yet fully explored.
Symptoms and diagnosis
Diagnosis and definition of congestive heart failure
The state of the art diagnosis of CHF is based on a combination of clinical symptoms combined with characteristic signs of myocardial dysfunction [5]. In clinical practice, CHF is commonly divided into systolic and diastolic myocardial dysfunction, the latter also being known as heart failure with preserved left ventricular function, with systolic dysfunction representing an impaired capacity to eject blood from the left ventricle and diastolic dysfunction an impaired ventricular filling caused by relaxation abnormalities. Echocardiography is the preferred method for documentation of such dysfunction, and left ventricular ejection fraction is the most commonly used expression for impaired systolic dysfunction. Evidence of abnormal left ventricular relaxation, reduced diastolic distensibility or diastolic stiffness are the echocardiographic signs of diastolic dysfunction. Echocardiography, including tissue Doppler imaging (TDI), is useful in detecting myocardial diastolic dysfunction in people with diabetes as well as in the non-diabetic population [15,16]. Plasma concentrations of natriuretic peptides or their precursors are also helpful for diagnosing CHF in patients, including those with diabetes [5,17]. The main clinical classification of the severity of CHF is that presented by the New York Heart Association (Table 41.1). This classification is used for all patients with CHF irrespective if seen in a hospital or outpatient setting and irrespective of etiology.
Table 41.1 Classification of congestive heart failure (CHF) according to New York Heart Association (NYHA).
| NYHA Class I | No limitation of physical activities |
| Patients without symptoms during ordinary activities | |
| NYHA Class II | Slight to mild limitation of physical activity |
| Patients comfortable at rest and mild exertion | |
| NYHA Class III | Marked limitation of activity |
| Patients comfortable only at rest | |
| NYHA Class IV | Confined to complete rest in a bed or a chair |
Diagnosis and definition of glucose abnormalities
Diabetes and other glucose abnormalities are a group of metabolic disorders characterized by hyperglycemia caused by defects in insulin secretion, insulin action or both. Diabetes is associated with damage, dysfunction and failure of various organs [18]. The metabolic syndrome is an entity that has been defined in various ways [19,20], combining different cardiovascular risk factors including abnormalities in glucose homeostasis. Further details on the classification and diagnosis of different glucose abnormalities are given elsewhere in this book.
Epidemiology
Risk factors for CHF and diabetes
The most important risk factors for cardiovascular disease (CVD) and MI are family history, smoking, abnormal blood lipids, hypertension, diabetes, obesity and socioeconomic factors [21]. Many of the risk factors for CHF are by necessity similar to those for CVD, with IHD and hypertension as leading causes. Other common factors influencing the occurrence of CHF are male gender, smoking, overweight, physical inactivity and valvular heart disease [5]. Type 2 diabetes mellitus (T2DM) as well as poor glucose control observed as high fasting plasma glucose and elevated HbA1c are also of considerable importance [22–27].
Risk factors for T2DM are family history, age, overweight or increased waist: hip ratio and a sedentary lifestyle [28,29]. The morbidity is known to increase progressively with the number of existing risk factors [30]. Particular risk factors for CAD in T2DM are lipid perturbations, including small, dense, easily oxidized low density lipoprotein (LDL) particles, low high density lipoprotein (HDL) cholesterol and increased triglycerides. Moreover, poor glucometabolic control observed as high fasting plasma glucose and elevated HbA1c contribute [31]. Hypertension is another important factor. The Reykjavík Study showed a strong relation between fasting and post load glucose levels and subsequent risk for hypertension, even after adjustment for age, body mass index (BMI) and weight gain, which is interesting because hypertension is one of the main risk factors for CHF [24,25]. Patients with diabetes and CHF have more IHD, increased systolic blood pressure (BP), lower diastolic BP and a higher HbA1c than their counterparts without diabetes [26]. Accordingly, there are many mutual risk factors for CHF and glucose abnormalities.
Prevalence of CHF and glucose abnormalities
The prevalence of CHF varies somewhat in different studies, partly because of differences in the definition of this disease [5]. The demand that a heart failure diagnosis should be supported by evidence of systolic dysfunction on echocardiography may be difficult to fulfill in epidemiologic studies. Modern echocardiographic techniques did not exist when several of the studies, still serving as important sources of information, were conducted [4,32]. The prevalence of CHF has been estimated to be 0.6–6.2% in Swedish men with an increase by age [32]. This is similar to the overall prevalence of CHF among both genders in the Rotterdam population and in the Reykjavík Study [33,34]. The prevalence of CHF was 1–10% in a British outpatient population [35]. It increases considerably when looking at elderly populations as exemplified by the Italian Campania study, in which the prevalence was 9.5%, once more underlining the impact of age [14].
It has been estimated that at least 30% of patients with diabetes are undetected [20]. When screening a Belgian outpatient population with one known cardiovascular risk factor, diabetes was detected in 11% and an additional proportion of 3% had impaired glucose tolerance (IGT) [36]. The prevalence of diabetes was 7.8% in Swedish men and 5.1% in women aged 35–79 years with similar proportions reported from a Finnish middle-aged population [37,38]. The prevalence of diabetes may be considerably higher in selected high risk populations not the least those with CAD. In the Euro Heart Survey Diabetes and the Heart, patients admitted to hospital because of acute and stable CAD were investigated for the presence of diabetes and IGT. Only 29% of the 4961 patients had a normal glucose metabolism while 31% had known and 12% previously unknown diabetes. The remaining 28% of the patients had IGT [39]. Similar proportions were detected in patient populations with cerebral and peripheral vascular disease [40]. Thus, the combination of CVD and glucose perturbations is very common but underestimated in many previous studies because of lack of diagnostic accuracy in combination with a thorough investigation of the glucometabolic state.
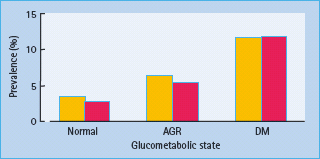
Considerably less is known about the prevalence of the combination of diabetes and CHF. The most recent and extensive study of the prevalence of diabetes and CHF is that from the Reykjavík population [34], in which the prevalence of the combination of CHF and diabetes was 0.5% in men and 0.4% in women, increasing by age. Diabetes was found in 12% of those with CHF compared to only 3% of the controls without CHF. Thus, there was a strong association between diabetes and CHF (Figure 41.1).
Figure 41.1 Prevalence of congestive heart failure (CHF) in relation to glucometabolic state. Yellow bars, males; red bars, females; AGR, abnormal glucose regulation; DM, diabetes. Reproduced from Thrainsdottir et at. [34], with permission from American Diabetes Association.
Based on Framingham data, Rutter et al. [41] noted that the heart is prone to changes in the form of increased left ventricular mass and wall thickness with worsening glucose tolerance. Kannel et al. [4] and Gustafsson et al. [42] reported on the role of diabetes in CHF from a general and a hospitalized population, respectively. Their findings indicated a strong association between CHF and diabetes. Iribarren et al. [43],Bertoni et al. [44] and Nichols et al. [26], focusing on the role of CHF in patients with diabetes, noted that the prevalence of CHF varied between 1.9 and 22.3%. Finally, Amato et al. [14] found a strong association between diabetes and CHF in a population of elderly people. The outcomes of these studies have been summarized in Table 41.2.
Table 41.2 Comparison of the prevalence, incidence and prediction of heart failure and diabetes in general populations and among patients.
Incidence of CHF and glucose abnormalities
Recent results from the Framingham study indicate that the incidence of CHF has declined during the last five decades [45], but these data are not supported in other studies [46]. On the contrary, it seems that hospital admissions for CHF are increasing, resulting in higher health care expenditures for patients with this diagnosis [47]. Among British outpatients, the incidence of CHF has been reported to be 4.4/1000 person-years in men and 3.9/1000 in women, rising with age in both genders [48]. The incidence in Finland is similar among men, 4.0/1000 person-years, but lower in women 1.0/1000 person-years [49].
The age-standardized annual incidence of diabetes, reported to be 2.2 and 2.3/1000 person-years in Dutch men and women, respectively, is rather uniform in several European countries [50]. When turning to an elderly population, however, as in the Italian Campania study, the incidence was considerably higher at 6.1% per year. This is somewhat different from the observation in the Netherlands where the incidence decreased in the oldest age group [14,50].
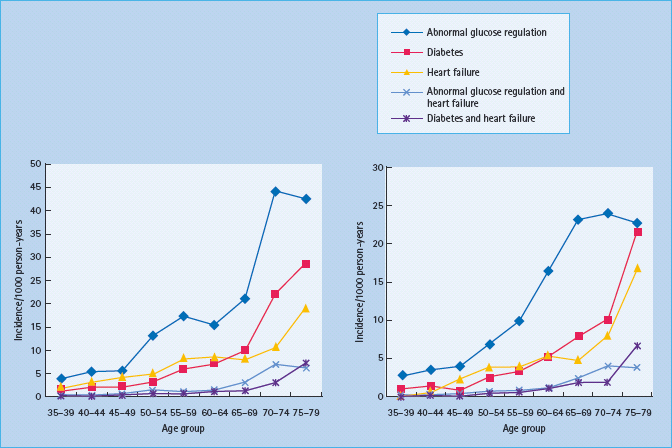
Considerably less is known about the incidence of the combination of diabetes and CHF. Once more it seems as if the most recent and extensive study originates from the Reykjavík population. In this study, the age and gender standardized incidence of abnormal glucose regulation was 12.6/1000/year, diabetes 4.6/1000/year and CHF 5.3/1000/year (Figure 41.2). In addition, there was a strong association between the incidence of glucose abnormalities and CHF [51].
Figure 41.2 Incidence of abnormal glucose regulation, diabetes, congestive heart failure (CHF), abnormal glucose regulation and CHF, and diabetes and CHF expressed as number of incident cases/1000 person years among men (left) and women (right) by age. Reproduced from Thrainsdottir et al. [51], with permission from Oxford University Press.
In the Framingham study, the incidence of CHF was twice that among males and five times higher in females with diabetes during 18 years of follow-up than patients free from diabetes. The excessive risk of CHF remained high even after the exclusion of patients with prior CAD [4]. In a general population of elderly Italians, the prevalence of diabetes was 9.6% per year in CHF patients [14].
Pathophysiology
Myocardial structural and biochemical alterations can be identified in the failing heart, and many of them seems to be independent of the etiology of myocardial dysfunction. They include changes in myocardial energy production, altered expression of contractile proteins, a desynchronized excitation–contraction coupling, β-adrenergic receptor stimulation, myocyte depletion and increased activity of a number of cytokines. Many of these aberrations are found in diabetic heart. Here, some general features of the pathophysiology are followed by a discussion of more diabetes-specific factors.
Congestive heart failure
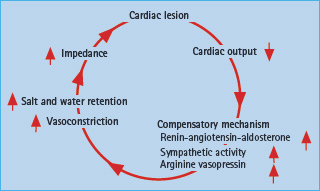
CHF is a clinical syndrome originally induced by myocardial damage but subsequently influenced by the induction of an untoward neurohormonal response. Thus, norepinephrine, angiotensin II, endothelin and aldosterone are all linked to the vicious cycle of myocardial remodeling (Figure 41.3) which unopposed will cause successive deterioration of myocardial performance [52].
Figure 41.3 Neurohormonal activation caused by depressed myocardial function leads to a vicious circle further compromising the already compromised myocardial function.
Metabolic conditions have a significant role in cardiac adaptation and remodeling. This leads to an increase of myosin heavy chain beta, altered troponin T (TnT) molecules, diminished storage of creatinine phosphatases, and decreased sarcoplasmatic ATPase activity, which may result in myocyte hypertrophy associated with impaired contractile function and less effective energy supply [53,54].
The myocardium has a high energy turnover with ATP as an important source of energy. The two pathways for energy supply are via the breakdown of free fatty acids (FFA) and of carbohydrates (Figure 41.4). The lipolytic pathway transfers FFA via β-oxidation to acetyl co-enzyme A (ACA), which enters the citric acid or Krebs cycle. The carbohydrate pathway produces pyruvate via glycolysis, glycogenolysis and lactate oxidation. Pyruvate is decarboxylated via pyruvate dehydrogenase to ACA, which enters the Krebs cycle. The dominant pathway for myocardial energy production is β-oxidation of FFA, but the myocardium is also dependent on glucose oxidation [55].
Figure 41.4 Schematic illustration of myocardial energy production of relevance for congestive heart failure (CHF) patients with and without diabetes. The site of action for metabolic modulators are indicated. See text for further information. CoA, co-enzyme A; FFA, free fatty acids; PDH, pyruvate dehydrogenase.
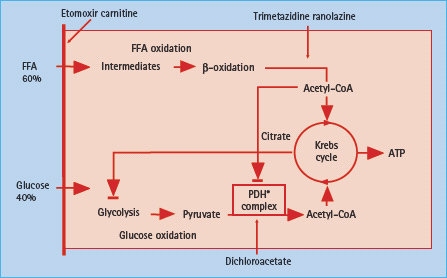
When the heart is subjected to ischemic stress or exposed to sustained enhancement of intraventricular pressure, it tends to change towards a more dominant glucose oxidation [56]. This may be counteracted by a reduction of the glucose transporter 4 (GLUT-4), which becomes reduced in CHF, hampering glucose transport over the cell membrane. At the same time, the heart is subjected to increased FFA concentrations, released via stress influenced by an increased sympathetic tone [57]. It is assumed that prolonged intracellular accumulation of FFA and its metabolites may cause myocardial dysfunction [58].
Besides these mechanisms, alterations in gene expression and inflammatory activity have been suggested to cause metabolic and mechanical disturbances in CHF [59–62]. All nucleated cells, including the cardiomyocyte, can produce pro-inflammatory cytokines as a response to injury such as MI, myocarditis or when the heart fails. Both tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6) levels increase in proportion to the severity and duration of CHF [59,60]. This cytokine release may trigger a cascade of events leading to myocardial structural alterations further deteriorating the clinical picture of CHF.
CHF and diabetes
The main myocardial energy production is normally based on β-oxidation of FFA (70%) with a smaller contribution from glucose oxidation (30%) and lactate. FFA are produced by lipolysis of endogenous cardiac or exogenous stores of triglycerides. Oxidation of FFA is an effective supplier of energy in the form of ATP if the oxygen supply is sufficient. In conditions with limited oxygen availability, glucose oxidation will provide more energy per mole oxygen and support more work than FFA [63]. In the person with diabetes, glucose utilization for energy production is substantially lower, about 10% (Figure 41.4). The shift to an even more pronounced β-oxidation of FFA causes a higher oxygen utilization than under normal circumstances [64]. The major restriction to glucose utilization in the diabetic heart is the slow rate of glucose transport across the sarcolemmal membrane in the myocardium [65,66]. The impaired glucose oxidation in the diabetic heart can also result from a decreased rate of phosphorylation of glucose which subsequently limits the entry of glucose into the cell. The depressed phosphorylation is triggered by the increased metabolism of FFA [64]. Insulin deficiency enhances lipolysis thereby increasing circulating FFA [67]. People with diabetes are also known to have increased risk for other disturbances such as reduced myocardial blood flow and blunted hyperkinetic response to myocardial ischemia resulting in diminished myocardial function [68–71]. Indeed, CHF is an insulin-resistant state with an increased release of non-esterified fatty acids which are taken up in muscular tissue and downregulate glucose uptake and utilization [72].
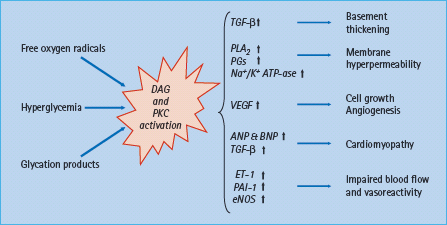
Another consequence of hyperglycemia is oxidative stress and activation of processes triggered by an increased level of diacylglycerol and protein kinase C as depicted in Figure 41.5. Besides, many other unfavorable effects of the increased levels of inflammatory cytokines in heart failure patients may enhance insulin resistance [71,72].
Figure 41.5 Metabolic effects of hyperglycemia induced activation of protein kinase C (PKC) and diacyloglycerol (DAG). See text for further explanation. ANP and BNP, atrial and brain natriuretic peptide; eNOS, endothelial nitrous oxide synthase; ET-1, endothelin 1; PAI-1, plasminogen activating inhibitor 1; PG, prostaglandin; PLA2, phospholipase A2; TGF-β, transforming growth factor β; VEGF, vascular endothelial growth factor.
Prognosis
CHF in general
During the past 30 years, mortality from CHD has declined markedly among patients free from diabetes. This decline has been substantially lower in men but is not seen in women with diabetes [73]. In the presence of CHF, the prognosis becomes poor [74]. In an English population, 1 month survival was 81% after incident CHF, declining to 57% after 18 months [8,75]. The annual mortality in a study of patients hospitalized for CHF was 10–20% with mild–moderate and 40–60% with severe symptoms [35]. The mortality in an elderly population with CHF recruited in Rotterdam was 47% during 6 years of follow-up. This is twice that of persons without CHF [74]. In a comparable Italian study, the mortality rate was 21.3% after 3 years [14]. Thus, CHF is a malignant disease irrespective of the underlying reason for myocardial dysfunction. Recent reports have been somewhat more encouraging. A 50-year follow-up of the Framingham data indicates that CHF survival has improved to some extent [45]. This observation is supported by a report based on the Swedish hospital discharge registry [76].
Diabetes and CHF
CVD is the most prevalent complication of diabetes and is of major importance. Cardiovascular mortality in men with diabetes lies between the mortality for men with angina and MI [77]. In the USA, it has been estimated that 77% of all hospitalizations for chronic complications of diabetes are attributable to CVD [78]. T2DM doubles the risk of death from CHD, and T2DM diagnosed at the age of 55 years reduces life expectancy by about 5 years [79]. This makes mortality from CVD in people with diabetes but without previous MI similar to the mortality in patients with a history of MI but without diabetes [80].
The prognosis of patients with diabetes becomes even worse in the presence of CHF [81–84]. In the first DIGAMI study, performed in patients with diabetes and acute MI, CHF was the most common reason for morbidity and mortality, accounting for 66% of the total mortality during the first year of follow-up [85]. Diabetes is a serious prognostic factor for cardiovascular mortality in patients with left ventricular dysfunction caused by IHD [86]. In a general population in Reykjavík, the survival decreased significantly with the concomitant presence of both CHF and glucose abnormality even after adjustment for cardiovascular risk factors and IHD [87], which may serve as an indicator of the serious implication of the presence of diabetes along with CHF.
Hyperglycemia and prognosis
The relationship between plasma glucose and mortality has been elucidated in several studies. According to the UK Prospective Diabetes Study (UKPDS), a 1% (11 mmol/mol) reduction in HbA1c is associated with a reduction of MI by 14% and CHF by 16%. In this study, the prognosis improved with a decrease in HbA1c without any threshold or observed upper limit [88]. In the DECODE study, a 2-hour post load glucose was a better predictor of mortality than fasting blood glucose [89]. This may perhaps be seen as an indicator of the importance of post-prandial hyperglycemia and the potentially serious implication of IGT for myocardial function.
Treatment
General aspects
Evidence-based treatment of CHF relies on a combination of angiotensin-converting enzyme (ACE) inhibitors and/or angiotensin receptor blockers (ARBs), beta-blockers, diuretics and aldosterone antagonists [5]. ACE inhibitors, ARBs and beta-blockers reduce mortality and improve symptoms in moderate to severe CHF with or without diabetes [90–93]. Diuretics are mandatory for symptomatic treatment of fluid overload, but should not be used in excess because they induce neurohormonal activation [94]. The addition of aldosterone antagonists is indicated in severe forms of CHF and may then improve longevity [95]; however, many patients are, although symptomatically improved, left with an unfavorable vital prognosis despite the best available pharmacologic treatment. A search for novel treatment modalities is therefore still ongoing. One of these is metabolic modulation with compounds that influence the disturbed metabolic pathways in CHF and which are thought to be of particular importance in patients with diabetes [55]. Attention has been paid to compounds that shift energy production from β-oxidation of FFA towards the energetically more efficient glucose oxidation under such conditions as in myocardial ischemia and CHF. Examples of such drugs are trimetazidine, ranolazine, etomoxir and dichloroacetate [96,97].
Various techniques have been used to study the efficacy of pharmacologic treatment in CHF. Among them are the general feeling of well-being as assessed by means of different questionnaires, and an estimation of physical capacity tested by exercise tolerance on a bicycle ergometer or treadmill. Two-dimensional echocardiography is the most commonly applied technique for investigating myocardial function [98–101]. A relatively newly developed technique, TDI assesses myocardial function in different myocardial segments. This technique is useful for diagnosing left ventricular dysfunction even before any symptoms or signs of CHF appear in subjects with diabetes [102–104].
Guidelines that specifically deal with diabetes and CVD including CHF have not been available until recently. Through a combined initiative by the European Society of Cardiology (ESC) and the European Association of the Study of Diabetes (EASD), guidelines on the management of diabetes, pre-diabetes and CVD were published in 2007 [105]. As outlined in this document, there are few trials that specificially address the treatment of patients with the combination of glucose abnormalities and CHF, causing a lack of specific evidence regarding the management of such patients. Current data are mostly based on analyses of subgroups of patients with diabetes in large CHF trials. This results in potential shortcomings related to a poor definition of diabetes and hidden diabetes, actual glucose lowering therapy and also a risk for selection biases with an overrepresentation of less severe diabetes. With these limitations in mind, available data favor a proportionately similar efficacy in patients with and without diabetes. Because the absolute prognosis is considerably worse in patients with diabetes, the impact of therapy expressed as number of patients needed to treat to avoid an event (e.g. hospitalization for CHF or death) is considerably lower among these patients than their counterparts without diabetes.
Pharmacologic therapy of CHF
ACE inhibition
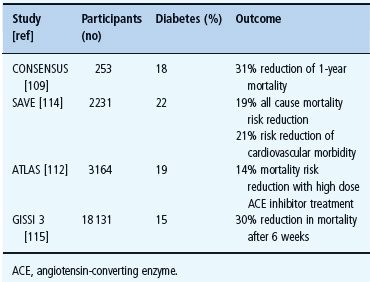
ACE inhibitors are recommended both in asymptomatic myocardial dysfunction and overt CHF. As shown in Table 41.3, they reduce mortality and improve symptoms in moderate to severe CHF with and without diabetes [106–110]. Patients with diabetes represent rather large subgroups in several trials. In SOLVD, the effect of enalapril on compromised left ventricular function was similar in patients with and without diabetes [111]. In ATLAS, comparing a high and a low dose lisinopril treatment strategy, mortality reduction was at least as good in patients with as in those without diabetes [112]. The GISSI 3 and the SAVE trials reported beneficial effects on morbidity and mortality of ACE inhibitor treatment in post-MI patients with diabetes [113–115].
Table 41.3 The effect of inhibition of the renin angiotensin system in congestive heart failure (CHF) trials by diabetic state.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







