Abstract
Chemotherapy and HER2-directed therapy for metastatic breast cancer represent an important and quickly evolving area. Much of the significant progress since 2010 has focused on advances in HER2-directed therapy, including introduction (and US Food and Drug Administration approval) of the agents pertuzumab and trastuzumab-emtansine (T-DM1), which has led to large improvements in overall survival for patients with HER2-positive metastatic breast cancer. Another important advance has been the improving understanding of triple-negative breast cancer as a heterogenous and distinct disease subtype and the demonstration of clinical efficacy for platinum-type agents (cisplatin and carboplatin) in triple-negative disease specifically. Progress in systemic therapy for brain metastases, an evolving understanding of treatments specific to BRCA -deficient breast cancer, and the ongoing introduction of many novel therapies including immunotherapy are all exciting recent developments in this field.
Keywords
chemotherapy, HER2, T-DM1, pertuzumab, triple-negative breast cancer, metastatic breast cancer, BRCA deficient, brain metastasis
Although the past decades have seen great growth in the arsenal of breast cancer treatments, approximately 40,000 individuals still die each year from metastatic disease, which remains incurable in the vast majority of patients. In recent years, there has been significant progress made in the development of HER2-targeted therapies, dramatically improving the prognosis of this historically unfavorable disease subset. New agents such as mammalian target of rapamycin (mTOR) inhibitors and cyclin-dependent kinase (CDK) 4/6 inhibitors have been approved for the treatment of hormone receptor–positive disease, and many additional agents are in development. Despite major efforts in the development of smarter, less toxic targeted therapies, in 2016 cytotoxic chemotherapy remains a central component of treatment for most women with advanced breast cancer. Current research strives not only to develop new therapies but also to identify populations in whom existing agents will be most effective and to optimize the activity of newer therapeutic modalities such as targeted and immune-based therapy by combining them with a chemotherapy backbone. Overall, women living with metastatic breast cancer (MBC) have far more options than in the past, and there has been a modest prolongation in overall survival (OS) over the course of the 21st century. Regardless the goal of cure remains unattained except in anecdotal cases, and ongoing progress is essential.
Epidemiology
Breast cancer remains the most common noncutaneous malignancy and the second leading cause of cancer death after lung cancer among women in the United States. In 2015 it was estimated that more than 230,000 new breast cancer diagnoses were made and that MBC was responsible for nearly 41,000 deaths. For women aged 20 to 59, MBC remains the leading cause of cancer death and is therefore an important public health concern. Although only approximately 6% of women with breast cancer initially present with metastatic disease, many women with localized disease or locoregional spread at diagnosis go on to develop distant disease despite adjuvant therapy. In patients diagnosed with MBC, 5-year survival is estimated to be 26%, and median OS is approximately 31 months, although this varies substantially based on disease characteristics. As such, a significant number of women with advanced breast cancer survive for many years. This statistic stands in sharp contrast to many other solid malignancies such as adenocarcinoma of the lung, pancreas, and stomach, in which 5-year survival remains less than 5%. Nevertheless, there is an urgent need for better therapies.
Therapeutic Goals
Although there are rare anecdotes reporting cure or long-term remission in patients with MBC, in the overwhelming majority of cases, the goal of therapy is symptom control and prolongation in survival. Unfortunately, the vast majority of women with MBC will experience disease progression within 1 to 2 years of treatment initiation and only 1% to 2% will be alive 20 years from diagnosis of MBC. Treatments should be selected to achieve three objectives simultaneously: prolong survival, control symptoms if present, and minimize therapy-associated toxicity. These goals are usually achieved through the administration of systemic therapy (hormonal therapy, chemotherapy, and targeted agents) with judicious use of both radiation therapy, and, less frequently, surgery.
Prognostication
Prognostic and predictive factors should be used to determine the most appropriate therapy for an individual. Although breast cancer was historically classified as a single disease, it is now known to be a heterogeneous group of diseases, all of which arise in the breast. Complex differences in tumor biology produce variable responses to therapy in individual patients. For this reason, the particular characteristics of a patient’s tumor may help the clinician predict the pace of disease, likelihood of response to certain therapies, and OS.
In general, OS and disease-free intervals are longer for patients with estrogen receptor (ER)- and/or progesterone receptor (PR)-positive disease; this is likely the result of a more indolent natural history, and, even more important, the availability of a large armamentarium of active endocrine therapies that are generally used before the administration of chemotherapy. Similarly, given the development of multiple highly effective HER2-targeted therapies in recent years, more patients with HER2-positive disease are cured with adjuvant therapy, and median OS for HER2-positive metastatic breast cancer has improved dramatically. By contrast, patients with “triple-negative” tumors that do not express ER, PR, or HER2 typically have a shorter disease-free interval before the development of metastatic disease, as well as a shorter median OS from the time of diagnosis of metastatic disease.
Other disease-specific characteristics that may help predict prognosis include disease-free interval before the development of metastatic disease, number of disease sites, visceral versus bone-only metastases, and disease volume. On the basis of data assembled more than 2 decades ago from the MD Anderson Cancer Center, the 5-year survival for patients with isolated bone metastases is 23%, compared with only 13% for MBC patients with visceral metastases. Patients with a prolonged relapse-free survival (>5 years) before diagnosis with MBC also have a more favorable prognosis. As a general rule, women with chest wall, nodal, bone, or soft tissue recurrences live longer than those with visceral or central nervous system (CNS) disease.
The other component to prognosis rests in individual patient characteristics. Younger age, better performance status, fewer comorbidities, and lower burden of disease all predict a better prognosis. This may be partially a result of a patient’s ability to tolerate toxic therapy and may also reflect differences in treatment approaches used for younger and older patients. In addition, past response to therapy or lack thereof may predict future response to therapy with new agents. Although mechanisms of chemotherapy resistance have not been fully elucidated, historical information suggests that tumor resistance to one agent is associated with an increased likelihood of resistance to other chemotherapy agents.
Despite a broad survival range for patients with MBC, the median OS remains approximately 3 years. Analysis of patients treated in the 1980s and 1990s suggests that independent of timing of detection of metastatic disease there has been an absolute improvement in prognosis for MBC patients, which has been largely attributed to the introduction of taxanes as first-line chemotherapy in the metastatic setting. Multivariate analysis of patients treated throughout the 1990s demonstrates that for the period from 1991 to 2001, access to new therapeutic agents for MBC resulted in improved survival. More recently, the slow uptrend in MBC survival has continued. Although the therapeutic driver of this trend is not entirely clear, presumably some credit is due to the introduction of highly effective anti-HER2 therapies.
Medical Evaluation in the Metastatic Setting
Large randomized trials have shown that routine surveillance testing after adjuvant therapy does not improve survival or health-related quality of life. As a result, the diagnosis of metastatic disease is typically made when a patient presents with new symptoms (e.g., bone pain, seizure, shortness of breath); has asymptomatic laboratory abnormalities; has a clinically detected local recurrence on the chest wall, in the regional lymph nodes, or within the breast itself; or has incidental findings on imaging performed for other purposes.
Once there is a suspicion for metastatic disease based on laboratory results, physical examination, or radiographs, it is generally important to biopsy a metastatic site to confirm that the lesion is consistent with breast cancer rather than another primary malignancy or some other benign process. It should be noted that there are multiple cases reported of women presenting with mediastinal lymphadenopathy on computed tomography (CT) scan that is assumed to be breast cancer but is found on biopsy to be sarcoidosis. Rebiopsy at the time of diagnosis with metastatic disease also provides a unique opportunity to reassess receptor status before the initiation of therapy. Biopsy can determine whether the patient’s MBC has the same receptor expression as the original lesion because in some cases, these properties have been known to change. One prospective study demonstrated discordant receptor status between primary and metastatic lesion in 16%, 40%, and 10% of cases for ER, PR, and HER2, respectively. In certain patients, the index of suspicion for MBC is high enough and/or the metastatic site is not amenable to biopsy, in which case one may choose to forgo biopsy. However, in almost all clinical scenarios, rebiopsy is feasible and recommended. Additionally, in the modern era, genomic profiling of metastatic biopsy tissue can have implications for clinical trial agent selection, and the use of molecular medicine will likely increase in the coming years.
Before initiation of therapy, one should assess the extent of disease with imaging of the chest and abdomen as well as with bone scan because bone involvement is quite common in MBC. The relative merits of fluorodeoxyglucose positron emission tomography (FDG-PET) scanning in this setting have been the subject of debate and at this time, PET scanning is an option but should not be considered essential. It is a sensitive imaging modality but not specific for malignancy, often resulting in findings that are difficult to interpret. Highly metabolic foci may be seen in inflammatory conditions such as rheumatologic disease or infection; FDG avidity may therefore result in unnecessary anxiety or inaccurate assumptions about the extent of disease. In patients with documented metastases, PET often does not offer enough clinical information over CT and bone scan to warrant the cost. With improvements in technology and changes in cost structure, recommendations may well evolve over time.
Imaging of the brain with contrast-enhanced CT or ideally gadolinium-enhanced magnetic resonance imaging (MRI) certainly should be performed in any MBC patient with focal neurologic findings or symptoms to suggest CNS involvement such as headaches, seizures, or cognitive changes. Given the high rate of CNS disease in women with HER2-positive breast cancer previously treated with trastuzumab and in triple-negative disease, there are some clinicians who advocate scanning asymptomatic patients with these disease subtypes, but there are no data to support that this approach improves survival or affects quality of life. In the absence of CNS signs and symptoms, brain imaging generally should not be performed when a patient is newly diagnosed with metastatic disease and is rarely required for clinical trial participation.
In addition to radiographic imaging, laboratory studies and a careful physical examination should also be performed. Physical examination should focus on identification of symptomatic foci such as bone tenderness, neurologic findings, chest wall disease, or lymphadenopathy. These findings may guide directed therapy with bisphosphonates or radiation and offer a baseline from which response to therapy may be assessed. Baseline laboratory studies can evaluate renal and hepatic function, electrolyte status, and bone marrow reserve in preparation for treatment with chemotherapy. Finally, serum tumor markers such as carcinoembryonic antigen (CEA) and cancer antigen (CA) 27-29 may be measured at the time of diagnosis and, if elevated, are often helpful in monitoring response to therapy. Tumor markers need to be used with caution because they do not always correlate with the course of the disease.
Local Therapy for Metastatic Breast Cancer
Although the general treatment paradigm for MBC is systemic therapy, there are unique clinical scenarios in which local therapy to a metastatic site may further the goals of therapy, namely symptom control and improvement in survival. Possible reasons to consider local therapy include oligometastatic disease, local symptoms that are unlikely to respond to systemic therapy, and impending local complications such as spinal cord compression, hydronephrosis, or bone fracture. Surgical interventions for metastatic disease may include resection of a CNS lesion, chest wall lesion, isolated pulmonary nodule, or isolated hepatic nodule, or drainage of a pleural effusion. Longitudinal data indicate that metastasectomy is becoming increasingly common in breast cancer and many other solid malignancies. However, metastasectomy for reasons other than resection of a CNS lesion or other local palliation remains a nonstandard approach, and there are no prospective data demonstrating that it improves disease outcomes. Local radiation therapy is indicated in the event of cord compression or unstable bone lesions, although the need for radiation has been reduced by the widespread use of bone-modifying agents for lytic bone disease.
Breast Surgery in Patients With Metastatic Disease
The decision of whether to offer breast surgery to a woman presenting with metastatic disease has long been debated, and practice styles vary greatly. In general, local therapy to the primary tumor is not thought to have an impact on clinical outcome and offers only palliation in symptomatic patients. In a retrospective examination of the National Cancer Database from 1990 to 1993, more than 16,000 women presenting with stage IV disease were identified, and 42.8% of these patients did not undergo definitive resection of their tumors, whereas 57.2% of patients underwent partial or total mastectomy. Women treated with surgical resection in whom negative margins were achieved had a superior prognosis compared with women who did not receive surgery (hazard ratio [HR] 0.61). Multiple smaller studies have reported similar results. Another large study that addressed this question was a retrospective, population-based cohort study evaluating more than 9000 women with stage IV breast cancer from the Surveillance, Epidemiology, and End Results database. In this patient cohort, 47% underwent breast cancer surgery, and 53% did not. After controlling for confounding variables and propensity scores, patients who underwent surgery were less likely to die during the study period compared with women who did not undergo surgery (HR 0.63). In retrospective studies, this benefit has only been seen in women whose tumors were resected with negative margins. Although these results suggest that local therapy may improve outcome, they likely also reflect a significant selection bias; women who underwent surgery were almost certainly different in ways that can and cannot be quantified from those in whom the primary tumor was not resected. Given the biases inherent in these retrospective analyses, this question can be answered only by prospective study.
Therefore numerous prospective evaluations have been undertaken, with inconclusive results thus far. The Turkish MF07-01 study was a phase III trial randomizing women with de novo MBC to systemic therapy with or without standard locoregional resection for their metastatic breast disease. The trial was powered to detect an 18% improvement in survival in the resection arm, and at a mean follow-up of 21.1 months, failed to demonstrate any difference in OS between the two groups. In a subgroup analysis, patients with solitary bone metastasis experienced significantly longer OS with surgery versus no surgery. In a similar study conducted in India, 350 women with de novo metastatic breast cancer and an objective response to initial chemotherapy were randomized to standard locoregional management, or no locoregional management. No significant OS difference was detected between the two groups at a median follow-up of 17 months.
The Translational Breast Cancer Research Consortium study 013 prospectively followed metastatic breast cancer patients who had de novo metastatic disease and intact primary, or who developed metastatic disease within 3 months of primary breast surgery. In this nonrandomized design, patients who underwent breast surgery had significantly better 2-year OS than patients who did not undergo surgery. However, when the cohort was limited to patients with de novo metastatic disease and intact primary who responded to systemic therapy, there was no improvement in 2-year OS in patients who underwent elective breast surgery. Overall, the therapeutic value of primary breast surgery in de novo MBC is still unclear, and many additional prospective studies of this question are underway. At present, the consideration of local resection in a woman with MBC should be managed on an individual basis.
Selecting Therapy for Metastatic Breast Cancer
Before initiating treatment in the metastatic setting, there are many factors to be weighed. The primary objective is to select the regimen that is most likely to yield a clinical response while minimizing toxicity and side effects. Tumor characteristics either from the original breast biopsy, or ideally from a metastatic site, determine which classes of agents are likely to be active against a given tumor. Chemotherapy is used invariably in the course of treatment for metastatic breast cancer of all subsets, but the appropriate time and context for its use varies by subtype (hormone receptor–positive vs. HER2-positive vs. triple-negative). ER-positive and/or PR-positive disease is often initially sensitive to endocrine therapy and cytotoxic agents are reserved for the scenarios outlined subsequently. HER2-directed therapies will form the backbone of all systemic therapy in HER2-positive disease but are generally used in combination with chemotherapy, as discussed later in the chapter. Lastly, in women with triple-negative cancers, cytotoxic therapy is the only treatment option with documented clinical activity and has a role in all lines of treatment, outside of a clinical trial.
In patients with hormone receptor–positive tumors, endocrine therapy should be the initial course of treatment unless they have extensive visceral metastases (“visceral crisis”), rapid disease progression, or symptoms that need rapid palliation ( Fig. 69.1 ). Of course, the decision to use endocrine therapy also will be influenced by prior endocrine treatment in the adjuvant setting. Response rates to first-line treatment for ER-positive/PR-positive, ER-positive/PR-negative, and ER-negative/PR-negative tumors in patients who have never received endocrine therapy are approximately 70%, 40%, and 10%, respectively. (Of note, the 10% response rate for ER-negative/PR-negative disease is based on old literature; this value has not been systematically evaluated with more accurate modern testing and is almost certainly far lower.) Although patients whose disease displays primary resistance to upfront endocrine therapy should usually proceed directly to treatment with chemotherapy, women with an initial response to endocrine therapy may receive multiple additional lines of endocrine therapy. Treatment options for women receiving endocrine therapy include tamoxifen, aromatase inhibitors, ovarian suppression with gonadotropin-releasing hormone (GnRH) agonists, fulvestrant, progestins, high-dose estrogens, and androgens. Aromatase inhibitors (letrozole, anastrozole, and exemestane) are often used in the first-line setting for postmenopausal women given the low risk of “tumor flare” with agents such as tamoxifen and evidence that they offer superior time to progression (TTP) and response rates compared with first-line tamoxifen for MBC. Recent evidence demonstrates that the combination of palbociclib (Ibrance, Pfizer, New York, NY), a cyclin-dependent kinase (CDK) 4/6 inhibitor, dramatically improves progression-free survival (PFS) when combined with both first- and second-line endocrine therapy in the treatment of metastatic disease. Palbociclib gained US Food and Drug Administration (FDA) accelerated approval in 2016 and is currently standard of care in combination with endocrine therapy in women with hormone-responsive metastatic breast cancer.
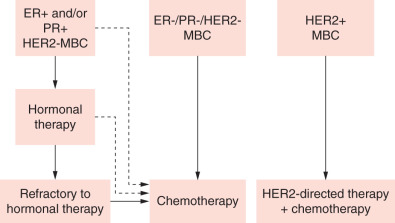
In patients who have previously been treated either in the adjuvant or metastatic setting, shorter disease-free interval is a predictor of more aggressive disease. The degree of symptoms, tempo of disease, and extent of disease (visceral vs. bone-only involvement) are critical criteria for drug selection and timing of therapy in the palliative setting. Response to prior treatment has also been shown to be a predictor of response to the next line of therapy, so early relapse after adjuvant therapy is suggestive of resistant disease. If there is a short disease-free interval, generally defined as less than 1 to 2 years, strong consideration should be given to the use of new non–cross-resistant agents based on the presumption that the tumor is now resistant to drugs administered in the past. In general, women who have received anthracyclines in the adjuvant setting are not retreated with anthracyclines in the first-line setting for MBC, largely related to concerns about cumulative cardiac toxicity.
A final dimension to individualizing the treatment plan incorporates patient preference, performance status, and comorbidities. A patient’s unique constellation of comorbidities, age, and general health status will likely predict her ability to tolerate a given treatment. The toxicity profile of a given agent may limit its use (e.g., avoiding anthracyclines in a patient with borderline cardiac function or risk factors for cardiac dysfunction or avoiding taxanes in a patient with preexisting neuropathy). In addition, patient preference regarding alopecia or concerns about toxicities (e.g., neurotoxicity), which may have an impact on occupation and quality of life, should also be considered in the decision-making process.
Selecting a First-Line Regimen in HER2-Negative Metastatic Breast Cancer
There is a panoply of chemotherapeutic agents known to be active in MBC, including anthracyclines, vinca alkaloids, antimetabolites, alkylating agents, and microtubule inhibitors ( Table 69.1 ). Selection of a first-line agent is often based primarily on a patient’s previous treatment because likelihood of response is more a function of line of therapy than it is of the agent used. At this time, the National Comprehensive Cancer Network (NCCN) breast cancer panel does not recommend a specific first-line agent because there is no evidence to support the use of drugs in a particular sequence. For this reason, treatment is tailored to the individual, as described previously. In patients who have received anthracyclines and/or taxanes in the adjuvant setting, agents with different mechanisms are chosen in the first-line setting for metastatic disease. Similarly, because no agent has been found to be superior, individualized therapy based on patient preference, comorbidities, and known drug toxicities is recommended. Multivariate analyses have demonstrated that the characteristics of the patient’s tumor and pace of disease are better predictors of response and survival than the class of drug used.
| Agent | RR (%): Prior Therapy | RR (%): No Prior MBC Therapy |
|---|---|---|
| 5-Fluorouracil | 21 | |
| Capecitabine | 26–29 | |
| Carboplatin | 19–31 a | |
| Cisplatin | 33 a | |
| Cyclophosphamide | 35 b | |
| Docetaxel | 34–35 | |
| Eribulin | 12 | |
| Doxorubicin | 26 | |
| Gemcitabine | 1–30 | 14–37 |
| Ixabepilone | 12 | 42 |
| Liposomal doxorubicin | 26 | |
| Paclitaxel | 32 | |
| Vinorelbine | 32–36 | 34 |
a Triple-negative breast cancer patients only. Some patients with one prior line of therapy in the metastatic setting.
b Small, historical study. Cyclophosphamide is seldom used as a single agent.
Single-Agent Versus Combination Chemotherapy in HER2-Negative Breast Cancer
It is a common and logical assumption that combination chemotherapy for MBC should result in superior response rates, as well as improved palliation, disease-free survival, and OS. For many decades, it was theorized that combining drugs with nonoverlapping toxicities and different mechanisms of action would overcome drug resistance in tumor cells via synergy. This approach has been successful in the treatment of lymphoma, leukemia, and germ cell tumors but has not been confirmed in MBC. Multiple clinical trials from the pretaxane era supported the superiority of polychemotherapy over monotherapy with respect to response rates, although improvements in OS were generally marginal at best. It should be noted, however, that none of these trials that demonstrated even a modest improvement in survival compared combination chemotherapy with the same single agents used in sequence ( Table 69.2 ).
| Study | Treatment Arms | RR (%) | TTP/TTF (mo) | OS (mo) | Superior QOL? | Crossover Permitted |
|---|---|---|---|---|---|---|
| Joensuu et al., 1998 | E→M | 48→16 | Equal | Equal | E→M | Y |
| CEF→MV | 55→7 | |||||
| Norris et al., 2000 (MA8) | AN | Equal | Equal | 13.8 | Equal | Y |
| A | 14.4 | |||||
| O’Shaughnessy et al., 2002 | D | 30 | 4.2 | 11.5 | N/A | N |
| XD | 42 | 6.1 | 14.5 | |||
| Sledge et al., 2003 (ECOG 1193) | A | 36 | 5.8 | 18.9 | Equal | Y |
| T | 34 | 6 | 22.2 | |||
| AT | 47 | 8 | 20.0 | |||
| Albain et al., 2004 | GT | 41 | 6.1 | 18.5 | GT | N |
| T | 26 | 4 | 15.8 | |||
| Martin et al., 2007 (GEICAM) | GN | 36 | 6 | 15.9 | N/A | N |
| N | 26 | 4 | 16.4 |
Dose reductions and missed doses are more common with polychemotherapy, and as a result, the total dose of each agent received over a given time period may be decreased. In addition, when more than one agent is used, it is difficult to determine which drug was effective when the time comes to dose-reduce or change lines of therapy.
An Intergroup trial (E1193) compared doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as first-line therapy in 739 patients with MBC. Patients receiving single-agent therapy crossed over to the other agent at the time of disease progression. Although a superior response rate and time to treatment failure were observed in the combination therapy arm, there was no significant improvement in median OS or quality of life compared with sequential single-agent therapy.
In contrast to the findings of E1193, an international phase III trial comparing the efficacy and tolerability of docetaxel plus capecitabine (Xeloda, Hoffman-La Roche, Nutley, NJ) to single-agent docetaxel did demonstrate superior response rate, TTP, and OS in the combined treatment arm. It should be noted, however, that this study design did not facilitate crossover for the sequential use of the two agents as was done in the earlier Eastern Cooperative Oncology Group (ECOG) trial, so the two trials cannot be directly compared. Indeed, only a small minority of the patients randomized to docetaxel went on to receive capecitabine, and in this subset, there was no suggestion of an improvement in OS with the combination therapy.
The results of these and other similar studies published over the past decade have helped address the controversy over combination versus sequential single-agent therapy. Combination therapy likely offers superior response rate and TTP but at the expense of increased toxicity and difficulty of customization. Because no known combination offers a substantial survival benefit, the general treatment paradigm in hormone-refractory MBC is to begin with sequential single-agent chemotherapy ( Table 69.3 ). In highly symptomatic patients or those with a large tumor burden, it is also valid and acceptable to select a strategy of combination therapy with the goal of more rapid and effective cytoreduction ( Table 69.4 ). It is unclear whether this aggressive approach offers a survival advantage, but on the basis of available data, it appears unlikely. As patients move beyond first-line therapy, they should generally be treated with single-agent chemotherapy; the likelihood of response decreases with each subsequent line of therapy, and toxicity with combination regimens is consistently higher than with single agents. Of note, the question of combination versus single-agent chemotherapy has not been adequately evaluated within biologically defined subgroups of women with MBC. It is conceivable, although unproven at this time, that combination therapy could be more effective than single-agent therapy in triple-negative disease.
| Drug | Dosing Schedule | Study |
|---|---|---|
| Albumin-bound paclitaxel | 260 mg/m 2 IV q 3 wk or 100 mg/m 2 or 125 mg/m 2 IV, days 1, 8, 15 q 4 wk | Gradishar et al., 2005 |
| Capecitabine | 1000–1250 mg/m 2 PO BID, days 1–14, q 3 wk | Bajetta et al., 2005 |
| Cisplatin | 75 mg/m 2 q 3 wk | Silver et al., 2010 |
| Carboplatin | AUC 6 IV q 3–4 wk | Isakoff et al., 2016 |
| Cyclophosphamide | 50 mg PO daily, days 1–21, q 4 wk | Licchetta et al., 2010 |
| Doxorubicin | 20 mg/m 2 IV q wk or 60–75 mg/m 2 IV q 3 wk | Gasparini et al., 1991 Chan et al., 1999 |
| Epirubicin | 60–90 mg IV q 3 wk | Bastholt et al., 1996 |
| Eribulin | 1.4 mg/m 2 IV days 1, 8 q 3 wk | Cortes et al., 2011 |
| Gemcitabine | 800–1200 mg/m 2 days 1, 8, 15, q 4 wk | Seidman et al., 1995 |
| Ixabepilone | 40 mg/m 2 IV q 3 wk | Perez et al., 2007 |
| Paclitaxel | 175 mg/m 2 q 3 wk or 80 mg/m 2 q wk | Seidman et al., 1995 Perez et al., 2001 |
| Pegylated liposomal encapsulated doxorubicin | 50 mg/m 2 IV q 4 wk b | Ranson et al., 1997 O’Brien et al., 2004 |
| Vinorelbine | 25 mg/m 2 IV q wk | Zelek et al., 2001 |
a Doses often need to be adjusted with all regimens based on individual toxicity and cumulative toxicity over time.
b Dose is often modified from 50 mg/m2 to 40 mg/m 2 to minimize toxicity.
| Drug Combination | Dosing Schedule | Cycle Length (Days) | Study |
|---|---|---|---|
| AC | A:60 mg/m 2 IV day 1 C: 600 mg/m 2 IV day 1 | 21 | Fisher et al., 1990 |
| FAC | F: 500 mg/m 2 IV days 1, 8 A: 50 mg/m 2 IV day 1 C: 500 mg/m 2 IV day 1 | 21 | Hortobagyi et al., 1979 |
| CAF | C: 500 mg/m 2 IV day 1 A: 50 mg/m 2 IV day 1 F: 500 mg/m 2 IV days 1 and 8 | 21 | Smalley et al., 1977 |
| FEC | F 500 mg/m 2 IV day 1 E: 60 mg/m 2 IV day 1 C: 500 mg/m 2 IV day 1 | 28 | Blomquist et al., 1993 |
| FEC | F: 400 mg/m 2 IV days 1, 8 | 28 | Ackland et al., 2001 |
| E: 50 mg/m 2 IV days 1, 8 | |||
| C: 500 mg/m 2 IV days 1, 8 | |||
| Oral CMF | C: 100 mg/m 2 PO days 1–14 M: 40 mg/m 2 IV days 1, 8 F: 600 mg/m 2 IV days 1, 8 | 28 | Bonadonna et al., 1976 |
| IV CMF | C: 600 mg/m 2 IV day 1 M: 40 mg/m 2 IV day 1 F: 600 mg/m 2 IV day 1 | 21 | Bonadonna et al., 1985 |
| Docetaxel/capecitabine | D: 75 mg/m 2 IV day 1 C: 1250 mg/m 2 PO BID day 14 | 21 | O’Shaughnessy et al., 2002 |
| Paclitaxel/gemcitabine | P: 175 mg/m 2 IV day 1 G: 850–1250 mg/m 2 IV days 1, 8 | 21 | Albain et al., 2004 |
| Ixabepilone/capecitabine | I: 40 mg/m 2 C: 1000 mg/m 2 PO BID days 1–14 | 21 | Thomas et al., 2007 |
Previous trials have explored the role of combination chemoendocrine therapy in metastatic breast cancer, and have not shown significant improvement in response rates or in OS. In 2009, a large randomized trial conducted in the adjuvant setting by the Breast Cancer Intergroup of North America demonstrated a trend toward worse breast cancer outcomes when tamoxifen was given concurrent with, as opposed to in sequence with, adjuvant chemotherapy. After the publication of these data, standard practice has tended not to favor concurrent chemoendocrine therapy.
Chemotherapy for Metastatic Breast Cancer
Anthracyclines
Before the advent of taxanes and the widespread use of anthracyclines in the adjuvant setting, doxorubicin was thought to be the drug with the greatest single-agent activity in MBC. It has long been known to be a very active drug for the treatment of MBC. Response rates for monotherapy in anthracycline-naive women are on the order of 35% to 50%. The likelihood of response in heavily pretreated patients is generally significantly lower. Phase II studies have shown that anthracycline rechallenge can be done with acceptable cardiac safety and reasonable activity, although given the multitude of available agents, retreatment with an anthracycline is often reserved for later in the course of disease treatment.
Epirubicin, a doxorubicin analog, is also a highly active drug for the treatment of MBC. In multiple randomized trials of every-week or every-3-week dosing, epirubicin and doxorubicin were found to have equivalent response rates and TTP. On a milligram-to-milligram basis, epirubicin is generally considered less toxic than doxorubicin, with a lower reported rate of gastrointestinal and cardiac toxicity.
Anthracyclines can be safely combined with other agents as they are in the adjuvant setting. Well-studied combinations include doxorubicin/cyclophosphamide/5-fluorouracil (CAF/FAC) and epirubicin/cyclophosphamide/5-fluorouracil (FEC). In general, the inclusion of anthracyclines in these regimens increases toxicity but also raises objective response rates.
The major long-term toxicity seen with anthracyclines is myocardial damage, which can result in heart failure. The proposed mechanism for this is oxidative stress, which can cause arrhythmia, pericarditis, or myocarditis acutely, or death of cardiac myocytes chronically. Late-onset arrhythmias and ventricular dysfunction may be seen many years after treatment with anthracyclines. Risk of heart failure appears to be directly correlated with total lifetime dose, with a marked increase in risk of heart failure with cumulative doses greater than 400 to 450 mg/m 2 of doxorubicin as plotted on the Von Hoff dose-response curve.
Multiple strategies have been devised to minimize anthracycline cardiac toxicity, including prolonged infusions, dose divisions, liposomal preparations, and the administration of cardioprotectant drugs such as dexrazoxane. Weekly dosing provides comparable efficacy with a lower rate of significant toxicity. Liposomal preparations of doxorubicin such as pegylated liposomal doxorubicin (PLD/Doxil), nonpegylated liposome-encapsulated doxorubicin (NPLD; Myocet/D-99, Liposome Company, Elan Corporation, Princeton, NJ), and Evacet (TLC-99) have been shown to have at least equivalent response rates but less cardiac toxicity, allowing for the administration of greater cumulative doses. NPLD was studied in conjunction with cyclophosphamide for first-line treatment of MBC and found to have a safer therapeutic index than doxorubicin with a notable reduction in the rate of cardiotoxicity and grade 4 neutropenia. PLD has been shown to be safe and effective in combination with cyclophosphamide, the taxanes, vinorelbine, gemcitabine, or trastuzumab. Patients treated with PLD have minimal alopecia, nausea, or vomiting but a high incidence of stomatitis and hand-foot syndrome (HFS), which can be ameliorated with alterations in dosing schedule. By contrast, the toxicity profile of NPLD is similar to that seen with conventional doxorubicin with the exception of cardiac toxicity.
Although free radical scavengers such as dexrazoxane have been shown to lower the rate of adverse cardiac events in patients receiving both doxorubicin and epirubicin, most patients unfortunately have clinical progression before reaching cumulative doses concerning enough to warrant the drug’s use. For this reason, it is rarely used in clinical practice.
Of note, in the modern era anthracyclines are given less and less often for metastatic breast cancer. This is likely due to a combination of factors. As anthracycline-based regimens are used with increasing frequency for localized disease, less opportunity remains for additional dosing in the metastatic setting before the safety threshold for cumulative lifetime dose is reached, although in some patients there remains room for safe anthracycline rechallenge in later lines of therapy. Even in patients with metastatic disease and no prior anthracycline exposure, there is growing wariness about the issue of cardiac toxicity (with a reported incidence of approximately 3% in patients receiving 400 mg/m 2 of doxorubicin), which has the potential both to impair patients’ quality of life and to limit their eligibility for subsequent lines of standard and clinical trial-based therapy.
Taxanes
In the early 1990s, Holmes and colleagues first noted that paclitaxel resulted in objective response in 56% of patients with MBC. This class of drugs generated great excitement not only because of its effectiveness but also because of its unique mechanism of action. Paclitaxel (Taxol, Bristol-Myers Squibb, Princeton, NJ), which is isolated from the bark of the Pacific yew tree (Taxus brevifolia), shifts the dynamic equilibrium in microtubule assembly from tubulin to microtubules, resulting in highly stable microtubules that are rendered dysfunctional.
Docetaxel (Taxotere, Sanofi-Aventis, Bridgewater, NJ), which is synthesized from extracts of the needles of the European yew (Taxus baccata), was originally selected for development in the hope that it would improve on the efficacy of paclitaxel. It has a similar mechanism of action, which results in G2/M cell cycle arrest. Although similar, the effects of these drugs are not identical. In vitro, docetaxel has greater affinity for the tubulin binding site, longer intracellular retention time, and higher intracellular concentrations in target cells. In preclinical models, docetaxel has also been shown to both upregulate thymidine phosphorylase and induce bcl-2 phosphorylation to a greater degree than paclitaxel, suggesting that it may have more potent antitumor apoptotic effects. Nabholtz and associates conducted a phase III trial comparing docetaxel at 100 mg/m 2 every 3 weeks to mitomycin 12 mg/m 2 every 6 weeks plus vinblastine 6 mg/m 2 every 3 weeks. This demonstrated the efficacy of single-agent docetaxel for MBC with a response rate of 30% and a median survival of 11.4 months in patients with anthracycline-refractory disease. Clinically, differences in pharmacokinetics and side effect/toxicity profiles have been observed between paclitaxel and docetaxel. When used in the commonly prescribed doses, neuropathy is more common with paclitaxel and fluid retention, myelosuppression, and fatigue are more commonly seen with docetaxel. Paclitaxel is also more commonly associated with hypersensitivity reactions, although these remain quite rare.
Since taxanes were originally introduced, they have become a cornerstone of effective therapy for MBC and are now often administered before anthracyclines because they are perceived as a safer, equally effective alternative. A Cochrane meta-analysis indicated that for most patients, a taxane-containing regimen improved OS, TTP, and objective response rate compared with non–taxane-containing regimens. This was true for comparison with some, but not all, non–taxane-containing regimens.
The schedule of administration of taxane chemotherapy has also been a topic of debate. Although paclitaxel was historically administered every 3 weeks, many heavily pretreated MBC patients develop neutropenia with this approach. Seidman and colleagues investigated and established the feasibility of weekly low-dose paclitaxel in this population. Cancer and Leukemia Group B (CALGB) trial 9840 further addressed this question, comparing once-a-week to every-3-week paclitaxel in women with MBC. The study demonstrated that weekly paclitaxel is superior to standard (3-hour infusion) paclitaxel with respect to response rate (40% vs. 28%), TTP (9 vs. 5 months), and OS (24 vs. 16 months). Weekly paclitaxel was associated with more grade 3 sensory/motor neuropathy but less grade 3/4 neutropenia. In the treatment of MBC, weekly paclitaxel is therefore the preferred schedule of administration.
The ideal dosing schedule for docetaxel has also been examined. A phase II randomized trial compared weekly to every-3-week docetaxel in 83 women with MBC. Grade 3 and 4 toxicities were more common in the every-3-week arm, but relatively more patients withdrew from the weekly treatment arm because of toxicity. The objective response rate, TTP, and median time to treatment failure were not significantly different between the two study arms. Overall, it is generally believed that every-3-week docetaxel may be slightly more efficacious and less toxic than weekly treatment, and thus every-3-week treatment is favored.
Attention has also focused on taxane dose intensity with the hope of achieving improved OS. In the metastatic setting, higher doses of docetaxel can improve response rates but not OS. Similarly, CALGB 9342 demonstrated that a higher dose of paclitaxel failed to improve outcome in women with MBC. It should therefore be concluded that in the metastatic setting, lower doses are less toxic and probably more appropriate for achieving the goals of palliative chemotherapy.
There is consensus on the efficacy of taxanes for MBC, but some debate remains as to whether paclitaxel and docetaxel have equal efficacy. A phase III randomized head-to-head trial comparing every-3-week docetaxel to every-3-week paclitaxel after an anthracycline for MBC found that docetaxel was superior in terms of OS, TTP, and objective response rate. However, both hematologic and nonhematologic toxicities, particularly pain, stomatitis, asthenia, and neurotoxicity, were greater in the docetaxel arm. Febrile neutropenia occurred in 14.9% of docetaxel-treated patients compared with 1.8% of patients in the paclitaxel arm. Quality of life was similar for patients treated with the two agents. However, as discussed earlier, weekly administration is the optimal schedule for paclitaxel in the metastatic setting. The question of whether or not docetaxel is actually a superior drug to weekly paclitaxel in MBC can be answered only through a direct comparison with weekly paclitaxel, which has not been performed. Both paclitaxel and docetaxel remain reasonable options for the treatment of MBC. Of note, docetaxel is less influenced by multidrug resistance proteins and is incompletely cross-resistant with paclitaxel. Therefore treatment with docetaxel is a consideration in patients who have previously been treated with paclitaxel, but there are generally equally effective and less toxic options available as well.
Common side effects/toxicities seen with taxanes include glove-and-stocking sensory/motor neuropathy, fluid retention, stomatitis, alopecia, myalgias, arthralgias, nausea/vomiting, nail disorders, and hypersensitivity/allergic reaction due to the vehicle, polyoxyethylated castor oil, and alcohol (Cremophor EL). The syndrome of arthralgias and myalgias usually begins 1 to 3 days after infusion and can last for 2 to 4 days. Neuropathy is a common toxicity and often results in dose reductions or treatment discontinuation, particularly during treatment with weekly paclitaxel. Preliminary studies have shown promise for some prophylactic neuroprotective agents, including glutamine, glutathione, vitamin E, and acetyl- l -carnitine, but final recommendations await prospective confirmatory studies. Recent retrospective data suggest that venlafaxine may be an effective treatment for neuropathy secondary to taxane chemotherapy.
Granulocytopenia is often seen with taxane therapy, particularly when every-3-week docetaxel is used. This toxicity can be mitigated by the use of prophylactic white blood cell–colony stimulating factors. The NCCN recommends the prophylactic use of myeloid growth factors for any regimen with a greater than 20% risk of febrile neutropenia as has been documented with every-3-week docetaxel administration. Toxicities more commonly ascribed to docetaxel are epiphora (excessive tearing) as the result of lacrimal gland stenosis and nail changes. The nail changes are not merely cosmetic, with as many as one-third of patients reporting functional problems. In an effort to minimize this problem, a small trial in 45 patients used a gel-filled “frozen glove” with each infusion. This technique lowered the rate of both nail toxicity (51% vs. 11%) and skin toxicity (53% vs. 24%). However, additional investigations have shown the “frozen glove” strategy to be minimally effective and uncomfortable for patients. Hypersensitivity reactions are less common with docetaxel because it does not use the Cremophor vehicle but are still observed in a small percentage of patients.
In an effort to minimize the risk of taxane hypersensitivity reactions and eliminate the need for antiallergic corticosteroid premedication, nanoparticle albumin-bound (nab)-paclitaxel (Abraxane, Abraxis BioScience, Los Angeles, CA), an albumin-bound paclitaxel nanoparticle, was developed; it does not use Cremophor for drug delivery. In the absence of synthetic solvents, premedication with antihistamines and corticosteroids is not necessary. It offers additional convenience by shortening infusion duration from 3 hours to 30 minutes. Preclinical studies comparing nab-paclitaxel to paclitaxel in the standard, non–albumin-bound form demonstrated lower toxicity, with a maximum tolerated dose 50% higher for nab-paclitaxel. Two subsequent multicenter phase II studies of nab-paclitaxel in MBC showed response rates at least equal to those seen with standard taxanes and an acceptable toxicity profile. Numerous different doses and schedules of nab-paclitaxel have been examined, and phase II data indicate that nab-paclitaxel 150 mg/m 2 weekly for 3 of 4 weeks appears to be the most effective regimen in metastatic disease, although its side effect profile (particularly with regard to peripheral neuropathy) may prohibit long-term dosing.
A phase III trial comparing every-3-week nab-paclitaxel to every-3-week standard paclitaxel in metastatic breast cancer patients demonstrated higher overall response rate and longer time to tumor progression with nab-paclitaxel. On the basis of these data, albumin-bound paclitaxel was approved by the FDA for use in MBC after failure of combination anthracycline-containing chemotherapy. Nab-paclitaxel and docetaxel have also been compared head-to head in a phase II trial, which demonstrated significantly longer PFS and OS with nab-paclitaxel 150 mg/m 2 weekly. However, more recent phase III data comparing weekly paclitaxel (the optimal paclitaxel dosing schedule in advanced disease) to nab-paclitaxel, in which both agents were given with bevacizumab in chemotherapy-naive advanced breast cancer patients, showed that nab-paclitaxel was nonsuperior to paclitaxel, with a trend toward inferiority. Moreover, toxicity (including both neuropathy and hematologic effects) was greater with nab-paclitaxel. Therefore a standard role for nab-paclitaxel in the early lines of metastatic breast cancer treatment remains unclear. Data do indicate that nab-paclitaxel has some activity in patients previously treated with standard paclitaxel. Therefore, like docetaxel, it is a therapeutic option in paclitaxel-refractory patients.
The combination of taxanes with other chemotherapeutic agents has been shown to improve response rates in advanced breast cancer. Data from ECOG 1193 discouraged the combination of taxanes with anthracyclines because of increased toxicity and no demonstrable effect on long-term survival. The avoidance of this combination is further supported by the results of the Etude RegionAle dans le cancer du Sein MEtastatique (ERASME) 3 trial, which also combined anthracyclines and taxanes. More convincing evidence is available to support the combination of taxanes with either gemcitabine or capecitabine. Randomized trials have shown modest gains with these combination regimens, again at the expense of added toxicity. The decision to combine taxanes with other antineoplastics must carefully consider the risk/benefit ratio. Doublet therapy is generally reserved for patients who are very symptomatic or who have rapidly progressive disease in which the timing and degree of response are critical.
Alkylating Agents
The efficacy of alkylating agents in both the adjuvant and metastatic settings has been documented over many decades. Cyclophosphamide is the alkylating agent most commonly used in breast cancer because it is highly effective and can safely be combined with many other agents such as anthracyclines (e.g., doxorubicin) and antimetabolites (e.g., methotrexate). On the basis of data from the 1970s, single-agent cyclophosphamide was estimated to have a response rate between 10% and 50% in the first-line treatment of MBC. Estimated response rates in the modern era are difficult to calculate because cyclophosphamide is used commonly as a component of combination regimens in the adjuvant setting (AC, FEC, CMF) and rarely used in the first- or second-line setting for the management of metastatic disease.
Common toxicities seen with alkylating agents include nausea, vomiting, alopecia, and myelosuppression. The risk of hemorrhagic cystitis is quite low and nearly eliminated with the use of adequate hydration. Although the issue of secondary myelodysplasia or leukemia after treatment with alkylating agents is of concern in the adjuvant setting, this should not influence treatment decisions for patients with metastatic disease, given the risk/benefit ratio in the setting of shortened life expectancy.
In recent years, there has been increasing excitement about the platinum agents cisplatin and carboplatin for treatment of MBC, particularly in triple-negative disease. The use of platinums in MBC was originally investigated in the 1980s, when a small single-arm trial demonstrated a 47% response rate to cisplatin as first-line chemotherapy for MBC. In the 1990s, carboplatin was found to have a response rate of 20% to 35% in relatively untreated breast cancer, and less than 10% in pretreated patients. Because of concerns about the platinum agents’ toxicity and the concurrent development of the taxanes, further development of single-agent platinum in breast cancer largely stalled after that point. However, in the last 10 years there has been a new recognition that platinums have significant activity specifically in triple-negative breast cancer. Platinums work through a mechanism of DNA cross-linking, which may be particularly effective in tumors with DNA repair defects conferred by BRCA mutations. Triple-negative breast cancers, in turn, share many features with BRCA mutant counterparts. A small study of neoadjuvant single-agent cisplatin in triple-negative disease demonstrated proof-of-principle for platinum efficacy in this cohort; 22% of patients achieved pathologic complete response, and 50% achieved good pathologic response by Miller-Payne criteria.
In metastatic disease, a single-arm, phase II trial of cisplatin or carboplatin in first- or second-line treatment of triple-negative MBC demonstrated a response rate of 25.6%. The randomized phase III TNT trial compared docetaxel to carboplatin in patients with metastatic or recurrent locally advanced triple-negative breast cancer; eligible patients had received no nonanthracycline chemotherapy for metastatic disease. Overall response rates were not statistically different (31.4% and 35.6% for carboplatin and docetaxel, respectively) in the overall population. Likewise, there were no significant differences in PFS or OS. There were significantly higher rates of grade 3/4 neuropathy and febrile neutropenia in the docetaxel arm. In the BRCA -mutant population specifically, there was a significantly higher response rate to carboplatin (68.0%, vs. 33.3% for docetaxel; p = .03). The management of BRCA mutant patients is addressed later in this chapter. There is interest in developing genomic or genetic scores for BRCA-like phenotype in non- BRCA -mutant tumors, which could predict for platinum responsiveness; this pursuit is currently at an early stage.
Antimetabolites
Fluoropyrimidines
Fluoropyrimidines have been known for decades to be active in breast cancer and have been widely used in both the adjuvant and metastatic settings. Bolus 5-fluorouracil, both alone and in combination with leucovorin (LV), is known to be active in MBC and can be administered on various dosing schedules. In the past, 5-fluorouracil was preferred by some patients because of its low incidence of alopecia, nausea, and vomiting, and it was a reasonable choice in patients who had received multiple lines of therapy. Infusional 5-fluorouracil is now rarely used because of the widespread availability of the oral agent, capecitabine.
Capecitabine ( n 4-pentyloxycarbonyl-5′-deoxy-5-fluorocytidine) is an oral fluoropyrimidine carbamate that was rationally designed to generate 5-fluorouracil preferentially in tumor tissue, mimicking the activity seen with infusional 5-fluorouracil. This agent is converted to 5-fluorouracil through a cascade of enzymes, the last of which is thymidine phosphorylase. It is believed that capecitabine selectively targets malignant tissue over healthy tissue because the former possesses higher thymidine phosphorylase activity.
Multiple clinical trials have demonstrated the safety and efficacy of capecitabine in MBC that has progressed after treatment with anthracycline and taxane chemotherapies. In general, response rates of 20% to 29% are seen in the first-line setting, and as one would expect in the first-line setting, median survival is consistently greater than 1 year. Capecitabine’s ease of administration and documented activity in heavily pretreated breast cancer make it a popular choice for the treatment of metastatic disease. Other available oral fluoropyrimidines include tegafur (Ftorafur) and uracil/LV (Orzel), but neither is commercially available in the United States.
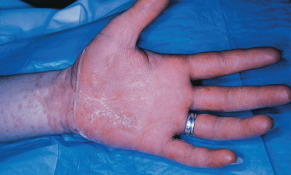
The most common treatment-related toxicities seen with capecitabine are hand-foot syndrome (HFS), diarrhea, nausea, vomiting, and fatigue. Unlike many other chemotherapeutic agents, capecitabine has a low incidence of myelosuppression, facilitating its use in heavily pretreated patients, in the elderly, and in combination with other agents that do cause myelosuppression. HFS, characterized by erythema, numbness, tingling, and either dysesthesias or paresthesias of the palms or soles, is the dose-limiting toxicity in many patients and occurs in up to half of patients treated with capecitabine ( Fig. 69.2 ). In severe cases of HFS, there can be painful swelling of the cutaneous tissues, and even desquamation, ulceration, or blistering. The syndrome may occur with initial courses of therapy or develop slowly over time. In a multicenter phase II study of capecitabine used to treat women with chemotherapy-refractory advanced breast cancer, 56% of patients developed HFS and 10% had severe HFS characterized by severe pain and/or skin breakdown. Strategies that may be used to minimize toxicity include manipulation of the daily dose/dose schedule; prophylactic pyridoxine (50–150 mg orally each day) has been proposed as a prophylactic treatment to reduce HFS, but multiple small trials have failed to demonstrate its efficacy. Topical emollients, particularly 10% urea cream, may prevent HFS and may provide symptomatic relief when it occurs.
Gemcitabine
Gemcitabine (Gemzar, Eli Lilly, Indianapolis, IN) is a nucleoside analog that replaces cytidine during DNA replication. This process arrests tumor growth, because new nucleosides cannot be attached to the “faulty” nucleoside, resulting in apoptosis. Response rates to gemcitabine in the metastatic setting vary from 14% to 37% in chemotherapy-naive patients to 1% to 30% in patients who have previously received a taxane and/or an anthracycline. Although alopecia and gastrointestinal toxicity are generally mild, the drug’s effects can be highly myelosuppressive, especially in patients who have received radiation or multiple lines of chemotherapy in the past. Flulike symptoms are reported in a small percentage of patients but are usually mild, transient, and treatable with acetaminophen. A rare but serious toxicity observed with gemcitabine is pulmonary toxicity (pneumonitis), which may occur more often when it is used in combination with a taxane.
In a phase III trial of 529 patients treated with paclitaxel alone versus paclitaxel/gemcitabine for first-line therapy in the metastatic setting, the combination showed an OS improvement from 15.8 months to 18.6 months for paclitaxel versus gemcitabine/paclitaxel, respectively. Of note, the paclitaxel was administered every 3 weeks. This trial led to the approval of gemcitabine for breast cancer in combination with paclitaxel. Subsequently, a trial of docetaxel alone versus docetaxel/gemcitabine in first- or second-line MBC showed no significant difference in response rate or OS. A recent phase randomized phase III trial compared cisplatin/gemcitabine with paclitaxel (every-3-week)/gemcitabine first-line MBC treatment and showed that cisplatin/gemcitabine was both noninferior and superior to paclitaxel/gemcitabine in terms of PFS. Gemcitabine also can be safely and effectively combined with a number of different agents in pretreated MBC, including taxanes, vinorelbine, and cisplatin. In practice, gemcitabine is given infrequently in the first-line setting but is used as a single agent or as a component of doublet therapy in more refractory MBC.
Other Microtubule Inhibitors
Initially discovered in 1993, the epothilones are a class of nontaxane microtubule polymerizing agents obtained from the fermentation of myxobacterium Sorangium cellulosum. Similar to taxanes, epothilones cause cell cycle arrest at the G2/M transition leading to cytotoxicity; however, these novel agents retain a much greater toxicity against P-glycoprotein–expressing multiple drug-resistant cells. Epothilones disrupt microtubule dynamics by stabilizing the microtubule from depolymerization, thereby enhancing microtubule polymerization. They compete for the same β-tubulin binding site as paclitaxel, but they are more powerful polymerizers of tubulin. Although they occupy the same binding site, they bind in a different fashion, compared with paclitaxel. This may explain the lack of cross-resistance between these two drug classes.
Although the family of epothilone compounds comprises many compounds, the most clinically advanced is ixabepilone (BMS-247550). Preclinical data have shown that epothilones work through partially nonoverlapping mechanisms with taxanes. Phase I and II studies have shown epothilones to be active even in patients who have progressed through taxane therapy. Ixabepilone (Ixempra, Bristol-Myers Squibb, Princeton, NJ) is a semisynthetic analog of epothilone B, which is a much more potent tubulin polymerizer in vitro than any of the commercially available taxanes. A phase III study of 752 patients with MBC who had previously received both an anthracycline and a taxane compared capecitabine monotherapy to capecitabine plus ixabepilone. The combined treatment arm had a longer median TTP (5.8 vs. 4.2 months) and an improved objective response rate (35% vs. 14%). Grade 3/4 treatment-related sensory neuropathy, fatigue, and neutropenia were more frequent with combination therapy. A second randomized phase III study confirmed these findings, demonstrating improved response rate and PFS for ixabepilone/capecitabine compared with capecitabine alone in pretreated MBC, although with no OS difference. Accordingly, ixabepilone was approved by the FDA in 2007 in combination with capecitabine to treat patients with metastatic or locally advanced breast cancer after failure of an anthracycline and a taxane. On the basis of a phase II trial demonstrating ixabepilone activity in patients with disease refractory to an anthracycline, taxane, and capecitabine, it was also approved as monotherapy. Small trials have examined the use of every-3-week versus weekly ixabepilone dosing, and it appears that every-3-week dosing is associated with better activity, at the expense of increased toxicity.
The toxicity profile of ixabepilone consists primarily of sensory neuropathy, fatigue/asthenia, myalgias, nausea/vomiting, and cytopenias. Both the hematologic and nonhematologic side effects of ixabepilone are more common when the drug is administered in combination with capecitabine. Pretreatment with H1- and H2-antagonists is recommended because hypersensitivity reactions have been observed in patients receiving epothilones.
Eribulin mesylate is a second nontaxane microtubule inhibitor commonly used in MBC. Eribulin is a synthetic analog of halichondrin B, a natural product that is isolated from the marine sponge Halichondria okadai. The compound inhibits the growth phase of microtubules. In preclinical models, it retains activity in paclitaxel-treated cells. After promising results in early-phase clinical studies, eribulin was investigated as a single agent for MBC in the phase III EMBRACE study.
In EMBRACE, patients who had received two to five prior chemotherapy regimens were randomized to either eribulin (administered on days 1 and 8 of a 21-day cycle) or treatment of the physician’s choice. In the accrued cohort, 99% of patients had received prior anthracycline and taxane, and 73% of patients had received prior capecitabine. OS was significantly higher with eribulin, compared with treatment of physician’s choice (13.1 months vs. 10.6 months, respectively). PFS was 3.6 months with eribulin versus 2.2 months with treatment of physician’s choice. The most common side effects in both treatment arms were neutropenia (52% with eribulin, 30% with treatment of physician’s choice) and asthenia/fatigue (54% with eribulin, 40% with treatment of physician’s choice). Other notable potential side effects of eribulin include neuropathy and alopecia. On the basis of these data, eribulin was FDA approved in 2010 for the treatment of MBC in the third line and beyond. Trials for its evaluation in earlier lines of therapy are ongoing.
Eribulin has also been formally compared with capecitabine in pretreated MBC. In a recent phase III trial, patients with locally advanced/MBC previously treated with up to three prior chemotherapy regimens, including an anthracycline and a taxane, were randomized to either eribulin or capecitabine treatment. There was no significant difference in OS or PFS between the two groups: 15.9 months versus 14.5 months for OS, and 4.1 months versus 4.2 months for PFS, for eribulin and capecitabine, respectively. Therefore, although eribulin clearly has a role in the management of refractory MBC, its sequence with capecitabine remains at the treating physician’s discretion.
Vinca Alkaloids
Vinorelbine (Navelbine, Pierre Fabre Pharmaceuticals, Parsippany, NJ), is a semisynthetic vinca alkaloid that prevents microtubule assembly (in contrast to taxanes, which stabilize microtubules). It has a well-established role in the treatment of MBC, demonstrating response rates of 16% to 50% depending on dosing and line of therapy; however, it has never been approved by the FDA for use in breast cancer. Dose-limiting toxicity is generally bone marrow suppression, most notably neutropenia, although grade 1 or 2 peripheral neuropathy is also reported by many patients. Vinorelbine’s side effect profile does not include cardiac toxicity, hypertension, or alopecia, thus making it an attractive agent for study in combination regimens as well as for use in the elderly. As has been the case with many other agents in MBC, the addition of a second chemotherapeutic agent to vinorelbine has not been shown to alter survival and generally increases toxicity.
An oral preparation of vinorelbine is also available for use in patients who prefer oral administration over intravenous infusion. Investigators in France have completed a phase II trial of first-line oral vinorelbine in women with locally advanced and MBC and have confirmed that it is both well tolerated and effective in this patient population. Data from clinical trials suggest that it is as active as the intravenous formulation. Of note, a higher incidence of gastrointestinal toxicity has been reported with the oral preparation. Despite its ease of administration, weekly blood counts must still be monitored given the risk of neutropenia; in the phase II trial, grade 4 neutropenia was seen in 17% of patients.
Treatment of HER2-Positive Metastatic Breast Cancer
The epidermal growth factor family of receptors consists of four transmembrane proteins (HER1 [EGFR], HER2, HER3, and HER4); each has different properties, but all are involved in the regulation of cell proliferation. The HER2 gene encodes the human epidermal growth factor receptor (HER2), which is known to be overexpressed in approximately 20% of all breast cancers. These tumors are associated with many adverse prognostic markers, including high tumor grade, high rate of cell proliferation, increased rate of nodal metastases, and relative resistance to certain types of chemotherapy. Retrospective studies suggest that HER2-positive disease is uniquely sensitive to anthracycline-based chemotherapy, and additional data support the efficacy of paclitaxel against this class of tumors. HER2-overexpressing tumors are more aggressive, and amplification of this gene was an independent risk factor for shortened disease-free and OS before the advent of HER2-directed therapy with trastuzumab (Herceptin, Genentech, South San Francisco, CA).
Trastuzumab is a humanized monoclonal antibody targeting the extracellular domain of the HER2 protein. Its exact mechanism is not certain but is thought to involve at least three main components: (1) attraction of immune cells to HER2-positive tumor cells through Fc domain interactions, resulting in antibody dependent cell-mediated cytotoxicity; (2) prevention of HER2 shedding, resulting in the inhibition of constitutive tyrosine kinase activity; and (3) internalization and degradation of HER2. First studied in the metastatic setting, in HER2-overexpressing patients trastuzumab monotherapy exhibited response rates of 25% in the first-line setting and 10% to 15% in previously treated MBC. It is important to note that historically not all clinical trials have required fluorescent in situ hybridization (FISH) testing, and many early trials included patients with 2+ staining by immunohistochemistry (IHC). Therefore some of the patients entered into early trastuzumab trials may have had tumors that would today be regarded as HER2 nonamplified. The inadvertent enrollment of HER2-negative patients is likely to have attenuated some of the effects of targeted therapy observed in these large treatment trials.
Beyond its single-agent activity, trastuzumab is highly effective when administered in combination with chemotherapy for MBC. In vitro models have shown that trastuzumab has synergistic effects when used in combination with cisplatin, carboplatin, docetaxel, and vinorelbine, and it has additive effects when administered with doxorubicin, cyclophosphamide, methotrexate, and paclitaxel. Although trastuzumab monotherapy does yield clinical responses and is sometimes used in patients who cannot tolerate combined therapy, the likelihood of a meaningful response is greater with combination therapy. For this reason, it is generally recommended that this HER2-directed therapy be administered in combination with chemotherapy to increase the likelihood of an objective response ( Table 69.5 ). Clinical data demonstrate that trastuzumab can be safely combined with many classes of drugs, including taxanes, vinorelbine, capecitabine, platinum, and ixabepilone.
| Agents | Study | Regimen |
|---|---|---|
| Docetaxel/trastuzumab/pertuzumab | Swain et al., 2015 | D: 75 mg/m 2 q 3 wk a + T: 8 mg/kg day 1 → 6 mg/kg q 3 wk + Pz: 840 mg/kg day 1 → 420 mg/kg q 3 wk |
| Paclitaxel/trastuzumab/pertuzumab | Dang et al., 2015 | P: 80 mg/m 2 q wk + T: 8 mg/kg day 1 → 6 mg/kg q 3 wk + Pz: 840 mg/kg day 1 → 420 mg/kg q 3 wk |
| T-DM1 (trastuzumab emtansine) | Verma et al., 2012 | T-DM1: 3.6 mg/kg q 3 wk |
| Paclitaxel/trastuzumab | Seidman et al., 2001 | P: 80 mg/m 2 q wk + T: 4 mg/kg day 1 → 2 mg/kg q wk |
| Docetaxel/trastuzumab | Marty et al., 2005 | D: 80–100 mg/m 2 day 1 + T: 4 mg/kg day 1 → 2 mg/kg q wk (q 3 wk) |
| Docetaxel/trastuzumab | Esteva et al., 2002 | D: 35 mg/m 2 days 1, 8, 15 + T: 4 mg/kg day 1 → 2 mg/kg q wk (q 4 wk) |
| Vinorelbine/trastuzumab | Burstein et al., 2001 | V: 25 mg/m 2 weekly + T: 4 mg/kg day 1 → 2 mg/kg q wk |
| Paclitaxel weekly/carboplatin/trastuzumab | Burris et al., 2004 Perez, 2004 | P: 70–80 mg/m 2 days 1, 8, 15 + C: AUC = 2 days 1, 8, 15 + T: 4 mg/kg day 1 → 2 mg/kg q wk (q 4 wk) |
| Paclitaxel q 3 wk/carboplatin/trastuzumab | Robert et al., 2006 | P: 175 mg/m 2 day 1 C: AUC = 6 day 1 T: 4 mg/kg day 1 → 2 mg/kg days 1, 8, 15 (q 3 wk) |
| Ixabepilone/trastuzumab | Tolaney et al., 2013 | I: 40 mg/m 2 q 3 wk + T: 8 mg/kg d1 → 6 mg/kg q 3 wk |
| Capecitabine/lapatinib | Geyer et al., 2006 | X: 1000 mg/m 2 PO BID days 1–14 L: 1250 mg PO QD days 1–21 (q 3 wk) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree