John E. Edwards Jr.
Candida Species
Written descriptions of oral lesions that were probably thrush date to the time of Hippocrates and Galen. Langenbeck, in 1839, found fungi in oral lesions of a patient.1 In 1861, Zenker described the first well-documented case of deep-seated Candida. The first case of Candida-induced endocarditis was described in 1940.2
The most interesting period in the history of Candida infections began in the 1940s, when the widespread use of antibiotics was introduced. Since then, the incidence of practically all forms of Candida infections has risen abruptly. Candida spp. have been the fourth most common organisms recovered from blood of hospitalized patients in the United States during recent decades.3 Between 2000 and 2005 the incidence of candidemia-related hospitalizations per 100,000 population rose by 52%.4 The burden of this illness in terms of morbidity, mortality, and expense is considerable. Estimates of the cost of candidemia in the United States are at least 2 billion dollars per year.5 A small sample of the numerous current reviews detailing the continued emergence of Candida as a common pathogen, the evolution of the disease in developing countries, and the shift to non-albicans species are available.6–9 The emerging Candida infections have included not only bloodstream infection but also arthritis, osteomyelitis, endophthalmitis, myocarditis, pericarditis, pacemaker-induced endocarditis, ventricular assist device infection, meningitis, peritonitis, myositis, pancreatitis, and others that are elaborated on in detail in their respective sections of this chapter. The increasing incidence of human immunodeficiency virus–1 infection, the use of therapeutic modalities for advanced life support, and certain surgical procedures, such as organ transplantation and the implantation of prosthetic devices, have expanded the incidence of Candida infections (Table 258-1).
Two interesting trends continue to develop with the extensive, rapidly evolving literature on Candida infections. First, as developing countries have introduced advanced medical care, including primarily more complex surgical procedures and more comprehensive cancer treatments, their increasing reports of the epidemiology and predisposing factors for Candida infections have recapitulated those that have been noted during the past 2 decades from countries with advanced medical care. Second, there has been a steady and significant increase in reports on the incidence and manifestations of Candida infections caused by non-albicans species.6
Pathogen
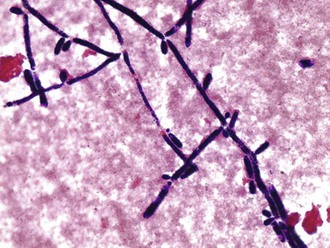
Candida organisms are yeasts (i.e., fungi that exist predominately in a unicellular form). They are small (4 to 6 µm), thin-walled, ovoid cells (blastospores) that reproduce by budding. They grow well in vented routine blood culture bottles and on agar plates and do not require special fungal media for cultivation. Several automated blood culture methods offer more rapid detection of Candida. Yeast forms, pseudohyphae, and hyphae may be found in microscopic examination of clinical specimens; identification of the hyphae and pseudohyphae is facilitated with 10% potassium hydroxide, which clears the epithelial cells, and with fluorescent microscopic examination of calcifluor10 white–stained smears. The organism also stains gram positive (Fig. 258-1).
Candida organisms form smooth, creamy white, glistening colonies that may resemble staphylococcal colonies. A rapid, presumptive identification of C. albicans can be made by placing the organism in serum and observing germ tube formation—small projections from the cell surface that appear within 90 minutes. However, both false-negative and false-positive germ tube formations may occur. The remainder of the identification and speciation procedures are based primarily on physiologic parameters rather than on morphologic characteristics. Metabolic tests include carbohydrate assimilation and fermentation reactions, nitrate utilization, and urease production. Chlamydospore formation is also used to identify C. albicans. Because of variation in species pathogenicity, speciation is desirable. There are more than 150 species of Candida, but only a small percentage is regarded as frequent pathogens for humans. They are C. albicans, C. guilliermondii, C. krusei, C. parapsilosis, C. tropicalis, C. pseudotropicalis, C. lusitaniae, C. dubliniensis, and C. glabrata (formerly classified as Torulopsis glabrata). When plated on CHROMagar Candida (Becton Dickinson, Cockeysville, MD), C. albicans, C. tropicalis, and C. krusei have distinctive colony colors that provide identification on primary isolation. C. dubliniensis was formerly included within C. albicans.11 C. dubliniensis forms germ tubes and chlamydospores and is identified as C. albicans by the most common methods. However, it will not grow at 45° C, is darker green when initially isolated on CHROMagar Candida, and hybridizes poorly to the Ca3 probe. Because it is not yet clear how the clinical features may differ from those of C. albicans, if at all, the two are considered synonymous in this chapter. Infections by other species are being reported with increasing frequency, such as the azole-resistant species Candida inconspicua and newer Candida species such as C. orthopsilosis and C. metapsilosis.12 The API Yeast 20C strip is a commercial kit that gives accurate identification of most Candida spp. in 2 to 5 days. Rapid methods have been developed for speciation, such as PNA FISH (AdvanDx, Woburn, MA), but they are not widely used at present.13
Epidemiology and Ecology
C. albicans organisms have been recovered from soil, animals, hospital environments, inanimate objects, and food. Non-albicans species may live in animal or nonanimal environments as well. Only rarely are Candida spp. laboratory contaminants. That principle has not been generally appreciated historically, and interpretation of positive cultures as laboratory or skin contaminants has led to important errors in patient management.
The organisms are normal commensals of humans and are commonly found on skin, throughout the entire gastrointestinal (GI) tract, in expectorated sputum, in the female genital tract, and in the urine of patients with indwelling Foley catheters. There is a relatively high incidence of carriage on the skin of health care workers. Although the vast majority of Candida infections are of endogenous origin, human-to-human transmission is possible. Examples are thrush of the newborn, which may be acquired from the maternal vagina, and balanitis in the uncircumcised man, which may be acquired through contact with a partner having Candida vaginitis. There is also important, emerging evidence that Candida infection can be acquired from the hospital environment. Molecular biology tools are improving considerably the understanding of Candida epidemiology.14–17
Pathogenesis and Immunology
An extensive discussion of the virulence factors, pathogenesis, and immunity of Candida infection is beyond the scope of this chapter and can be only highlighted herein. Currently, these are areas of intense investigation. A PubMed search using key words “Candida” and “pathogenesis” reveals well over 2500 publications in just the past 2 years.
Virulence factors responsible for the capability of the organism to damage the host have been studied extensively and reviewed recently.10,18 Of these, the capability of the organism to switch from yeast to hyphal phase, in vivo, has been perhaps the most intensively investigated through the years. In infected organs, the hyphal phase dominates. Locking the organism in yeast phase by genetic engineering renders it nonpathogenic.19 The precise role of this switch in conferring traits of pathogenicity is yet to be defined because C. glabrata can cause lethal infection but does not transform to a hyphal phase. By performing gene disruption studies, more than 150 pathogenicity factors (encoded gene products) have now been identified to attenuate virulence of the organism.10 Biofilm formation has been studied extensively and is important for the pathogenicity of the organism both on human tissues and on prosthetic materials.20,21 Numerous animal models for both deep visceral and mucocutaneous candidiasis have been explored. Mechanisms of adherence to host constituents and prosthetic devices have been examined. Hwp1 and genes within the ALS gene family have received the greatest attention for conferring adherence; however, numerous other genes have also been investigated as adhesins.22 Secreted aspartyl proteases23 and secreted phospholipases, capable of directly damaging tissue during invasion, have received considerable attention also.24
A primary defense mechanism against Candida is intact skin and mucosal membranes. Any process causing skin maceration or mucosal damage leaves the involved site susceptible to Candida invasion, even in healthy individuals. In recent years the importance of the dendritic cell for maintaining skin and mucosal integrity and as an immune effector cell has been recognized.25
Perhaps the greatest expansion in knowledge of the pathogenesis of Candida infections in recent years has been in the area of host defense mechanisms, innate immunity, and genetic susceptibility to infection. The organism is a normal commensal of humans and becomes pathogenic mainly as a result in alterations of the integrity of the immune system. For a more complete review of this topic the reader is directed to comprehensive, contemporary, and excellent reviews (only a partial list).26–30
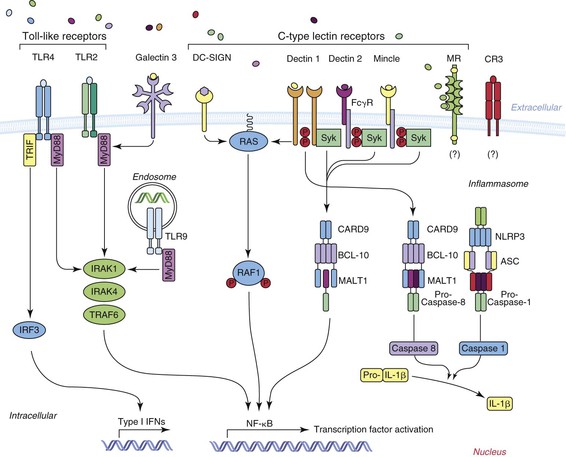
Although Figure 258-2, modified from Lionakis,26 summarizes current concepts regarding the cell surface pattern recognition receptors involved in the recognition of Candida by the innate immune system, findings related to STAT1, Dectin-1, and CARD9 deserve special emphasis. STAT1 is the gene encoding the STAT1 protein, which is a signal transducer and transcription activator31 that modulates cellular responses to a variety of cytokines and growth factors. Of great significance is that a subset of individuals with autosomal dominant chronic mucocutaneous candidiasis has been found to have mutations in the CC domain of the STAT1 gene in their peripheral blood monocytes, resulting in an insufficient production of interferon-γ, interleukin (IL)-17, and IL-22, leading to defective Th1 and Th17 responses, which are critical for antifungal defense of skin and mucosa.
Dectin-1 has been found to be a major recognition factor for β-glucan on Candida and it participates in the CARD9 pathway as shown in Figure 258-1.30 Defective surface expression of Dectin-1 results in impaired cytokine response in monocytes and macrophages but not neutrophils. Deficiency of this recognition factor has been found in some patients with recurrent vulvovaginitis or onychomycosis.32 However, deficiency of this recognition factor, to date, has not been shown to be associated with the acquisition of candidemia.31,33 Homozygous mutations in CARD9 have predisposed to chronic mucocutaneous candidiasis but also to Candida brain abscess and deep dermatophytosis.33a
Another area of intense evaluation has been the role of Th1 and Th17 cells in defense against invasion by Candida.34 There is evidence in mice that these cells play a role in defense against both mucosal candidiasis and disseminated disease.35 Additionally, IL-17 has been shown to increase in humans in response to vaccination with the encoded product of the N-terminus of Als3.36
Numerous other cells and components of the immune system play critical roles in defense against Candida damage. An incomplete list includes a broad array of cytokines, defensins, platelets, complement components, macrophages and monocytes, Treg and natural killer (NK) cells, extracellular traps, Th2 cells, and antibodies. The reader is directed to the comprehensive reviews of these additional inflammatory and immune components.37,38 The neutrophil is a key component for defense against this pathogen. Patients with neutrophil NADPH (nicotinamide adenine dinucleotide phosphate) oxidase deficiency and chronic granulomatous disease are known to have increased susceptibility to Candida infections. A potent predisposing factor for disseminated candidiasis is iatrogenic neutrophil deficiency, usually caused by cytotoxic chemotherapy, illustrating further the importance of the neutrophil in defense. Additionally, innovative recent studies have addressed the tissue-mediated injury caused by chemokine receptor Ccr1–driven neutrophil accumulation in the kidney, increasing mortality in the murine model.39 These studies are directed toward eventually improving neutrophil effectiveness in disseminated candidiasis. Of additional interest is the possibility that Candida-generated amyloid may inhibit neutrophil function.40
The most important predisposing factors to Candida infection, and especially to disseminated candidiasis, are iatrogenic. The introduction of newer therapeutic modalities for advanced life support into clinical medicine has been primarily responsible for the dramatic change in incidence of this disease. Of these factors, probably the most important have been the introduction of antibiotics and the widespread use of indwelling intravenous catheters. Antibiotics suppress normal bacterial flora and allow Candida organisms to proliferate, especially in the GI tract. The factors that predispose patients to disseminated candidiasis, which have been mentioned in countless reviews, are listed in Table 258-1. The increase in nearly all forms of Candida infections is a consequence of the advancement of modern medical therapeutics.
Clinical Manifestations
As the frequency of diseases due to Candida has increased, a relatively large number of manifestations, which were previously either not recognized or extremely infrequent, have become well documented. The discussion of these clinical manifestations is facilitated by their subdivision into mucocutaneous and deep organ involvement.
Mucous Membrane Infections
Thrush
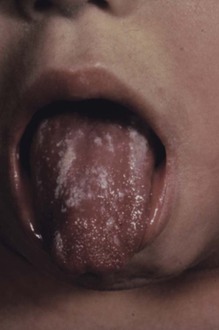
Oral Candida infections are common and have been reviewed extensively.41–44 The term thrush is applied to a specific form of oral candidiasis characterized by creamy white, curdlike patches on the tongue and other mucous membranes (Fig. 258-3); the patches are removable by scraping and leave a raw, bleeding, and painful surface. The patches are actually pseudomembranes consisting of Candida, desquamated epithelial cells, leukocytes, bacteria, keratin, necrotic tissue, and, in the mouth, food debris. The formation of Candida biofilm45 is important in its establishment, as well as epithelial cell invasion.46 The diagnosis is made by the clinical appearance of the lesion and confirmed by scraping, using either a potassium hydroxide smear or Gram stain to show masses of hyphae, pseudohyphae, and yeast forms. Simple culturing does not solidify the diagnosis because Candida grows easily from normal mouths. In addition to the classic lesions, which have been described by Lehner,47 other manifestations include (1) acute atrophic candidiasis, a nonspecific atrophy of the tongue that is thought to be a sequela of acute pseudomembranous candidiasis; (2) chronic atrophic candidiasis or “denture sore mouth,” which is a chronic inflammatory reaction and epithelial thinning under the dental plates; (3) angular cheilitis, an inflammatory reaction at the corners of the mouth (not due exclusively to Candida); (4) Candida leukoplakia, firm, white plaques affecting the cheek, lips, and tongue that have a protracted course (and, in rare instances, may be precancerous). Since the introduction of inhaled steroids for the treatment of asthma, especially in children, oral thrush has been reported extensively in patients treated with these agents.48 The incidence has ranged from 0% to 77%. Thrush developing in patients who use inhaled steroids usually resolves spontaneously without a change in the dosage of the agent or is successfully managed with topical nystatin or clotrimazole. Other patients with a high incidence of thrush are cancer patients and those with acquired immunodeficiency syndrome (AIDS). Patients with thrush for no obvious reason should be evaluated for AIDS. Because of the introduction of potent antiretroviral therapy, the incidence of colonization and symptomatic infection thrush has declined somewhat in patients with AIDS but remains common.49
Certain patients may have chronic thrush, and thrush may be a concomitant of the chronic mucocutaneous candidiasis (CMC) syndrome (see later). As is the case with CMC and recurrent Candida vaginitis, certain genetic defects are being discovered.31
Candida Esophagitis
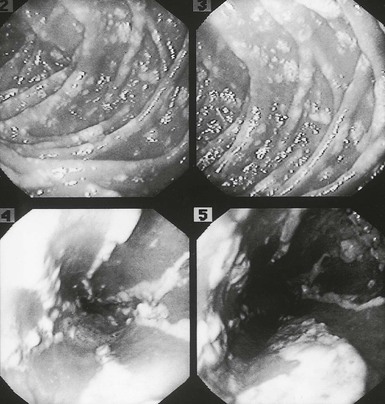
Although there have been a small number of reports of Candida esophagitis occurring in patients with no known underlying illness, it is more commonly associated with treatment of malignancy of the hematopoietic or lymphatic systems (Fig. 258-4) and in AIDS patients.50–52 Additionally, omeprazole has been implicated as a risk factor. Esophageal disease was believed to occur by direct spread from oral disease (thrush), but reviews have shown that Candida esophagitis may occur frequently without thrush; this is an important clinical concept. The most common symptoms of Candida esophagitis include painful swallowing, a feeling of obstruction on swallowing, and substernal chest pain. Nausea and vomiting may also occur. The diagnosis is made definitively by biopsy during endoscopy (Fig. 258-5). However, the appropriate clinical settings, associated with the endoscopic appearance of white patches resembling thrush, which show masses of hyphae and pseudohyphae on scraping, are enough evidence to initiate therapy without a histopathologic demonstration of the organisms invading the mucosa. It is important to recognize that Candida esophagitis can occur simultaneously with herpes simplex virus or cytomegalovirus infection in severely immunocompromised patients. Radiographic examination may be helpful in making a clinical diagnosis; irregularity of the esophageal mucosa as a result of ulcerations may be seen, as well as shoulder defects, diverticula, fistulas, and dilatation of the esophagus from denervation. Endoscopy is the preferred procedure for definitive diagnosis, however. The pseudomembrane that forms may become so extensive that it causes intraluminal protrusions and partial esophageal obstruction. Perforation of the esophagus due to esophageal candidiasis is rare. Generally, if perforation occurs, it is in the lower two thirds of the esophagus. Some patients have had extensive esophageal disease and been almost asymptomatic, probably as a result of denervation of the esophagus from the disease. Other complications include bleeding and, presumably, dissemination.

Nonesophageal, Mucous Membrane, Gastrointestinal Candidiasis
The most common clinical setting for GI-tract candidiasis is in patients with neoplastic disease. The esophagus is the most common site, followed by the stomach and small intestines. Gastric candidiasis has two forms: diffuse mucosal involvement (rare) and focal invasion of benign gastric ulcers. The most frequent lesions are single or multiple ulcerations containing Candida deep in the ulcer beds. In addition, but with less frequency, chronic gastric ulcer, gastric perforation, and malignant gastric ulcer with concomitant Candida infection are seen. Small bowel and large bowel infection also occur. Ulceration is the most common lesion. Pseudomembrane formation and ulceration in association with tumor also occur. As in other mucous membrane Candida infections, white plaques may be seen on endoscopy of the duodenum, and there may be thickening of mucosal folds in the duodenum and jejunum. Equal in frequency to the involvement of the small bowel is involvement of the large bowel, which again may be characterized by ulceration, superficial erosions, pseudomembrane formation, penetrating ulcers, and perforation. A succinct review of defensive mechanisms of mucosal candidiasis exists,53 as well as a more comprehensive review.54 The details are beyond the scope of this discussion. The importance of neutropenia facilitating hematogenous dissemination from the intestine has been illustrated in the experimental murine model.55 It is highly likely that hematogenous dissemination from the gastrointestinal tract occurs in patients with gastrointestinal mucosal damage due to cytotoxic chemotherapy for cancer treatment.
Candida Vaginitis
Candida has assumed the role of the most common cause of vaginitis with higher frequency rates than those of Trichomonas or bacterial vaginosis. This common infection is most frequently seen in a setting of diabetes mellitus, antibiotic therapy, and pregnancy. In addition, although controversial, the use of birth control pills may be a predisposing factor. Estimates are that 75% of women have an episode of candidal vaginitis during their lifetime, and 8% have recurrent vulvovaginal candidiasis. Recent investigations have shown that certain different mutations and polymorphisms in innate immune genes are likely to be responsible for recurrence in this subset of patients.26,29,56 Vaginal candidiasis is not clearly more common or more refractory to treatment in patients with AIDS.
The widespread use of antibiotic therapy may be the most important factor responsible for the emergence of Candida-induced vaginitis, and the importance of biofilm formation by Candida is under investigation.57 This biofilm formation may be influenced by the change in microbial flora caused by antibacterial antibiotics. Excellent reviews of the current trends in the epidemiology and pathogenesis of vaginal candidiasis are now available.58–60 In these reviews the rising incidence of non-albicans Candida species is emphasized, as well as a rising incidence of resistance in the species of Candida recovered.61,62
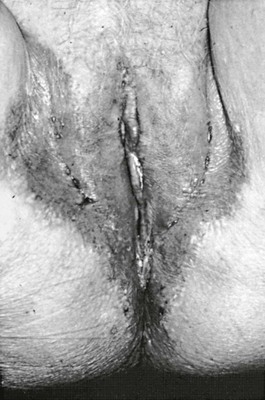
Although Candida-induced vaginitis may be accompanied by a thick, curdlike discharge, scanty discharge may instead characterize the infection. Edema and intense pruritus of the vulva is almost always present. The discharge consists of epithelial cells and masses of hyphae and pseudohyphae, accompanied by lymphocytes and neutrophils.63 The vagina and labia are usually erythematous, and extension onto skin of the perineum can occur (Fig. 258-6). In addition, endometritis due to Candida has been reported, and the urethra may become secondarily infected.
Cutaneous Candidiasis Syndromes
Generalized Cutaneous Candidiasis
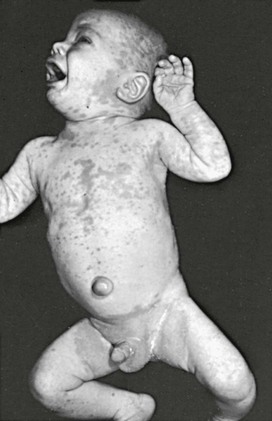
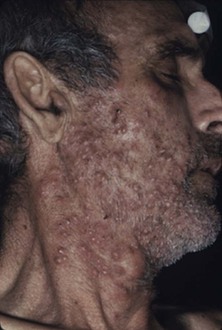
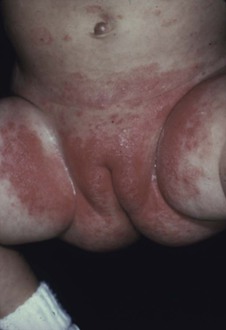
This condition is an unusual form of cutaneous candidiasis and is characterized by widespread eruptions over the trunk, thorax, and extremities, with increased severity in the genitocrural folds, anal region, axillae, hands, and feet (Fig. 258-7). The process begins as individual lesions that spread into large confluent areas. It is more common in newborns and children but does occur in adults.64–66
Erosio Interdigitalis Blastomycetica
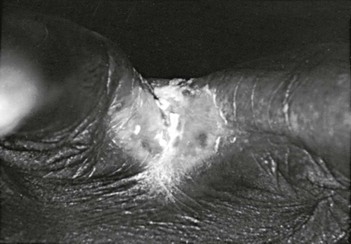
This term applies to Candida infection occurring between the fingers or toes (Fig. 258-8). It has a red base, may extend onto the sides of the digits, is painful, and is predisposed to by maceration.67,68
Candida Folliculitis
Infection at the hair follicles with Candida can occur (Fig. 258-9). Rarely, the condition may become extensive. It must be distinguished from folliculitis caused by the dermatophytes and tinea versicolor. This folliculitis has been described in immunocompromised hosts and intravenous drug abusers. Its incidence is increased in obesity.69
Candida Balanitis
This process begins as vesicles on the penis that develop into patches resembling thrush and are accompanied by severe itching and burning. Candida is one of the more common causes of balanitis.70 It may spread to the thighs, gluteal folds, buttocks, and scrotum. It can be acquired through sexual intercourse with a partner who has vaginal candidiasis.71
Cutaneous Lesions of Disseminated Candidiasis
Four distinct types of lesions associated with disseminated candidiasis have been described. The macronodular lesions (Fig. 258-10) are 0.5 to 1 cm in diameter, pink to red, and may either be single or occur widely distributed over the entire body.72,73 The most accurate method of making a specific diagnosis is by punch biopsy and demonstration of organisms on histologic section. Most patients with these lesions are neutropenic and all have disseminated candidiasis, not local inoculation. Additionally, lesions resembling ecthyma gangrenosum,74,75 purpura fulminans,76 and leukocytic vasculitis77 have been described. Chronic lesions of pyoderma gangrenosa may become superinfected with Candida and delay their definitive diagnosis.
Intertrigo
This common skin condition affects any site in which skin surfaces are in close proximity and provide a warm, moist environment. It begins as vesicopustules, which enlarge and rupture, causing maceration and fissuring. The area of involvement has a scalloped border with a white rim consisting of necrotic epidermis, which surrounds an erythematous, macerated base. Frequently, satellite lesions that may coalesce and extend the affected area are found. A variant form of cutaneous candidiasis in the intertriginous region has a miliary appearance resembling miliaria rubra with erythematous macules or vesicopustules.
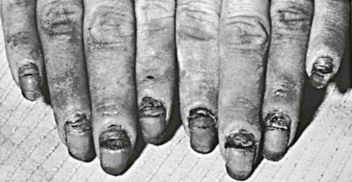
Paronychia and Onychomycosis
Candida is one of the most common causes of paronychia. Species other than albicans may be causative.78 Many skin bacteria, as well as Candida, can usually be recovered by culture of the infected area. The appearance of the reaction is that of a relatively well-localized area of inflammation that becomes warm, glistening, and tense and may extend extensively under the nail (Fig. 258-11). Unless the disease process is stopped, secondary thickening, ridging, and discoloration occur, and nail loss may result.
Candida paronychia occurs in association with frequent immersion of the hands in water. People who may contract paronychia include dishwashers, laundry workers, and young mothers. There is also a higher incidence of paronychia among diabetic patients than in the nondiabetic population. Specific diagnosis is made by Gram stain or potassium hydroxide preparation and culture showing predominantly Candida organisms.
In addition to paronychia, Candida is the most common cause of onychomycosis, which is prevalent in the elderly.79,80
Diaper Rash
Candida is a common cause of diaper rash in infants.81 The condition generally starts in the perianal area and spreads over the perineum in the region of diaper contact (Fig. 258-12). The process is facilitated by maceration caused by wet diapers. The probable origin is the GI tract. Diagnosis is made by scraping the area and demonstrating the organisms on potassium hydroxide preparation.
Perianal Candidiasis
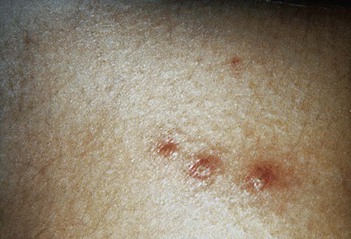
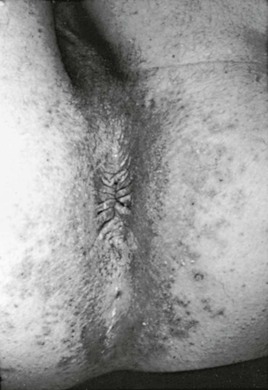
Although numerous organisms and combinations of organisms have been associated with pruritus ani either alone or in combination, Candida is a frequent cause.82 The perianal skin develops marked erythema and progresses to maceration (Fig. 258-13). Intense pruritus results. Complications include involvement of the anal canal and extensive spread over the perineum.
Chronic Mucocutaneous Candidiasis
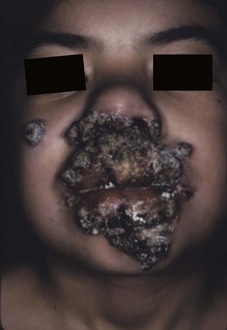
The term chronic mucocutaneous candidiasis (CMC) is used to describe a heterogeneous group of Candida infections of the skin, mucous membranes, hair, and nails that have a protracted and persistent course despite what is usually adequate therapy. The major complication is disfiguring lesions of the face, scalp, and hands. Candida esophagitis can be a long-term complication and cause esophageal stenosis. Alopecia in areas of infection is common and may be permanent. The subject has been reviewed comprehensively in a classic publication.83 The different forms of CMC are generally categorized as follows: familial CMC, autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED), chronic localized candidiasis, chronic mucocutaneous candidiasis with thymoma, candidiasis with keratitis, and candidiasis with hyper-IgE syndrome. Most forms of CMC begin in infancy or within the first 2 decades; rarely, the onset may be after the age of 30 years. The first manifestation is usually oral thrush followed by nail infections and then skin involvement. There is a broad spectrum of severity, ranging from chronic involvement of an isolated nail to a severely disfiguring form (Candida granuloma) (Fig. 258-14).
Of great interest in recent years has been the discovery of certain, specific innate immunity defects that result in CMC. These discoveries have elucidated certain constituents of the innate immune system that play active roles in normal defense against Candida infections. The topic has been reviewed comprehensively.31 Briefly, the genes discovered so far include AIRE (21q) (APECED), STAT3, DOCK8 (9p), TYK2 (19p), CARD9 (9q), Dectin-1 (12p), IL17RA (22q), IL17F (6p), STAT1 (2q), and IL12Rβ1 (19p).
Some patients have other immune abnormalities than those described earlier, such as cutaneous anergy to such antigens as streptokinase-streptodornase, mumps virus, and tetanus toxoid; defective lymphocyte transformations to nonspecific mitogens (e.g., phytohemagglutinin); defective monocyte chemotaxis; a lack of anti-Candida antibody in salivary IgAs; plasma inhibitors to Candida-stimulated lymphocyte transformations; suppressor lymphocytes; and various degrees of thymic aplasia.
In general, most patients with CMC do not have identifiable B cell or neutrophil dysfunction. However, a group of patients with selective immunoglobulin deficiencies has been described.84 Hematogenous, disseminated candidiasis is rare, probably due to intact, functional neutrophils.
Deep Organ Involvement
Central Nervous System Candidiasis
Candida infects both parenchymal brain tissue and the meninges, usually as a complication of hematogenously disseminated candidiasis. Approximately 50% of patients with Candida meningitis have had disseminated disease in other organs. When infection occurs in brain parenchyma, it generally forms multiple microabscesses and small macroabscesses scattered throughout the tissue. Rarely, larger abscesses have occurred and may be visualized by computed tomography. Mechanisms by which this organism localizes to the brain in the mouse model have been elucidated.85
Virtually all patients with Candida meningitis have had cerebrospinal fluid pleocytosis. Fifty percent have had a lymphocyte pleocytosis with an average count of 600 cells/mm3. Sixty percent have had hypoglycorrhachia and elevated protein levels; organisms have been present on wet mount or Gram stain in approximately 40%.C. albicans has been the responsible pathogen in 90% of cases. Occasionally, cases caused by C. tropicalis or other species are reported.
The clinical manifestations of central nervous system involvement with diffuse microabscesses may be variable. If the patient is comatose or noncommunicative, detection of abnormalities may be exceptionally difficult. When meningitis is present, the signs of meningeal irritation (headache, stiff neck, irritability), typical of any meningeal infection, are frequently present. In the newborn, particularly the very low birth weight neonate, diagnosis is often difficult and delayed, leading to permanent neurologic sequelae. Lumbar puncture should be considered when the blood culture of such infants contains Candida.
In addition to occurring as a complication of disseminated candidiasis, Candida meningitis may result from infection of a ventricular shunt or may be introduced by lumbar puncture, trauma, and neurosurgery86 or may complicate bacterial meningitis. The signs and symptoms are nonspecific. Untreated, the mortality rate is high; it is reduced substantially with antifungal therapy. Hydrocephalus is a reasonably frequently occurring complication of the infection. An increase in the number of cases of Candida meningitis reported in neonates is occurring. AIDS is now considered a predisposing factor for Candida meningitis.
Respiratory Tract Candidiasis
In general, Candida pneumonia occurs in two forms: (1) either local or diffuse bronchopneumonia originating from endobronchial inoculation of the lung, a rare event, or (2) as a hematogenously seeded, finely nodular, diffuse infiltrate, which in its early stages may be difficult to distinguish from congestive heart failure or Pneumocystis pneumonia. Other forms of Candida pneumonia are rare; those that have been described are necrotizing pneumonia, Candida pulmonary fungus ball, and transient infiltrates due to Candida. Radiographic and computed tomographic findings are nonspecific, and definitive diagnosis depends on biopsy-proven fungal invasion of pulmonary tissue. Because of a relatively high prevalence of yeasts colonizing the respiratory tract, especially in ill patients, a diagnosis of Candida pneumonia cannot be made based on radiographic findings and recovery of yeasts from sputum or endotracheal tube aspirate.87,88 Candida has also caused bronchial infection, laryngitis, epiglottitis, and infection of laryngeal prostheses. The entity of “fungal empyema thoracis” has been described as an emerging clinical entity.89 Infection with Candida alone or in association with bacteria has been frequent within the population of patients with this entity. Hospitalized patients who develop bacterial pneumonia, are treated with broad-spectrum antibiotics, and then begin growing Candida from the sputum only rarely have Candida pneumonia (i.e., pneumonia that is predominately caused by Candida).
Cardiac Candidiasis
In addition to causing endocarditis, Candida infects both the pericardium and the myocardium. Candida myocarditis occurs as diffuse microabscesses scattered throughout the myocardium with normal intervening myocardial tissue. The relatively high incidence of myocarditis has been stressed by Franklin and coworkers,90 who found that 62% of their 50 patients with disseminated candidiasis had myocardial involvement. Other retrospective autopsy studies have shown a range from 8.4% to 93%. Candida myocarditis has also occurred in AIDS patients. Autopsy series of disseminated candidiasis reveal a surprisingly high incidence of myocarditis (without associated valvular involvement) and point to the importance of thorough cardiac evaluation in patients who may have disseminated candidiasis. Of interest has been the emergence of Candida organisms as a cause of pericarditis. A review of purulent pericarditis spanning the years 1960 to 1974 revealed that Candida organisms were either the single cause or combined with Aspergillus in 15% of the 26 cases.91 The association of Candida pericarditis with either cardiac surgery or burns has been emphasized.
Candida Endocarditis
This manifestation of Candida was once a distinctly rare phenomenon, but its true incidence has increased simultaneously with the generalized increase in Candida infections. Of all the forms of fungal endocarditis, Candida is by far the most common. In the past 4 decades, there have been more than 214 reported cases. In a detailed review of 319 cases of fungal endocarditis, Candida accounted for 67% of the cases.92 The entity of Candida endocarditis has been reviewed extensively.93–95,96,97 Additional cases have been reported in children.98 Candida endocarditis occurs in association with six clinical factors: (1) underlying valvular heart disease, (2) heroin addiction,99 (3) cancer chemotherapy, (4) implantation of prosthetic valves,100 (5) prolonged use of intravenous catheters (endocarditis, right atrial fungal masses, and infection of atrial myxomas have all been described), and (6) preexisting bacterial endocarditis, on which it is superimposed. Of these associations, by far the most frequent is the postoperative cardiac surgery, accounting for approximately 50% of the cases. Of interest is the frequency of species other than C. albicans that have caused endocarditis; a minimum of 41% of the cases have been due to organisms of other species, some of which have been rarely recovered species. Newer species continue to be reported. In heroin addicts, C. parapsilosis has been the most common causative organism.101 Of interest is that heroin abuse has diminished in a percentage of underlying predisposing conditions relative to iatrogenic causes.
The pathogenic mechanisms for fungal endocarditis are not fully understood, but patients who undergo cardiac valve replacement are at risk for candidemia by being exposed to multiple antibiotics, prolonged intravenous fluid administration, and intravenous plastic catheters. Both the damaged endocardium and prosthetic material apparently serve as foci for the localization of Candida organisms. Also, contamination of suture material has been implicated in cases reported with concentration along the suture line. Contamination of homografts and heterografts before insertion has also been documented. Experimental evidence for a role in the pathogenesis of adherence of Candida to platelet fibrin complexes and/or fibronectin is accumulating.102,103 The mechanisms for adherence and the potential for blocking the adherence remain under investigation.
The most common valves involved in Candida endocarditis have been the aortic and mitral. In postoperative Candida endocarditis, the type of surgery has not been as important as the length of the postoperative course and complications during the postoperative period. Candida infection has been seen in simple valvulotomies and in prosthetic material placement, heterografts, and homografts. Pacemaker endocarditis has also been described.104 The physical findings and usual symptoms of Candida endocarditis are not significantly different from those of bacterial endocarditis with the exception of the occurrence of large emboli to major vessels. Osler’s nodes, Janeway lesions, splinter hemorrhages, hepatosplenomegaly, hematuria, proteinuria, pyuria, and casts all can occur. In addition, although the lesions of hematogenous ocular candidiasis have been described much more frequently in the setting of disseminated candidiasis without endocarditis, they may also be seen with endocarditis.
The complications of Candida endocarditis are similar to those of bacterial endocarditis and include valve perforation, myocarditis, congestive heart failure, and major emboli. Although most cases of postoperative Candida endocarditis occur in the first 2 postoperative months, some have occurred later, and some patients who have been treated have had recurrent active disease after 2 years, and perhaps as long as 8 years. Therefore, in following patients treated for postoperative endocarditis, careful follow-up must be extended over a prolonged period.
Most patients with Candida endocarditis have positive blood cultures. Modern blood culture methods likely provide better sensitivity. Echocardiography is becoming progressively more helpful, and vegetations may be detected with this technique. False-negative results are common, especially in cases of mural endocarditis without valvular involvement. Transesophageal echocardiography has improved the sensitivity, particularly in the mitral valve. The largest prospective experience with various serum diagnostic tests for Candida endocarditis has been published recently.95 Detection of (1→3)-β-d-glucans and antimannan antibody had a high negative predictive value. Studies of serodiagnostic tests were inconclusive regarding their impact on treatment.
The therapy for Candida endocarditis is discussed in detail in the section on therapy. Before the introduction of surgical procedures for the management of Candida-induced endocarditis, the mortality rate from this disease was approximately 90%. With combined surgical and medical therapy, this high mortality rate has dropped to approximately 45%. Because of the introduction of newer antifungals, there has been a greater propensity for their use in chronic suppression for selected patients.
Candida endocarditis has been seen in association with bacterial endocarditis as a polymicrobial infection. In general Candida has been a superinfection introduced by prolonged intravenous catheterization for antibiotic administration. Interesting investigations are developing showing how Candida/bacterial interactions enhance the pathogenicity of a coinfection.105,106
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree